Abstract
Water scarcity is a global crisis and of particular concern in arid regions like Morocco. One creative solution is mining unusual water sources, such as landfill leachate. The presence of nitrogen in the sediment was studied as part of the use of sugar lime sludge in treating landfill leachate for irrigation purposes. A volume of 40 L of landfill leachate was treated with three different concentrations of sugar lime sludge (25%, 35%, and 50%). After homogenization and agitation of the mixture for 24 to 36 h, it was permitted to settle through the concrete decantate and supernatant. Nitrogen was efficiently decanted into the sediment during the composting process with green waste, enhancing the quality of the finished compost. The supernatants underwent physicochemical and microbiological analyses to ascertain their suitability for irrigation. The findings showed that the number of fecal streptococci was decreased by 99.13% at a 25% concentration of sugar lime sludge. The percentage of organic matter in the sediment rose from 10% to 40%, suggesting that the leachate had partially depolluted. The pH and electrical conductivity of the supernatants were within irrigation guidelines. The safety of diluted supernatants for plant germination was verified by phytotoxicity experiments conducted on maize seeds. The compost made from the decantate and green waste showed acceptable physical and chemical properties. Statistical analysis was conducted using JAMOVI software version 2.6.26. One-way ANOVA was used to assess the significance of treatment effects on microbiological and physicochemical parameters. The results confirmed statistically significant differences (p < 0.05) between the sludge concentrations, supporting the effectiveness of the treatment process. This study demonstrates how sugar lime sludge can be used to turn landfill leachate into a sustainable and safe irrigation water source, resolving environmental issues and promoting creative water management techniques.
1. Introduction
Waste generation has significantly increased in recent decades due to globalization. Globally, more than two billion metric tons of municipal solid waste (MSW) are produced per year; by 2050, this amount is expected to increase by almost 70% [1].
Leachate, the liquid produced by the decomposition of waste in landfills, is a hazardous mixture containing organic and inorganic pollutants [2], heavy metals [3], and different compounds like endocrine-disrupting chemicals [4] and microplastics [5]. The physical, chemical, and biological processes inside a landfill vary depending on waste composition, moisture, temperature, and time, leading to significant variations in leachate composition and pollution potential [6]. Based on the landfill’s age, leachates can be categorized into three types:
- Young leachate (<5 years): high biodegradable organic load, low pH;
- Intermediate leachate (5–10 years): decreased biodegradability;
- Stabilized leachate (>10 years): complex and refractory organics with high molecular weight.
The concentration and the type of micropollutants in landfill leachate vary considerably based on factors such as landfill, age, and physicochemical properties [7].
There are different types of leachates, categorized into three types depending on the age of the landfill. The first type is young leachate, which is less than 5 years old; it has a high organic load and is characterized by good biodegradability and a low pH. The second category is intermediate leachate, aged between 5 and 10 years, during which biodegradability decreases. For leachates older than 10 years, we refer to stabilized leachate, which contains substances with high molecular weight [7].
Various physicochemical parameters are measured to assess the characteristics of landfill leachates, including pH variations, suspended solids, chemical oxygen demand (COD), biological oxygen demand (BOD), and the concentration of chloride, nitrogen, and ammonia [8].
The microbial communities present in leachate are central to the degradation of organic contaminants, making their analysis critical for understanding both the biodegradability of the leachate and its broader ecological impact [9].
The environmental impact of landfill leachate is a significant concern in the management of municipal solid waste. Due to its complex composition, this contaminant effluent poses a serious threat to both groundwater and surface water resources [10,11].
The implications for humans are equally concerning. Exposure to pollutants from contaminated water can result in various health issues, including neurological, respiratory, and gastrointestinal disorders [12,13].
1.1. Key Pollutants in Landfill Leachate
1.1.1. Xenobiotic Organic Compounds (XOCs)
XOCs include synthetic compounds such as hydrocarbons (e.g., benzene, toluene), halogenated hydrocarbons (e.g., chlorobenzene, tetrachloroethylene), and phenols (e.g., 4-chlorophenol). These compounds originate from household items like aerosol sprays and disposable diapers and are known for their mobility and persistence in the environment [10,14]. However, no data are available on the final concentrations of these elements in the leachate stored in the landfill.
1.1.2. Dissolved Organic Matter (DOM)
DOM in landfill leachate is mainly generated by the organic matter coming from household waste, which represents 65% in Morocco [15], and is secondary from anaerobic degradation in the landfill with four phases: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [16].
1.1.3. Heavy Metals
Heavy metals (e.g., Hg, Cd, Pb, Cr, Ni, Cu, Zn) originate from household waste, electronic waste, batteries, and thermometers. Their mobility depends on leachate pH, COD, and the affinity of metals for organic matter [17,18,19].
1.1.4. Inorganic Macro-Compounds
Leachates also contain major inorganic ions such as Na+, Mg2+, NH4+, SO42−, Cl−, and HCO3−, which contribute to salinity and increase treatment complexity [10].
1.2. Treatment Methods for Landfill Leachate
Several methods are used worldwide for the treatment of landfill leachate Table 1. In the case of Morocco, the most common methods used are osmose reversis and biological treatments. However, the cost of such methods is high; moreover, no recovery of the treated leachate in the watering of green spaces, for example, is observed. The aim of this study is to investigate the potential of sugar lime sludge, an agricultural by-product from the sugar industry, as a low-cost adsorbent for landfill leachate and to reuse the liquid obtained from the treatment for watering green spaces. Being a waste material itself, sugar lime sludge offers both economic and environmental advantages, especially in developing countries where budget-friendly and sustainable solutions are critical. Notably, this material has already been used within our laboratory for composting purposes [20,21], demonstrating its valorization potential in waste management applications [20]. This paper is the first part of this research, which corresponds to the tests carried out with sugar lime sludge and the physicochemical, bacteriological, and biological analysis of the liquid obtained after treatment.

Table 1.
Various treatment methods for landfill leachate.
2. Materials and Methods
2.1. Materials
2.1.1. Sugar Lime Sludge
The sugar lime sludge used in this study originates from the sugar industry in Morocco, from the Doukkala region. The composition of this sludge is given in Table 2.

Table 2.
Chemical composition of sugar lime sludge.
2.1.2. Leachate from the Marrakech Landfill
The leachate analyzed was collected from the Marrakech landfill site, Morocco, on February 2024. It’s young leachate. The characteristics of the Raw Leachate are given in Table 3.

Table 3.
Characterization of raw leachate.
2.2. Methods
Concentrations of Sugar Lime Sludge and Treatment Durations:
Three concentrations of sugar lime sludge, 25%, 37%, and 50%, along with two treatment durations, 24 h and 36 h, were established. Following these durations and after the decantation process, a clear separation was observed between the supernatant and the decantate. The supernatant is intended for irrigation purposes and will undergo further analysis to ensure its suitability and compliance with irrigation standards, while the decantate will be mixed with green waste for composting.
2.3. Bacteriological Analysis
2.3.1. Dilution Preparation
Dilutions were made from the raw leachate sample and from each supernatant obtained after treatment. Dilutions for each sample were prepared in sterile physiological water following ISO 6887-1.
Bacterial analysis was carried out on raw leachate as well as on the supernatants obtained after leachate treatment.
2.3.2. Enumeration of Total Aerobic Mesophilic Flora
According to ISO 4833, the enumeration of total mesophilic flora (FMAT) is carried out through inoculation on nutrient agar using the dilution–spread technique. For each sample, 100 µL of each dilution (10−1 to 10−5) was inoculated into Petri dishes in three replicates using the rake method.
2.3.3. Enumeration of Fecal Streptococci
ISO 7899-2 specifies the use of Bile Esculin Azide (BEA) medium for the identification of fecal streptococci. In this procedure, 100 µL of each decimal dilution (10−1, 10−2, 10−3, 10−4) is placed in sterile Petri dishes containing BEA agar. The dishes are incubated at 37 °C for 24 h. The presence of fecal streptococci is confirmed by the appearance of small translucent colonies surrounded by a black halo, which are then counted.
2.3.4. Enumeration of Total Coliforms
According to NF ISO 9308-1, lactose agar with tergitol 7 and TTC is used for the detection and enumeration of total coliforms. Four series of four Petri dishes containing the culture medium were inoculated with 100 µL of the appropriate dilution. The dishes were then incubated at 37 °C for 24 and 48 h.
2.3.5. Enumeration of Fecal Coliforms
The experimental protocol for the detection and enumeration of fecal coliforms is identical to that of total coliforms, except for the incubation temperature. After incubation at 44.5 °C for 24 h, dishes showing specific colonies were counted.
2.3.6. Composting of the Decantate and Nitrogen Recovery
A total volume of 40 L of landfill leachate was treated with sugar lime sludge, and the decantates were mixed with green waste and composted for 90 days. Nitrogen content in the sediment was measured before and after composting to assess nutrient recovery. The C2D2 treatment (corresponding to a 37% concentration of sugar lime sludge with 36 h of contact time) was selected for composting, as it showed the most promising results in terms of organic matter enrichment in the sediment.
2.3.7. Analysis on Supernatants and Decantates
Chemical and biological characteristics of the supernatants and decantates were analyzed. The organic matter (OM) content was determined by calcining samples at 600 °C. The sample was calcined to combust remaining organics and was used to confirm the organic inclusions through weight analysis pre- and post-heating to determine organic matter. The Kjeldahl method was used to determine total nitrogen in decantates (before and after samplings).
pH Measurements:
Provably to the AFNOR NF ISO 10-390 standard, which describes an accurate method for measuring the pH of the samples, measurements were also made.
Electrical Conductivity:
First, electrical conductivity (EC) was measured, as it gives an idea of samples’ salinity by indicating the dissolved ion concentration. A BioBase 900 multiparameter meter, which can be used to measure multiple parameters (i.e., pH, conductivity, etc.) at the same time, guarantees accuracy and consistency in measurements.
Phytotoxicity Assessment:
The bioassay of phytotoxicity was performed using the Zucconi method [40] with maize seeds (Zea mays) as the indicator species. This approach was modified to evaluate the ecotoxicological consequences of the treatment on the supernatant.
Seed Germination: The treated leachate with varying dilution levels was infused with maize seeds for 7 days. Germination data were raw on a daily basis, and the final germination rate was calculated from the 7th day.
Root Elongation: Finally, on the last day of the experiment, the length of each radicle (first root) of all the germinated seeds was measured for inhibition or stimulation of growth.
With these parameters, the germination index (GI), a widely used phytotoxicity parameter, was calculated. In addition, analysis at several dilutions made it possible to assess concentration-dependent toxicity, as well as the potential for treatment leachate to be fit-for-purpose for irrigation or environmental recycling.
2.3.8. Statistical Analysis
Statistical validation was performed using the Jamovi application. An ANOVA test was applied to determine the significance of the effect of different concentrations and treatment durations. Student’s t-test was used to compare each treated sample with the raw leachate (control). Results were expressed as mean ± standard deviation, and a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Physico-Chemical Characteristics of Supernatants
3.1.1. pH Values After Leachate Treatment with Three Concentrations of Sugar Lime Sludge
The raw leachate has a pH of 8.48, which is slightly alkaline, categorizing it as young leachate [41]. The alkaline nature of sugar lime sludge, when added to the leachate, gives an alkaline supernatant with pH values ranging from 8.69 to 8.91 (Figure 1). These pH levels are moderately alkaline and suitable for irrigation purposes, particularly for green spaces, as they fall within acceptable ranges for safe use without being excessively alkaline.
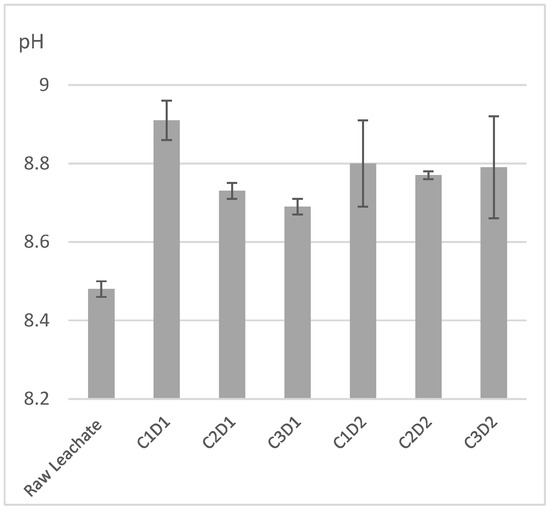
Figure 1.
pH of raw leachate and supernatants after treatment with sugar lime sludge. C1: 25%, C2: 37%, C3: 50%, D1 = 24 h, D2 = 36 h.
A one-way ANOVA was performed to quantitatively validate these observations, showing that the treatment had a significant effect on pH (F = 15.6, p < 0.001). Statistical comparisons indicated that all treatments significantly elevated pH relative to raw leachate (p < 0.0001) in post-hoc pairwise comparisons with adjusted p-values. The estimated differences were between +0.213 and +0.424 units, and all comparisons were found to be significant with p < 0.001 (Table 2). The most significant increase was seen in the condition C1D1 (25% sugar lime sludge at 24 h), where the pH increased significantly by +0.424 units when compared to raw leachate (p < 0.001), (Table 4).

Table 4.
t-Test Results: pH variation in raw leachate compared to treated leachate.
The pH results presented here illustrate the statistical methodology employed throughout this study. All subsequent parameters were analyzed using one-way ANOVA, and significant differences were assessed via post-hoc pairwise comparisons with adjusted p-values.
3.1.2. Electrical Conductivity Values After Leachate Treatment with Three Concentrations of Sugar Lime Sludge
The initial conductivity of the raw leachate is very high (around 300 mS/cm), indicating a significant presence of dissolved salts and ions, characteristic of untreated leachate [11]. After treatment with sugar lime sludge, the electrical conductivity of the supernatant decreases (Figure 2), suggesting the removal of salts from the decantate and indicating a partial depollution of the leachate. However, this effect is more dependent on the contact duration between the leachate and the sludge than on the concentration of the sludge (Figure 2). A contact duration of 36 h resulted in the greatest decrease in electrical conductivity, with a reduction of 87%. Although the resulting electrical conductivity does not yet meet the Moroccan standard for irrigation (12 mS/cm) [42], this treatment represents a significant step toward the depollution of leachate (Figure 2). The ANOVA test shows that the differences between the treatment groups are statistically significant (p < 0.001), indicating that the treatment with sugar lime sludge has a real effect on the electrical conductivity of the leachate. Specifically, the sum of squares for the treatment is much higher than that for the residuals, suggesting a significant variability due to the treatment compared to the random variability.
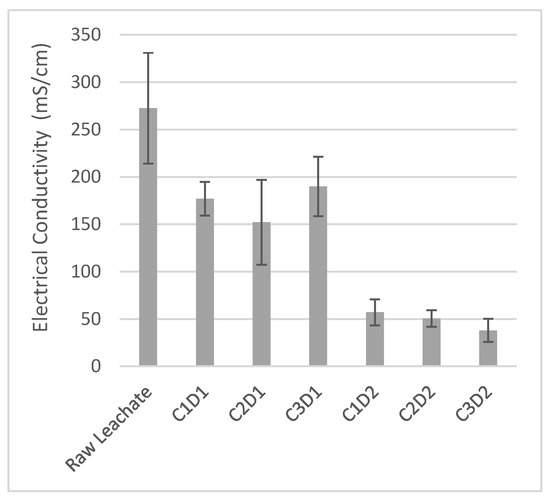
Figure 2.
Electrical conductivity of raw leachate and supernatants after treatment with sugar lime sludge. C1: 25%, C2: 37%, C3: 50%, D1 = 24 h, D2 = 36 h.
3.2. Organic Matter Content in the Decantate After Leachate Treatment
All treatments with sugar lime sludge showed an increase in the organic matter (OM) content of the decantate compared to the raw sugar lime sludge (Figure 3). The increase ranged from 10.4% to 40.8%. This result may be attributed to the adsorption of organic matter onto the lime and calcium carbonate present in the sugar lime sludge (Table 2). The best result was observed with a sludge concentration of 37% and a contact duration of 36 h (Figure 3). This depollution process improves the quality of the supernatant by reducing its organic matter concentration, making it more suitable for reuse in irrigation.
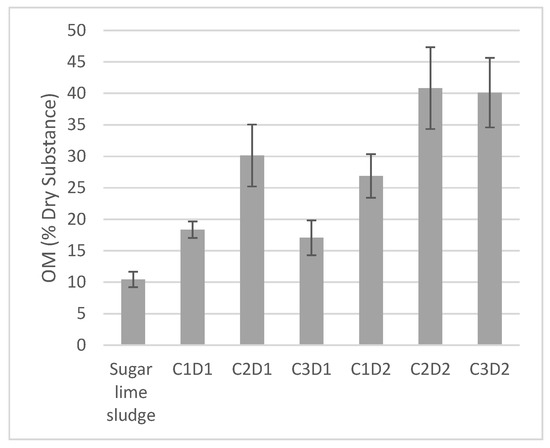
Figure 3.
Organic matter content (% dry substance) in the raw sugar lime sludge and in the decantate obtained after leachate treatment. C1: 25%, C2: 37%, C3: 50%, D1 = 24 h, D2 = 36 h.
3.3. Phytotoxicity Assessment of Treated Leachate for Irrigation Potential
3.3.1. Germination Percentage
Over a period of seven days, the germination percentage of leachate samples that were treated and those that were not was assessed (Figure 4). The ideal circumstances for seed germination were demonstrated by the control sample (C), where germination began rapidly on the second day and reached about 100% by the fourth day. On the other hand, even after seven days, no germination was seen in the raw leachate, indicating its severe toxicity, which totally prevents seed development. The dilution level affected the germination % for the treated leachate. Germination started on the second day at a dilution of 1/10 and increased gradually until it reached 100% on the sixth day. This indicates that the 1/10 dilution effectively reduces toxicity to control levels. At 1/6 dilution, germination was marginally delayed, with 80% germination on day 7, with partial reduction in toxicity. Also, like the 1/5 dilution, germination for 1/4 was similar to the 1/6 dilutions; however, it only reached 70% by day 7. These results show that treatment and proper dilution of the leachate greatly lower its toxicity, allowing successful germination in all tested conditions.
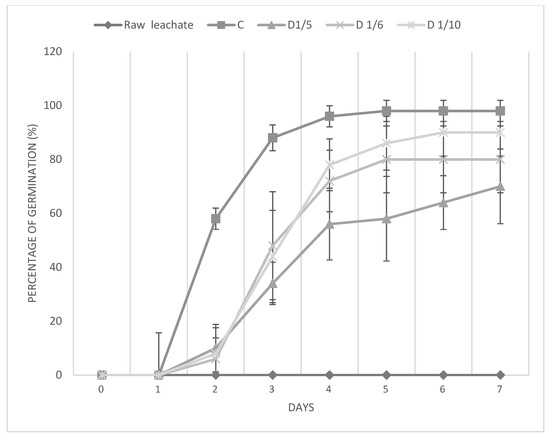
Figure 4.
Seed germination percentage over time for raw leachate and diluted supernatants after treatment with sugar lime sludge. Control C = Distilled water.
3.3.2. Germination Index
The germination index values verified that the treated leachate was phytotoxic. For raw leachate, the germination index falls nearly to zero, but for threatened and diluted leachate (1/6 and 1/10), it rises to or just beyond the 50% threshold (shown by the red line). This implies that toxicity is decreased to a point where seeds can sprout when sugar lime sludge treatment is used in conjunction with the proper dilution (Figure 5). We assessed the phytotoxicity of both the raw leachate diluted at 1/6 and the treated leachate diluted at 1/6 in order to show that the treatment, not merely dilution, is what works.
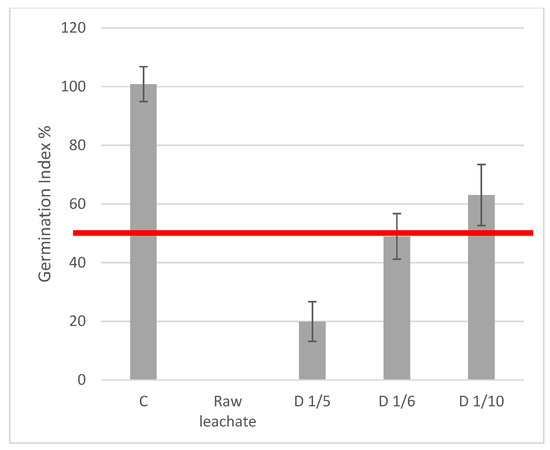
Figure 5.
Germination index of raw leachate and diluted supernatants after treatment with sugar lime sludge. Control C = Distilled water. The red line indicates the threshold value of 50% for the Germination Index (GI). Values below this threshold suggest phytotoxicity, while values above indicate non-toxic or acceptable conditions for plant growth.
The germination rate falls to 26.3% when raw leachate is diluted at 1/6 (D 1/6 RL, Figure 6), highlighting strong phytotoxic effects due to the presence of toxic compounds in the untreated leachate. However, for treated leachate diluted at the same dilution (D 1/6 TL), the germination rate improves to 48.97%. This indicates that the treatment with sugar lime sludge effectively reduces the leachate’s toxicity, nearly doubling the germination rate compared to the raw leachate. Although the treated leachate still shows some residual toxicity compared to the control, the significant improvement suggests that the treatment successfully mitigates a portion of the harmful effects. Further optimization of the treatment process may be needed to enhance its efficiency.
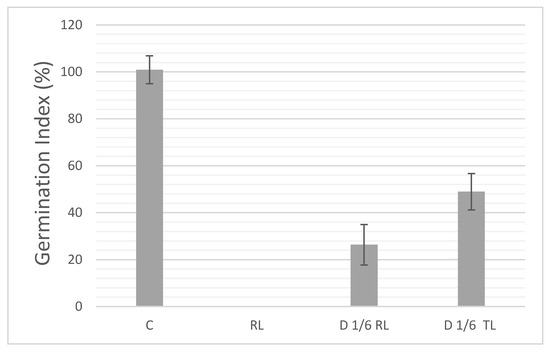
Figure 6.
Germination index of diluted raw leachate and diluted supernatants after treatment with sugar lime sludge (Control C = Distilled water, RL = Raw leachate, TL = Treated leachate).
3.4. Microbiological Results
3.4.1. Raw Leachate
Only fecal streptococci were identified in raw leachate. The fecal coliforms and the total coliforms were not identified (Table 5). A similar result was obtained by Mobaligh et al. [20] on the leachate coming from the same landfill. This may indicate that the conditions were not favorable for their growth. The lab results showed a strong concentration of fecal streptococci (40,000 CFU/mL). This indicates that microorganisms of fecal origin pollute the raw leachate, rendering it incompatible with irrigation or other agricultural uses without treatment. The concentration of Mesophilic flora (1,666,666.7 CFU/mL) signifies loads of mesophilic organisms. Those are non-specific microorganisms and aerobically ferment in moderate temperatures; indicative of the overall microorganism’s contamination in raw leachate.

Table 5.
Microbiological contamination of raw leachate.
3.4.2. Fecal Streptococci After Treatment with Sugar Lime Sludge
After treatment of sugar lime sludge, a significant reduction in fecal streptococci is observed in all treated samples compared to the raw leachate (Figure 7), indicating that the treatment process substantially decreases microbial contamination. This may be in relation to the pH of sugar lime sludge and the presence of lime, which is known to inhibit bacterial growth [5]. The reductions range between 93.1% and 99.1%, indicating a high overall efficiency of the process. The least reduction (99.1%—346 CFU/mL) indicates that there are some conditions of treatment that are very effective in the elimination of fecal streptococci. The efficiency of the treatment also relies on several variables, including contact time, sugar lime sludge concentration, and leachate characteristics, as noted by the variability in reduction percentages (93.1% to 99.1%). To sum up, the overall reduction is statistically significant, but the efficiency can be optimized for a more consistent maximized treatment (Table 6).
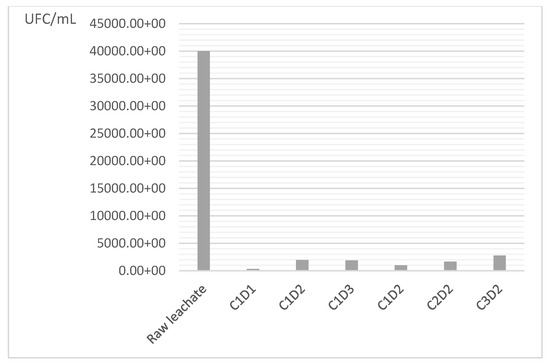
Figure 7.
Reduction in fecal streptococci levels in the supernatants after treatment with sugar lime sludge. C1: 25%, C2: 37%, C3: 50%, D1 = 24 h, D2 = 36 h.

Table 6.
Fecal streptococci reduction analysis. For the supernatants after leachate treatment.
The ANOVA results show that the differences between the treatment groups are highly significant (p < 0.001), indicating that the sugar lime sludge treatment effectively reduces fecal streptococci in the leachate. The large sum of squares for the treatment (3.85 × 109) compared to the residuals (2.01 × 108) highlights the strong influence of the treatment on microbial contamination reduction.
3.5. Composting of Decantate with Green Waste and Assessment of Compost Maturity
The composting process was closely monitored. The temperature curve exhibited the typical profile of a successful composting cycle: a rapid increase during the thermophilic phase, followed by a gradual decline during the maturation phase, confirming active microbial degradation (Figure 8). Simultaneously, the organic matter content decreased steadily over time, indicating effective mineralization and stabilization of the biodegradable fraction. These changes, illustrated in the temperature and organic matter evolution curves (Figure 9), confirm that the composting process was successful. The final compost showed a balanced nitrogen content and acceptable organic matter levels, making it suitable for agricultural or landscaping use. This demonstrates that landfill leachate, when properly treated and composted with green waste, can be safely and effectively transformed into a valuable product.
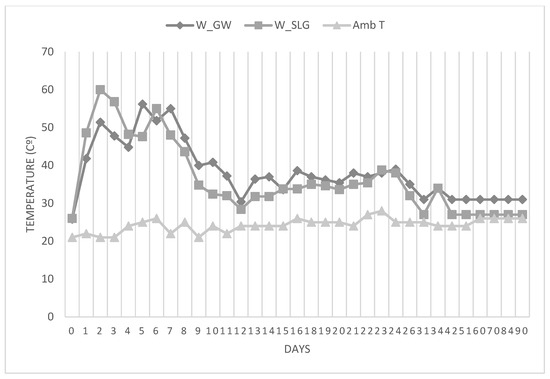
Figure 8.
Temperature evolution during composting: W_GW (Windrow with Green Waste Only), W_SLG (Windrow with Green Waste with sugar lime sludge), and Ambient Temperature (amb_T).
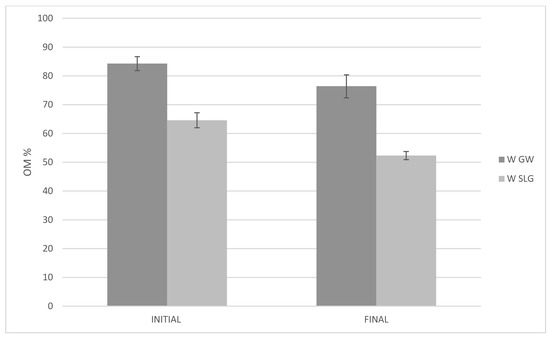
Figure 9.
Reduction in organic matter content during composting: initial vs. final for W_GW (Windrow with Green Waste Only) and W_SLG (Windrow with Green Waste with Sugar Lime Sludge.
3.6. Evolution of Total Nitrogen in the Decantate After Treatment and During Composting
The graph in Figure 8 illustrates the variation in total Kjeldahl nitrogen (TKN) content, expressed as TKN in g per 100 g of dry matter, across three stages: after decantation treatment and following composting at Day 1 (D1) and Day 90 (D90).
The results suggest that after the treatment, nitrogen was effectively concentrated in the decantate (0.33 g/100 g DS): the total nitrogen increases by 65% if we compare it with the relatively low TKN value of raw sugar lime sludge (0.2 g/100 g DS). This indicates that a significant fraction of nitrogen was removed from the liquid phase and retained in the solid phase (the sediment).
Subsequent composting of the decantate, mixed with green waste, resulted in a marked increase in TKN at Day 1 of composting, reaching approximately 1 g/100 g DS. This sharp rise may be attributed to the addition of nitrogen-rich organic matter from green waste, combined with initial microbial activity during the early stages of composting.
By Day 90 (the end of composting), a decline in NTK was observed (Figure 10), with the value reducing to approximately 0.5 g/100 g DS, which is quite a good concentration for the quality of the compost. This reduction reflects the mineralization and stabilization processes that occur during composting. Ammonia volatilization and/or leaching during composting can also cause nitrogen loss. Another reason could be the conversion of organic nitrogen into stable forms (Figure 9). The differences in nitrogen retention are mainly due to the chemical composition of sugar lime sludge, which is rich in calcium compounds, including calcium carbonate. By promoting the aggregation and flocculation of suspended particles, calcium holds great importance in the course of sewage treatment. The overall nitrogen recovery for treated water is higher once this mechanism has run its course. Thus, the success of nitrogen retention hinges upon how much free calcium is available to bind and stabilize nitrogen compounds.
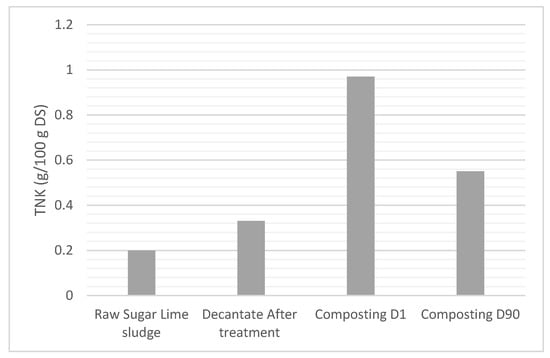
Figure 10.
Evolution of Total Nitrogen by Kjeldahl (TNK) in the decantate after treatment with sugar lime sludge and composting with green waste (D = day).
These results not only indicate that the primary treatment is successful in fixing nitrogen in the solid phase, but they also illustrate that composting is associated with a progressive transformation of the nitrogen content. It indicates the stabilization of organic matter and that matured final compost can be used for agricultural or landscaping purposes.
4. Discussion
Sugar lime sludge-treated leachate has displayed significant enhancements in lowering electric conductivity as well as stabilizing pH, improving microbiological quality, and decreasing phytotoxicity, which could foster its reuse for environmental and agricultural demands [43]. Experimental data show that the procedure can significantly reduce fecal streptococci. This was evident when the total microbial load was successfully reduced, rendering it safe for other potential uses, such as irrigation in accordance with regional regulations or for environmental discharge purposes. The treatment improved leachate alkalinity slightly, as the pH was stabilized at 8.7 to 8.9, which is preferable for irrigation purposes. Similarly, the extraction of dissolved species was evidenced by a strong reduction in leachate electrical conductivity caused by the addition of sugar lime sludge. Further treatment may still be necessary to meet the requirements of sensitive crops and stringent irrigation regulations. It also reduced phytotoxicity, as indicated by higher germination indices in samples treated with diluted leachate. Of the dilutions, 1/10 exhibited the most promising results, as the germination index surpassed the phytotoxicity threshold for safe irrigation, confirming that the treatment with sugar lime sludge and the appropriate dilution mitigate, in practice, the toxic effects of the sludge, thus allowing its reuse safely in agriculture. The best results can be obtained from 25% sludge concentration (C2) with longer treatment duration (D2) based on optimized parameters to reduce microbial load, stabilize pH, decrease electrical conductivity, and minimize phytotoxicity. However, while short-term indicators are promising, long-term studies are essential to fully confirm the agronomic safety of treated leachate reuse. In terms of efficiency, the sugar lime sludge method demonstrates notable advantages, especially cost-efficiency and simplicity, making it suitable for implementation in low-resource settings. However, compared to advanced treatment systems such as constructed wetlands, reverse osmosis, or membrane bioreactors, its performance is generally more limited in removing dissolved organics, nitrogen species, and trace pollutants. For example, reverse osmosis can achieve >95% reduction in COD and ammonium but is energy-intensive and expensive. Constructed wetlands, while slower, offer ecological treatment and polishing capacity, particularly when designed for tertiary treatment. Sugar lime sludge treatment could therefore be best utilized as a preliminary or intermediate treatment, especially when combined with post-treatment steps such as dilution.
5. Conclusions
Overall, this work shows how the sugar lime sludge treatment with a really simple method could be a new option to valorize the landfill leachate and convert a waste into an economical product, which could be used as irrigation water. It was observed that the treatment was most effective with a reduction ratio of the order of 10.43 to 40.84 g/L of organic matter, with valuable reductions shown in microbial load, pH, and conductivity at concentrations of 25%, 37%, and 50% at time spans for 24 h and 36 h of induced treatment. These reductions indicate that treating landfill leachate with sugar lime sludge is able to minimize its environmental hazards, in turn leading to improved irrigation utility.
Despite these promising results, there are important limitations to consider. Scalability remains a challenge, as the effectiveness of the treatment in larger-scale applications, especially in diverse environmental conditions, requires further validation. The cost-effectiveness of the sugar lime sludge treatment must also be thoroughly assessed, as the economic viability for large-scale implementation in real-world settings is uncertain. The impact on soil health, particularly its capacity to absorb and filter pollutants over time, and potential consequences for crop growth will be addressed in the second part of this study. This initial phase focused on evaluating the treated leachate under controlled laboratory conditions. Ongoing monitoring of soil composition, microbial diversity, and plant health will be essential to determine the sustainability of using treated leachate for irrigation in the long run. In conclusion, while sugar lime sludge treatment offers a promising approach for landfill leachate management and green space irrigation, further studies are required to optimize the process and address scalability, cost, regulatory, and long-term application concerns.
Author Contributions
Conceptualization, K.F. and T.B.; methodology, T.B.; software, T.B.; validation, T.B., A.C. and K.F.; formal analysis, T.B.; investigation, T.B.; resources, K.F.; data curation, T.B.; writing—original draft preparation, T.B.; writing—review and editing, A.C. and K.F.; visualization, T.B.; supervision, K.F.; project administration, K.F.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Cadi Ayyad University (UCA). The authors gratefully acknowledge their support.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baun, A.; A Reitzel, L.; Ledin, A.; Christensen, T.H.; Bjerg, P.L. Natural attenuation of xenobiotic organic compounds in a landfill leachate plume (Vejen, Denmark). J. Contam. Hydrol. 2003, 65, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; STATISTA. Global Waste Generation—Statistics & Facts. STATISTA. Available online: https://www.statista.com/topics/4983/waste-generation-worldwide/#topicOverview (accessed on 18 December 2023).
- Shafy, H.I.A.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Hosseini Beinabaj, S.M.; Heydariyan, H.; Mohammad Aleii, H.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef] [PubMed]
- Nuansawan, N.; Witthayaphirom, C.; Sawasdee, A.; Chiemchaisri, C.; Shoda, M. Removals of endocrine disrupting compounds during landfill leachate treatment in two-stage aerobic sequential batch reactor: Effect of Alcaligenes faecalis no.4 bio-augmentation. Emerg. Contam. 2023, 9, 100223. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Selvam, A. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. J. Hazard. Mater. 2009, 169, 882–889. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag. 2013, 33, 2129–2136. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Xu, T.; Ma, J.; Sun, Q.; Rong, G.; Ren, B. Corrosion characteristics of concrete by landfill leachates of different ages. Case Stud. Constr. Mater. 2024, 20, e03242. [Google Scholar] [CrossRef]
- Koffi, S.; Prasad, S.B.; Agbotsou, K.E.; Souley, H.S.; Mudigandla, R. Characteristics of landfill leachate and leachate treatment by biological and advanced coagulation process: Feasibility and effectiveness—An overview. Waste Manag. Bull. 2024, 2, 181–198. [Google Scholar]
- Marek, M.; Płaza, G.A.; Nałęcz-Jawecki, G.; Ulfig, K.; Markowska-Szczupak, A. Estimation of the environmental risk posed by landfills using chemical, microbiological and ecotoxicological testing of leachates. Chemosphere 2011, 82, 1017–1023. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; El-Wafai, N.A.; Abou-Aly, H.E.; Salem, H.M.; Soliman, S.M.; El-Mageed, T.A.A.; Elrys, A.S.; Selim, S.; El-Hack, M.E.A.; et al. Hazardous wastes and management strategies of landfill leachates: A comprehensive review. Environ. Technol. Innov. 2023, 31, 103150. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, H.; Li, X.; Qi, D.; Liu, R.; Kang, G.; Liu, J.; Li, N.; Zhang, S.; Hu, S. Pathogens and antibiotic resistance genes during the landfill leachate treatment process: Occurrence, fate, and impact on groundwater. Sci. Total Environ. 2023, 903, 165925. [Google Scholar] [CrossRef] [PubMed]
- Viraj, G.; Phillips, A.J.; Zanoletti, A.; Rajapaksha, A.U.; Vithanage, M.; Di Maria, F.; Pivato, A.; Korzeniewska, E.; Bontempi, E. Environmental pitfalls and associated human health risks and ecological impacts from landfill leachate contaminants: Current evidence, recommended interventions and future directions. Sci. Total Environ. 2024, 912, 169026. [Google Scholar]
- Seibert, D.; Quesada, H.; Bergamasco, R.; Borba, F.H.; Pellenz, L. Presence of endocrine disrupting chemicals in sanitary landfill leachate, its treatment and degradation by Fenton based processes: A review. Process Saf. Environ. Prot. 2019, 131, 255–267. [Google Scholar] [CrossRef]
- Belmakki, M.; Bartali, E.H.; Xiaoru, H.; Anne-Belinda, B. Identification and characterization of organic waste in Morocco, an important step towards the valorization of waste. Rev. Mar. Sci. Agron. Vét. 2015, 3, 37–45. [Google Scholar]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Li, Y.-J.; Yuan, Y.; Tan, W.-B.; Xi, B.-D.; Wang, H.; Hui, K.-L.; Chen, J.-B.; Zhang, Y.-F.; Wang, L.-F.; Li, R. Antibiotic resistance genes and heavy metals in landfill: A review. J. Hazard. Mater. 2024, 464, 132395. [Google Scholar] [CrossRef]
- Wang, Q.; Ko, J.H.; Liu, F.; Xu, Q. Leaching characteristics of heavy metals in MSW and bottom ash co-disposal landfills. J. Hazard. Mater. 2021, 416, 126042. [Google Scholar] [CrossRef]
- Ishchenko, V. Heavy metals in municipal waste: The content and leaching ability by wasre fraction. J. Environ. Sci. Health, Part A 2019, 54, 1448–1456. [Google Scholar] [CrossRef]
- Mobaligh, M.; Meddich, A.; Imziln, B.; Fares, K. The Use of Phosphate Washing Sludge to Recover by Composting the Leachate from the Controlled Landfill. Processes 2021, 9, 1735. [Google Scholar] [CrossRef]
- Mobaligh, M.; Saadani Hassani, O.; Ait Rahou, Y.; Fares, K. Impact of the Addition of Sugar Beet Lime Sludge on the Composting of Argan Oil By-Products. Catal. Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Yasamani Masouleh, S.; Mozaffarian, M.; Dabir, B. Landfill leachate treatment using sequential natural zeolite adsorption and peroxymonosulfate activated by UV/Fe2+ system: Effects of main operating parameters and application of fluorescence EEM to monitor organic matters’ transformation. J. Environ. Chem. Eng. 2024, 12, 113457. [Google Scholar] [CrossRef]
- Lavudya, S. Treatment of landfill leachate using advanced oxidation process and struvite precipitation processes. Mater. Today Proc. 2023, 76, 206–211. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Mota, A.; Vilarinho, C.; Gouveia, L.; Gonçalves, M. A circular approach for landfill leachate treatment: Chemical precipitation with biomass ash followed by bioremediation through microalgae. J. Environ. Chem. Eng. 2021, 9, 105187. [Google Scholar] [CrossRef]
- Chen, Q.; Lü, F.; Zhang, H.; Han, Y.; He, P. Dissolved organic nitrogen is a key to improving the biological treatment potential of landfill leachate. Water Res. 2024, 254, 121403. [Google Scholar] [CrossRef]
- del Amo, E.H.; Poblete, R.; Sánchez, O.; Maldonado, M.I. Biological treatment and microbial composition of landfill leachate using a compost process in an airlift bioreactor. J. Clean. Prod. 2023, 415, 137748. [Google Scholar] [CrossRef]
- De Carluccio, M.; Sabatino, R.; Eckert, E.M.; Di Cesare, A.; Corno, G.; Rizzo, L. Co-treatment of landfill leachate with urban wastewater by chemical, physical and biological processes: Fenton oxidation preserves autochthonous bacterial community in the activated sludge process. Chemosphere 2023, 295, 137578. [Google Scholar] [CrossRef]
- Huang, Y.; Guan, Z.; Xia, D. Effective remediation of leachate concentrate by peroxymonosulfate in a catalytic ceramic membrane filtration process: Performance and mechanism. Waste Manag. 2023, 172, 117–126. [Google Scholar] [CrossRef]
- Li, Y.; Yi, Q.; Wang, D.; Wu, Z.; Wang, Z. Efficient treatment of landfill leachate using an electrochemical ceramic membrane filtration system: Chlorine-mediated oxidation. Chem. Eng. J. 2022, 450, 138102. [Google Scholar] [CrossRef]
- Saleem, M.; Spagni, A.; Alibardi, L.; Bertucco, A.; Lavagnolo, M.C. Assessment of dynamic membrane filtration for biological treatment of old landfill leachate. J. Environ. Manag. 2018, 213, 27–35. [Google Scholar] [CrossRef]
- Bai, F. Landfill leachate treatment through the combination of genetically engineered bacteria Rhodococcus erythropolis expressing Nirs and AMO and membrane filtration processes. Environ. Pollut. 2020, 263, 114061. [Google Scholar] [CrossRef]
- Kundu, A. Optimization and kinetic analysis of electrocoagulation-assisted adsorption for treatment of young landfill leachate. J. Environ. Manag. 2024, 366, 121779. [Google Scholar] [CrossRef]
- Ameli, F.; Hashemi, H.; Samaei, M.R.; Asgari, E.; Fazli, M.M. Enhanced reducing leachate pollution index through electrocoagulation using response surface methodology. Heliyon 2024, 10, e38134. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Hernandez, J.; Marrugo-Negrete, J.; Pérez-Espitia, M.; Durango-Hernández, J.; Enamorado-Montes, G.; Navarro-Frómeta, A. A pilot-scale electrocoagulation-treatment wetland system for the treatment of landfill leachate. J. Environ. Manag. 2023, 351, 119681. [Google Scholar] [CrossRef] [PubMed]
- Parthenidis, P.; Evgenidou, E.; Lambropoulou, D. Landfill leachate treatment by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). Water Process Eng. 2023, 53, 103768. [Google Scholar] [CrossRef]
- Xu, L.; Siracusa, G.; Di Gregorio, S.; Yuan, Q. COD removal from biologically stabilized landfill leachate using Advanced Oxidation Processes (AOPs). Process Saf. Environ. Prot. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Rocha, E.M.R.; Vilar, V.; Fonseca, A.; Saraiva, I.; Boaventura, R. Landfill leachate treatment by solar-driven AOPs. Solar Energy 2011, 85, 46–56. [Google Scholar] [CrossRef]
- Bonu, R.; Anand, N.; Palani, S.G. Impact of thermal pre-treatment on anaerobic co-digestion of sewage sludge and landfill leachate. Mater. Today Proc. 2023, 2, 99–103. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, S.; Ji, Z.; Cui, J.; Pei, Y. Solidification/stabilization of organic matter and ammonium in high-salinity landfill leachate concentrate using one-part fly ash-based geopolymers. J. Environ. Chem. Eng. 2023, 11, 109379. [Google Scholar] [CrossRef]
- Zucconi, F. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Santos, J.M.; Amaral, L.M.; Martinho, G. Effects of landfill age, climate, and size on leachate from urban waste landfills in Portugal: A statistics and machine learning analysis. Waste Manag. 2023, 172, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Transition Energétique et du Développement Durable. Normes Qualité des Eaux usées Épurées Detinees a L’irrigation. Available online: https://www.environnement.gov.ma/fr/lois-et-reglementations/normes (accessed on 28 January 2024).
- Acarer, S.A. Microplastics in landfill leachate: Sources, abundance, characteristics, remediation approaches and future perspective. Desalination Water Treat. 2024, 319, 100445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).