Analysis of GmERF5 Response to the Rhizobial Type III Effector NopAA Underlying the Nodule in Soybeans
Abstract
1. Introduction
2. Materials and Methods
2.1. Function Domain Prediction of Candidate Gene GmERF5
2.2. Construction of Rhizobial nopAA Mutants
2.3. qRT-PCR Analysis of GmERF5 Expression
2.4. Yeast Two-Hybrid Analysis
2.5. Hairy Root Transformation and Detection of Positively Transformed Hairy Roots
3. Results
3.1. Predictive Analysis of GmERF5 Protein Structural Domains
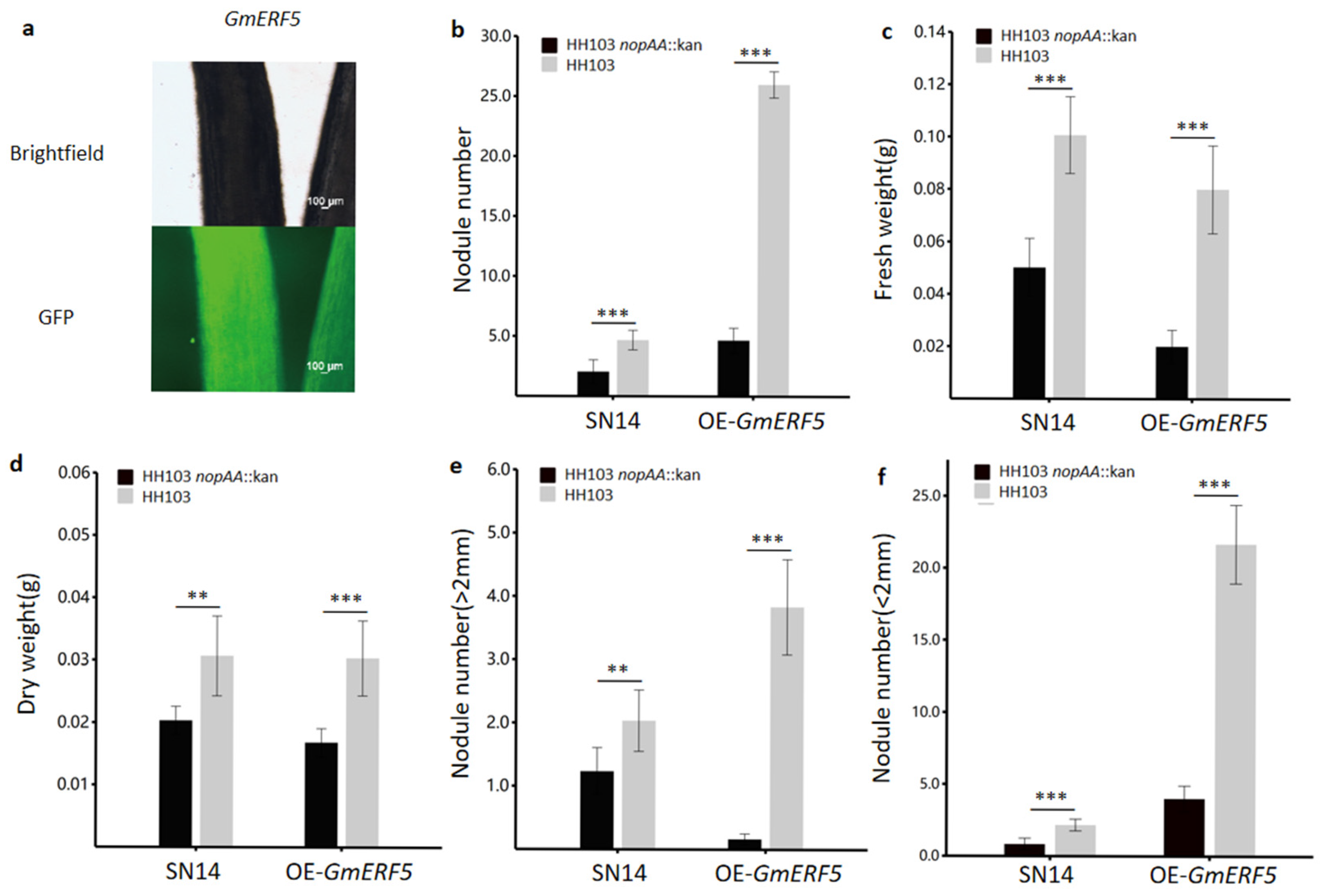
3.2. Nodule Capacity Identification of Mutant HH103 nopAA::kan
3.3. GmERF5 Responses to NopAA
3.4. GmERF5 Effect on Nodule of Soybeans
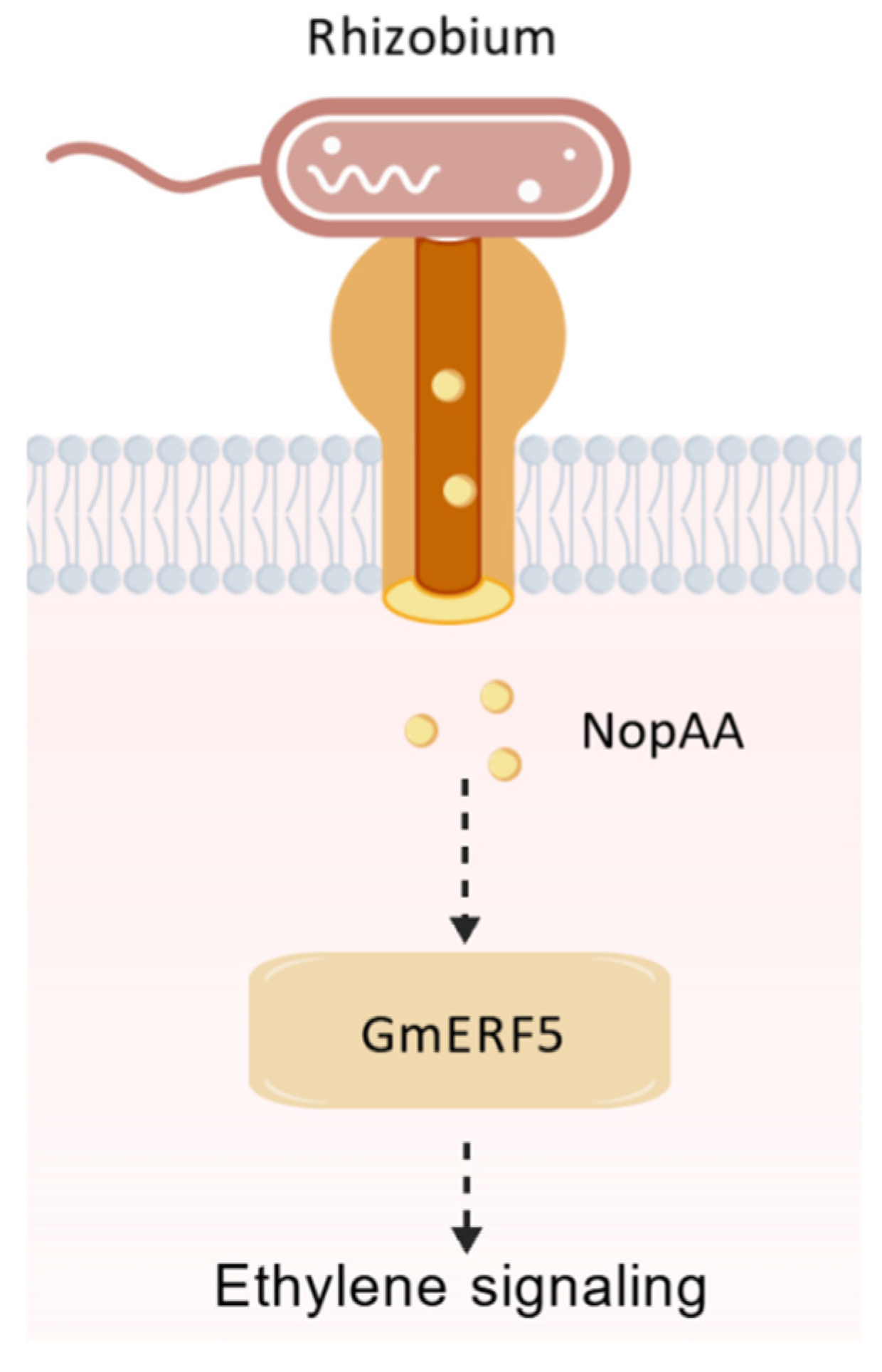
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirriam, A.; Mugwe, J.; Nasar, J.; Kisaka, O.; Ranjan, S.; Gitari, H. Role of Phosphorus and Inoculation with Bradyrhizobium in Enhancing Soybean Production. Adv. Agric. 2023, 2023, 3231623. [Google Scholar] [CrossRef]
- Sousa, W.d.S.; Soratto, R.P.; Peixoto, D.S.; Campos, T.S.; da Silva, M.B.; Vaz Souza, A.G.; Teixeira, I.R.; Gitari, H.I. Effects of Rhizobium inoculum compared with mineral nitrogen fertilizer on nodulation and seed yield of common bean. A meta-analysis. Agron. Sustain. Dev. 2022, 42, 52. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Wrona, D.; Przybylko, S. Effect of Nitrogen Fertilization of Apple Orchard on Soil Mineral Nitrogen Content, Yielding of the Apple Trees and Nutritional Status of Leaves and Fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Yao, Y.; Yuan, H.; Wu, G.; Yan, J.; Zhao, D.; Chen, S.; Kang, Q.; Ma, C.; Gong, Z. Nitrogen fixation capacity and metabolite responses to phosphorus in soybean nodules. Symbiosis 2022, 88, 21–35. [Google Scholar] [CrossRef]
- Zambon, L.M.; Umburanas, R.C.; Schwerz, F.; Sousa, J.B.; Barbosa, E.S.T.; Inoue, L.P.; Dourado-Neto, D.; Reichardt, K. Nitrogen balance and gap of a high yield tropical soybean crop under irrigation. Front. Plant Sci. 2023, 14, 1233772. [Google Scholar] [CrossRef]
- Thanthrige, N.; Weston-Olliver, G.; Das Bhowmik, S.; Friedl, J.; Rowlings, D.; Kabbage, M.; Ferguson, B.J.; Mundree, S.; Williams, B. The cytoprotective co-chaperone, AtBAG4, supports increased nodulation and seed protein content in chickpea without yield penalty. Sci. Rep. 2023, 13, 18553. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Zhuang, Q.; Xue, Y.; Yao, Z.; Zhu, S.; Liang, C.; Liao, H.; Tian, J. Phosphate starvation responsive GmSPX5 mediates nodule growth through interaction with GmNF-YC4 in soybean (Glycine max). Plant J. 2021, 108, 1422–1438. [Google Scholar] [CrossRef]
- Zeng, X.B.; Li, D.Z.; Lv, Y.F.; Lu, Y.; Mei, L.L.; Zhou, D.L.; Chen, D.S.; Xie, F.L.; Lin, H.; Li, Y.G. A Germin-Like Protein GLP1 of Legumes Mediates Symbiotic Nodulation by Interacting with an Outer Membrane Protein of Rhizobia. Microbiol. Spectr. 2023, 11, e0335022. [Google Scholar] [CrossRef]
- Clua, J.; Roda, C.; Zanetti, M.E.; Blanco, F.A. Compatibility between Legumes and Rhizobia for the Establishment of a Successful Nitrogen-Fixing Symbiosis. Genes 2018, 9, 125. [Google Scholar] [CrossRef]
- Teulet, A.; Camuel, A.; Perret, X.; Giraud, E. The Versatile Roles of Type III Secretion Systems in Rhizobium-Legume Symbioses. Annu. Rev. Microbiol. 2022, 76, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Dong, W.T.; Murray, J.; Wang, E.T. Innovation and appropriation in mycorrhizal and rhizobial Symbioses. Plant Cell 2022, 34, 1573–1599. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.W.E. Enhancing Rhizobium-Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia—The roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef]
- Gühl, K.; Holmer, R.; Xiao, T.T.; Shen, D.F.; Wardhani, T.A.K.; Geurts, R.; van Zeijl, A.; Kohlen, W. The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula. Genes 2021, 12, 988. [Google Scholar] [CrossRef]
- Teulet, A.; Busset, N.; Fardoux, J.; Gully, D.; Chaintreuil, C.; Cartieaux, F.; Jauneau, A.; Comorge, V.; Okazaki, S.; Kaneko, T.; et al. The rhizobial type III effector ErnA confers the ability to form nodules in legumes. Proc. Natl. Acad. Sci. USA 2019, 116, 21758–21768. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Chen, L.; Fu, Y.; Li, C.; Qi, Z.; Zou, J.; Zhu, R.; Li, S.; Wei, W.; et al. Mining for genes encoding proteins associated with NopL of Sinorhizobium fredii HH103 using quantitative trait loci in soybean (Glycine max Merr.) recombinant inbred lines. Plant Soil 2018, 431, 245–255. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Ma, S.; Zheng, H.; Feng, H.; Wang, Y.; Wang, J.; Liu, C.; Xin, D.; Chen, Q.; et al. GmARP is Related to the Type III Effector NopAA to Promote Nodulation in Soybean (Glycine max). Front. Genet. 2022, 13, 889795. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Yang, W.; Zhu, L.; Su, C.; Wang, X.; Wu, H.; Sun, Z.; Li, X. Genome Wide Identification and Expression Profiling of Ethylene Receptor Genes during Soybean Nodulation. Front. Plant Sci. 2017, 8, 859. [Google Scholar] [CrossRef]
- Van de Poel, B.; Smet, D.; Van Der Straeten, D. Ethylene and Hormonal Cross Talk in Vegetative Growth and Development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.F.; Wei, W.; Fan, Z.Q.; Deng, W.; Li, Z.G.; Bouzayen, M.; Pirrello, J.; Lu, W.J.; Chen, J.Y. MaXB3 Modulates MaNAC2, MaACS1, and MaACO1 Stability to Repress Ethylene Biosynthesis during Banana Fruit Ripening. Plant Physiol. 2020, 184, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-Aminocyclopropane-1-carboxylate (ACC) in Plant-Bacterial Interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Ma, B.; Ma, T.; Xian, W.H.; Hu, B.; Chu, C.C. Interplay between ethylene and nitrogen nutrition: How ethylene orchestrates nitrogen responses in plants. J. Integr. Plant Biol. 2023, 65, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.J.; Nascimento, F.X.; Glick, B.R.; Rossi, M.J. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018, 66, 252–259. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Li, Z.; Liu, M. Role of ethylene response factors (ERFs) in fruit ripening. Food Qual. Saf. 2020, 4, 15–20. [Google Scholar] [CrossRef]
- Xie, Z.L.; Nolan, T.M.; Jiang, H.; Yin, Y.H. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Vernie, T.; Moreau, S.; de Billy, F.; Plet, J.; Combier, J.-P.; Rogers, C.; Oldroyd, G.; Frugier, F.; Niebel, A.; Gamas, P. EFD Is an ERF Transcription Factor Involved in the Control of Nodule Number and Differentiation in Medicago truncatula. Plant Cell 2008, 20, 2696–2713. [Google Scholar] [CrossRef]
- Xin, D.-W.; Liao, S.; Xie, Z.-P.; Hann, D.R.; Steinle, L.; Boller, T.; Staehelin, C. Functional Analysis of NopM, a Novel E3 Ubiquitin Ligase (NEL) Domain Effector of Rhizobium sp Strain NGR234. PLoS Pathog. 2012, 8, e1002707. [Google Scholar] [CrossRef]
- Ni, H.; Peng, Y.; Wang, J.; Wang, J.; Yuan, Y.; Fu, T.; Zhu, Z.; Zhang, J.; Pan, X.; Cui, Z.; et al. Mapping of Quantitative Trait Loci Underlying Nodule Traits in Soybean (Glycine max (L.) Merr.) and Identification of Genes Whose Expression Is Affected by the Sinorhizobium fredii HH103 Effector Proteins NopL and NopT. Agronomy 2022, 12, 946. [Google Scholar] [CrossRef]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ma, C.; Zhou, Z.; Yang, D.; Zheng, J.; Wang, Q.; Li, H.; Zhou, H.; Sun, Z.; et al. QTL Mapping and Data Mining to Identify Genes Associated with the Sinorhizobium fredii HH103 T3SS Effector NopD in Soybean. Front. Plant Sci. 2020, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Li, S.; Liu, P.; Xi, L.; Wang, S.; Meng, Y.; Liu, J.; Yang, X.; Lu, Y.; Wang, H. Construction and identification of a yeast two-hybrid bait vector and its effect on the growth of yeast cells and the self-activating function of reporter genes for screening of HPV18 E6-interacting protein. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2010, 30, 8–12. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs Feed-Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Deng, X.; Zou, J.; Ma, C.; Li, C.; Yin, T.; Liu, C.; Wang, J.; Chen, Q.; et al. GmPBS1, a Hub Gene Interacting with Rhizobial Type-III Effectors NopT and NopP, Regulates Soybean Nodulation. Agronomy 2023, 13, 1242. [Google Scholar] [CrossRef]
- Marie, C.; Deakin, W.J.; Viprey, V.; Kopcinska, J.; Golinowski, W.; Krishnan, H.B.; Perret, X.; Broughton, W.J. Characterization of Nops, nodulation outer proteins, secreted via the type III secretion system of NGR234. Mol. Plant Microbe Interact 2003, 16, 743–751. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Y.; Li, Y.; Lei, T.; Yan, F.; Su, L.; Li, X.; Zhao, Y.; Sun, X.; Li, J.; et al. Isolation and molecular characterization of GmERF7, a soybean ethylene-response factor that increases salt stress tolerance in tobacco. Gene 2013, 513, 174–183. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Burger, M.; Wang, Y.; Chory, J. Two interacting ethylene response factors regulate heat stress response. Plant Cell 2021, 33, 338–357. [Google Scholar] [CrossRef]
- Dorival, J.; Philys, S.; Giuntini, E.; Brailly, R.; de Ruyck, J.; Czjzek, M.; Biondi, E.; Bompard, C. Structural and enzymatic characterisation of the Type III effector NopAA (=GunA) from Sinorhizobium fredii USDA257 reveals a Xyloglucan hydrolase activity. Sci. Rep. 2020, 10, 9932. [Google Scholar] [CrossRef]
- Bartsev, A.V.; Deakin, W.J.; Boukli, N.M.; McAlvin, C.B.; Stacey, G.; Malnoë, P.; Broughton, W.J.; Staehelin, C. NopL, an effector protein of Rhizobium sp NGR234, thwarts activation of plant defense reactions. Plant Physiol. 2004, 134, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J.; Gao, Y.; Dong, X.; Feng, H.; Yang, M.; Yu, Y.; Liu, C.; Wu, X.; Qi, Z.; et al. The type III effector NopL interacts with GmREM1a and GmNFR5 to promote symbiosis in soybean. Nat. Commun. 2024, 15, 5852. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Song, Y.; Yu, T.; Pei, Y.; Jiang, H.; Chen, Q.; Xin, D. Analysis of GmERF5 Response to the Rhizobial Type III Effector NopAA Underlying the Nodule in Soybeans. Nitrogen 2025, 6, 38. https://doi.org/10.3390/nitrogen6020038
Xia L, Song Y, Yu T, Pei Y, Jiang H, Chen Q, Xin D. Analysis of GmERF5 Response to the Rhizobial Type III Effector NopAA Underlying the Nodule in Soybeans. Nitrogen. 2025; 6(2):38. https://doi.org/10.3390/nitrogen6020038
Chicago/Turabian StyleXia, Lianheng, Yunshan Song, Tong Yu, Ying Pei, Hongwei Jiang, Qingshan Chen, and Dawei Xin. 2025. "Analysis of GmERF5 Response to the Rhizobial Type III Effector NopAA Underlying the Nodule in Soybeans" Nitrogen 6, no. 2: 38. https://doi.org/10.3390/nitrogen6020038
APA StyleXia, L., Song, Y., Yu, T., Pei, Y., Jiang, H., Chen, Q., & Xin, D. (2025). Analysis of GmERF5 Response to the Rhizobial Type III Effector NopAA Underlying the Nodule in Soybeans. Nitrogen, 6(2), 38. https://doi.org/10.3390/nitrogen6020038






