Abstract
The key objectives of contemporary agriculture are restoring biodiversity, preserving ecosystem health, reducing the effects of climate change, and producing safe and healthy foods. Maintaining high soil fertility while reducing greenhouse gas emissions requires a precise assessment of how fertilization and crop rotation affect carbon and nutrient cycles in agroecosystems. Fertilization affects soil conditions, which alters the environment for soil microbial development and influences the number and composition of soil microbial communities, leading to changes in nutrient and carbon cycling. There is a lack of long-term experimental data on the impact of fertilizer treatments on soil CO2 emissions, soil microbial communities, and their interactions. The novelty of this study is that it identified the fertilization effects on soil carbon sequestration, soil properties, and microbial communities in the context of a long-term fertilizer experiment in Luvic Chernozem. The fertilization treatments that were continuously pplied for 64 years under a four-crop (wheat, barley, corn, and bean) rotation were nitrogen (N), phosphorus (P), potassium (K), NP, NK, PK, NPK, and control. The chemical and microbiological soil properties and soil CO2 emissions were monitored. The highest organic carbon content was observed under the NPK (1.42%) and NP (1.43%) treatments. N fertilizer application most significantly affected soil properties, including pH, electrical conductivity, and soil organic carbon content, altering the environment for soil microbial development and influencing the number and composition of soil microbial communities. On average, the field-measured soil C-CO2 emissions were the most intensive under NP (2.76 kg ha−1 h−1), NPK (2.83 kg ha−1 h−1), and PK (2.51 kg ha−1 day−1) treatments.
1. Introduction
The primary agricultural technique that significantly increases crop yields is mineral fertilization. The biological, chemical, and physical processes of the soil are also impacted by altering the nutrient regime and the environment for plant growth [1,2,3,4,5]. To obtain information on the types and amounts of fertilizers used to increase agricultural yields and plant nutrition, numerous long-term fertilizer experiments have been set up [6]. These studies offer a chance to investigate how the long-term application of mineral fertilizers and their combinations affect soil characteristics. The effects of mineral fertilization on soil quality must be investigated over an extended period of time to understand how they impact soil parameters that change slowly. Additionally, they allow the development of a theoretical framework for sustainable crop yields and the preservation of a healthy soil environment [7].
The link between the carbon cycle and climate change is now well established. Land use is responsible for about one-third of the increase in atmospheric carbon dioxide over the past 150 years, mainly due to the loss of soil organic carbon (SOC) [8]. The main goals of modern sustainable agriculture are mitigating climate change, avoiding long-term environmental damage, and assuring human nutrition [9]. Fertilization has been the most effective management practice for ensuring global agricultural production since the discovery of the role of nutrients in crop growth and development. However, excessive mineral fertilization decreases soil fertility and increases nutrient loss [10]. This leads to many interrelated negative effects on the environment and human health, such as increased greenhouse gas emissions, and pollution of the surface, groundwater and food [11]. Nitrogen is a key element in plant growth and yield formation and a limiting factor in ecosystem productivity [12]. Nitrogen fertilization strongly affects biomass production, microbial activity, accumulated biomass decomposition, and SOC dynamics [13]. Previous research has shown that the effects of nitrogen addition on soil respiration have been controversial [14]. The effect of nitrogen mineral fertilization leads to higher yields and a higher amount of plant residues, supporting soil biota and humification, which are essential for soil structure stability and chemical buffering and lead to CO2 release [15]. Other studies have shown a reduction in soil CO2 emissions after nitrogen fertilizer application compared to non-fertilized treatments [16]. Thus, the impact of fertilization practices on soil carbon and nitrogen content in agroecosystems needs to be accurately assessed to maintain high soil fertility and mitigate greenhouse gas emissions [17].
In the current climate change context, another key goal of agriculture is optimizing fertilization management to improve soil carbon sequestration, and it is essential to understand how fertilizing strategies impact SOC dynamics [17]. A growing number of studies have suggested that agricultural soils have significant potential to mitigate climate change through carbon sequestration [18,19,20,21]. Agricultural lands have the potential to sequester approximately 5500–6000 Mg CO2-eq. yr−1 by 2030 [22]. SOC accumulation and greenhouse gas emissions in arable land can be managed by various agricultural practices [23,24,25], including applied fertilization [15]. The most significant regulator of soil carbon dynamics and carbon biogeochemical cycles is the microbial community [26]. Microorganisms play a crucial role in soil activities and are the primary drivers of SOC turnover. Therefore, understanding the structure and function of microbial communities is essential for better managing soil carbon sequestration [27]. Fertilization rates and crop rotations can affect soil pH, nutrients, and SOC through their effects on microbial processes [28]. Fertilization can indirectly affect soil microorganisms by changing soil properties, and directly through applied nutrients [29]. Soil microbiota play an essential role in the cycling of soil carbon, nitrogen, phosphorus, and sulfur and are an important component in maintaining soil quality and fertility. Changes in soil microbial communities also affect the productivity and functioning of agroecosystems [30,31].
A 64-year fertilization experiment under four crop rotations at the Institute of Agriculture and Seed Science (IASS) “Obraztsov Chiflik” Rousse is one of few long-term fields experiments in Bulgaria that have been maintained. Since the soil had undergone irreversible changes as a result of extended, continuous mineral fertilization in the long-term experiment, the study’s main goals were as follows: (1) to investigate the influence of mineral fertilization on soil carbon sequestration; (2) to determine the impact of mineral fertilization on soil microbial communities; and (3) to assess the trends in field C-CO2 emissions.
2. Materials and Methods
2.1. Site Description
A long-term fertilization experiment was carried out in the experimental field of IASS Obraztsov Chiflik, region Ruse, (43.807, 26.041). The area has the characteristics of a moderate continental climate and is situated on the Danube plain (Figure 1).
Figure 1.
Location of the site.
The soil type is Luvic Chernozem [32] with low organic carbon content and moderately acidic soil reaction (pH in H2O 5.5–6.0) at 0–40 cm depth. The texture is classified as Silty Clay Loam (SiCL) with 30% clay content (Table 1).

Table 1.
Textural fractions in the humus horizons of Luvic Chernozem under grass, IZ “Obratzov Chiflik”.
2.2. Experiment Design
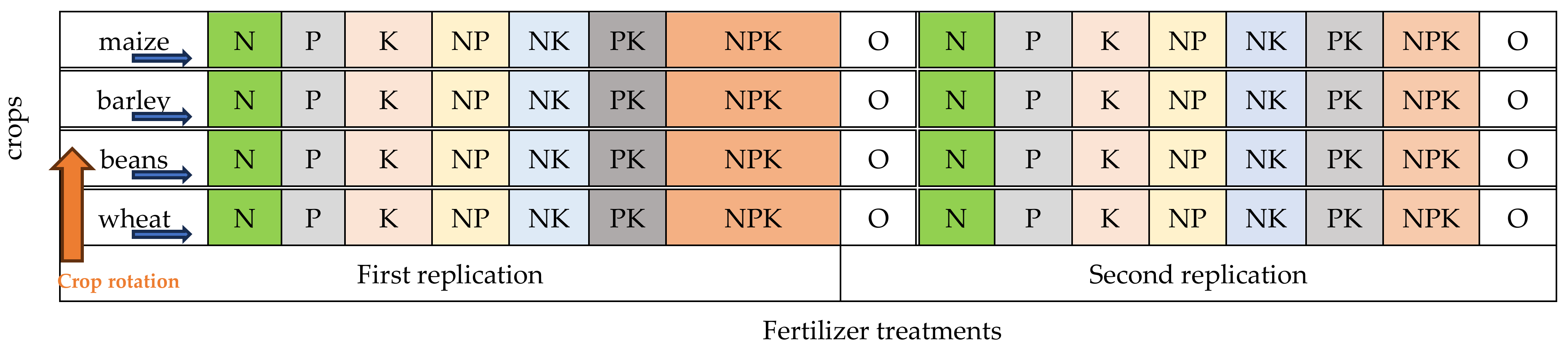
The experiment was created in its present form in 1958. It is located in an area of 1 ha. The size of the trial plots is 100 m2, and that of the harvest plots 60 m2. The applied methods of fertilization had been carried out at the same place for 64 years on four agricultural crops: wheat (variety “Danube”), beans (variety “Obratzov chiflik 12”), barley (variety “Agate”) and maize (hybrid “Ruse 464”) (Figure 2, Table 2). In the rotation, wheat replaces beans, maize replaces wheat, beans replace barley, and barley replaced maize. This experiment was configured according to the method and scheme of Georges Ville (1879) in two replicates with eight variants each.
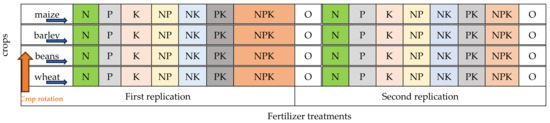
Figure 2.
Experimental design 2021–2022, two replicates, four crops, eight treatments.

Table 2.
Experimental setup.
Seven treatments were tested with individual (single) and combined application of the three macro-elements N, P, K. Nitrogen rates for wheat and maize were N—150 kg ha−1; for beans N—50 kg ha−1 and for barley N—100 kg ha−1. Phosphorus—120 kg ha−1 and potassium—70 kg ha−1 were applied for all crops. For comparison, the unfertilized control N0P0K0 is maintained. Phosphorus and potassium fertilizers were applied before the main tillage, and nitrogen fertilizers—after the sprouting of the crops, as an early spring feeding.
2.3. Chemical and Microbiological Analyses
In May and October of 2023, soil samples were collected for chemical and microbiological analyses from all treatments and two experiment replicates for all crops at a depth of 0–20 cm. The Kjeldal method was applied to determine the ammonium and nitrate nitrogen as well as the total nitrogen. Total organic carbon content (SOC, %) was determined by wet digestion via the modified Tjurin’s method (dichromate digestion at 125 °C, 45 min) [33]. The soil pH and electrical conductivity (EC) of the samples were measured in water suspension 1:2.5 by a combined pH–EC meter. The acetate–lactate method was used for determination of the plant-available forms of phosphorus and potassium by simultaneous extraction. The potassium was measured directly from the extract by a flame photometer. The phosphorus was determined by a spectrophotometer after a color reaction (according to Murphy–Riley, 1962).
The substrate-induced respiration method (SIR) was used for measuring the microbial biomass (Cmic) according to the Anderson–Domsch method, a modification of Hoper [34].
The number of microorganisms was determined by the plate counts technique on selective agar media. Ammonifying bacteria were cultivated on Nutrient broth agar, actinomycetes on starch–ammonium agar medium, microscopic fungi on Czapek’s agar medium, nitrifying bacteria on Winogradski medium, and nitrogen fixing bacteria on Ashby medium [35].
Soil CO2 emissions were measured during the three-year period 2021–2023, from May to October. A total of 12 complete measurements (of all variants and all crops) were made, regardless of when the crops were harvested, as in the autumn they were carried out on bare soil. CO2 fluxes were measured using infrared gas analyzer via the calibrated tester “ALMEMO” with a CO2 sensor. Soil emissions were measured, placing the plastic chambers in the plots after cleaning the surface from plants and residues, without disturbing the soil surface. The chambers were dark, with a volume of 5 L and openings for placing gas analyzer sensors. One meter above the ground, the atmospheric CO2 concentration was measured. After placing the chambers, they were closed and an incubation period of 30 min was maintained, after which the accumulated CO2 in the closed chambers was measured. All measurements were made in 3 replicates. Then the CO2 fluxes (kg ha−1 h−1) were calculated, according to [36]:
where E (CO2)—CO2 release from the soil (kg ha−1 h); M—molar mass of CO2 (kg mol−1); P—air pressure (Pa); V—chamber volume (m3); c1—initial concentration of CO2 (µmol mol−1); c2—concentration of CO2 after incubation time (µmol mol−1); R—gas constant (J mol −1 K−1); T—air temperature (K); A—chamber surface area (m2); t2 − t1—incubation period.
E (CO2) = (M × P × V × (c2 − c1))/(R × T × A × (t2 − t1))
2.4. Statistical Analyses
A multifactor ANOVA statistical analysis was conducted. For each significant factor, the Multiple Range Test was performed. Principal component analysis and Spearman correlation analysis were conducted to identify interactions between studied parameters. The purpose of the principal component analysis is to obtain a small number of linear combinations of the variables which account for most of the variability of the data. Spearman rank correlations between each pair of variables with p-value tests were performed to determine the statistical significance of the estimated correlations. The analysis of the obtained data was carried out using STATGRAPHICS Plus 2.1.
3. Results
3.1. Soil Chemical Properties and Organic Carbon Content
According to the applied treatments, all of the soil chemical indicators showed statistically significant differences (Table 2). The cultivated crop also affected some of the parameters, including pH, electrical conductivity (EC), available forms of phosphorus, and mineral nitrogen. Because two of the crops in the rotation were winter cereals, one a spring cereal (maize), and the other a spring leguminous crop (bean), the agricultural technologies differed greatly. This was reflected in the nitrogen fertilizer dosages, fertilization schedules, and crop growth stages, in which nutrient uptake varied.
As anticipated, long-term nitrogen-only (N) fertilization increased EC and decreased pH. On average, across all plots, the EC was highest in all variants with nitrogen fertilization (N), nitrogen, phosphorus, and potassium (NPK), and nitrogen and potassium (NK) treatments). In the non-fertilized variants, it was the lowest. Since the pH was greater in the potassium-only (K), phosphorus-only (P) and control treatments, the variants with nitrogen fertilizer application showed the greatest pH drop. In general, a decrease in pH was observed under N, nitrogen and phosphorus (NP; 5.44), NK (5.30), and NPK (5.42) treatments. The average pH of the control was 5.82.
On average, the SOC was most significantly increased by the NP and NPK treatments. The control had the lowest average SOC content (1.05%) (Table 3). The NK (1.25%), phosphorus and potassium (PK; 1.18%), and N (1.22%) treatments exhibited similar SOC contents as a result of long-term, consistent mineral fertilization. The highest increase in SOC was the result of fertilization with both nitrogen and phosphorus—1.42% in NPK treatment and 1.43% in NP treatment. The NP, NK, NPK, and N treatments had the most elevated levels of total nitrogen, as expected. The NK (9.87) and N treatments (10.25) had the lowest average carbon to nitrogen (C/N) ratios, indicating higher rates of mineralization and nitrogen and carbon release. P (12.34), NPK (12.19), and K (11.54) fertilization resulted in the highest C/N ratios, indicating a decreased rate of mineralization compared to other treatments.

Table 3.
Mean values by treatment of mineral N (N-NO3, N-NH4) (mg kg−1), available P2O5 (mg 100 g−1), available K2O (mg 100 g−1), EC (µS cm−1), pH, SOC (%), Cmic (mg kg−1), upper soil 0–20 cm.
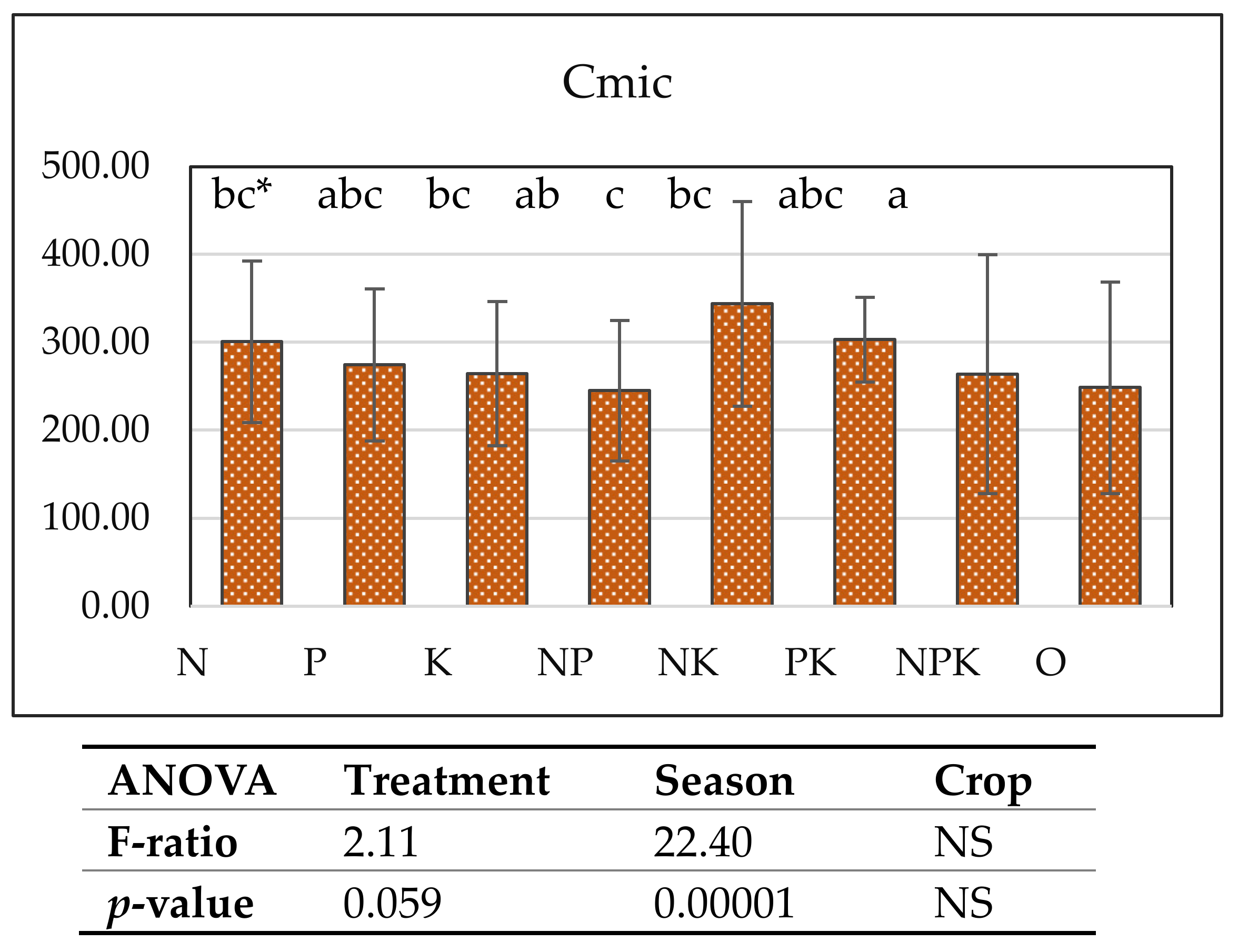
Microbial biomass carbon was stimulated most strongly by single and combined nitrogen and potassium fertilization. The microbial biomass carbon increased in the NK (343.8 mg kg−1), K (304.0 mg kg−1), N (300.9 mg kg−1), and PK (303.3 mg kg−1) treatments. Season and treatment had a significant influence on this parameter (Figure 3). Microbial biomass carbon was positively correlated with actinomycetes (r = 0.74, p = 0.05), potassium content (r = 0.40, p = 0.1), and ammonifying microorganisms (in wheat r = 0.35, p = 0.005, in maize r = 0.59, p = 0.02) and less with the number of microscopic fungi. The amount of actinomycetes was also correlated with potassium (r = 0.70, p = 0.068), and nitrogen fertilization led to a lower pH and higher amounts of microscopic fungi and ammonifying microorganisms, which could explain the increase in microbial carbon in the indicated treatments with nitrogen and potassium fertilization.
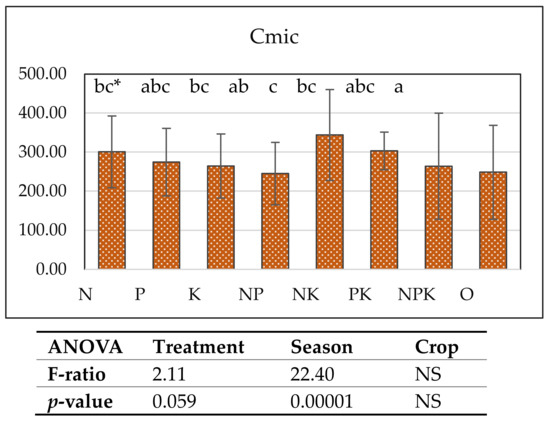
Figure 3.
Cmic (mg kg−1), upper soil 0–20 cm, vertical bars denote standard deviation.* a–c—groups that share the same letter belong to the same homogeneous subset, meaning that their means are statistically similar at the chosen significance level (e.g., p < 0.05).
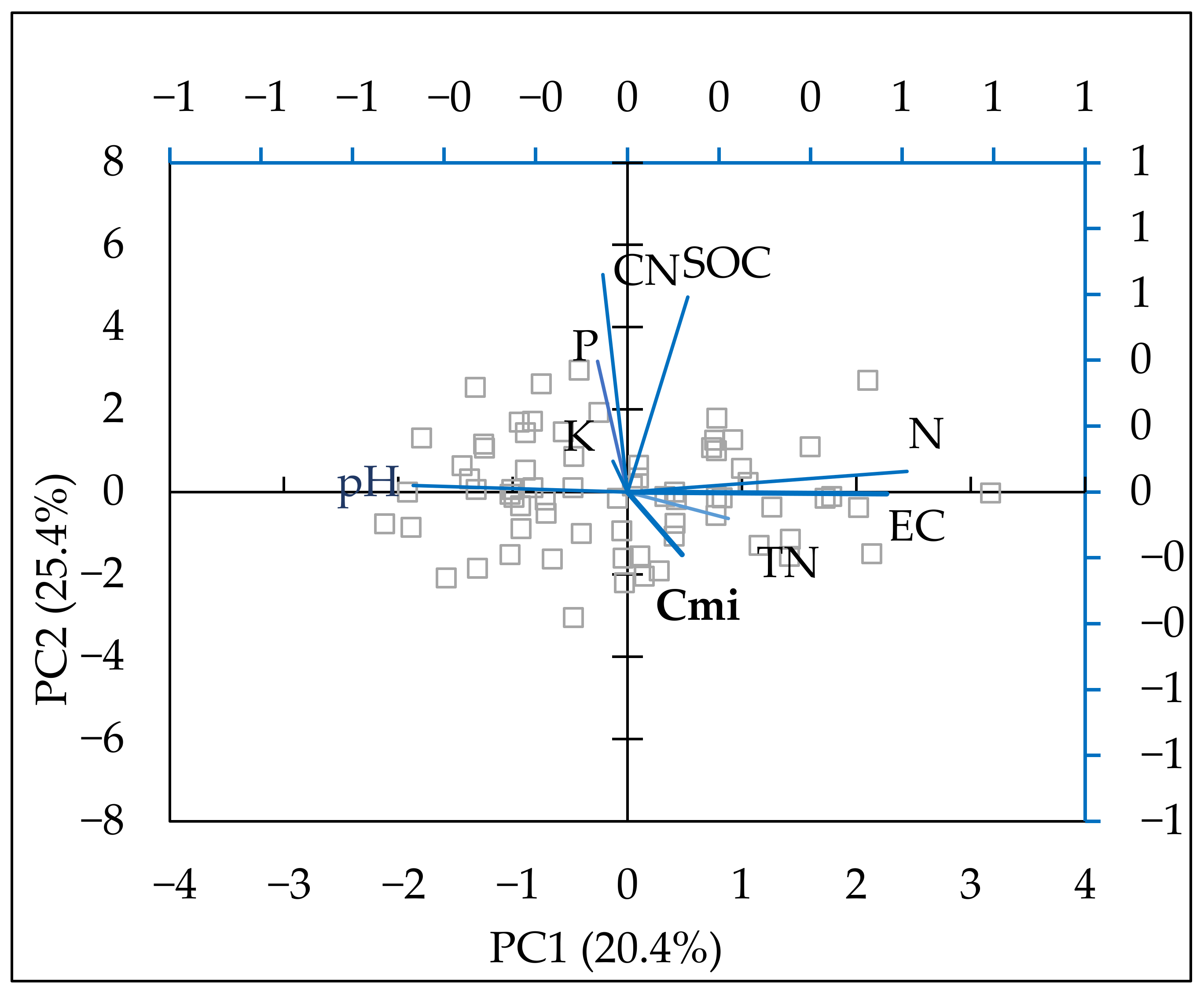
Principal component analysis (PCA) was performed to establish the main relationships between the available forms of macro-elements, physicochemical properties, SOC, total nitrogen, C/N ratio, and Cmic (Table 4). The data were fitted into 5 principal components and described 87.2% of data variability. The first two components are presented in Table 4. Component 1 reflected 25.36% of the variation in the indicators. The vector of principal component 1 (PC1) increased mineral nitrogen, which led to a higher EC, lower pH, and a very slight increase in SOC. The enhanced SOC was strongest in the second component (PC2), and the available forms of phosphorus and potassium also increased with PC2. Cmic grew in the first component. As shown in Figure 4, the parameters were considered to be positively correlated with each other when they were at an acute angle. When their vectors formed a right angle, they were not correlated and, when they were at an obtuse angle, they were negatively correlated. As shown in the PCA figure (Figure 4), a positive relationship was established between mineral nitrogen and SOC as well as between total nitrogen and microbial biomass carbon.

Table 4.
Variable loadings of the first two components of principal component analysis. Soil mineral N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), EC (µS cm−1), pH, SOC (%), Cmic (mg kg−1), TN (%), C/N ratio.
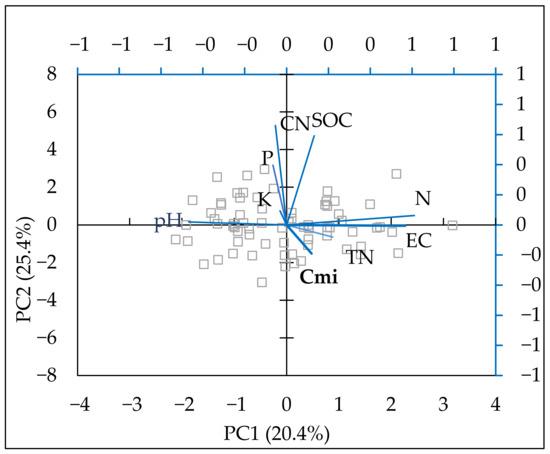
Figure 4.
Biplot of the first two components in the analysis carried out on a dataset including soil mineral N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), EC (µS cm−1), pH, SOC (%), Cmic (mg kg−1), TN (%), C/N ratio.
According to PCA, available forms of phosphorus and potassium had a stronger impact on the SOC content, and mineral nitrogen had a stronger positive relationship with microbial carbon. However, SOC was improved most strongly in plots with combined nitrogen and phosphorus fertilization (Table 2), but the nitrogen effect in the N treatment was low.
3.2. Effects of Mineral Fertilization on Soil CO2 Emissions
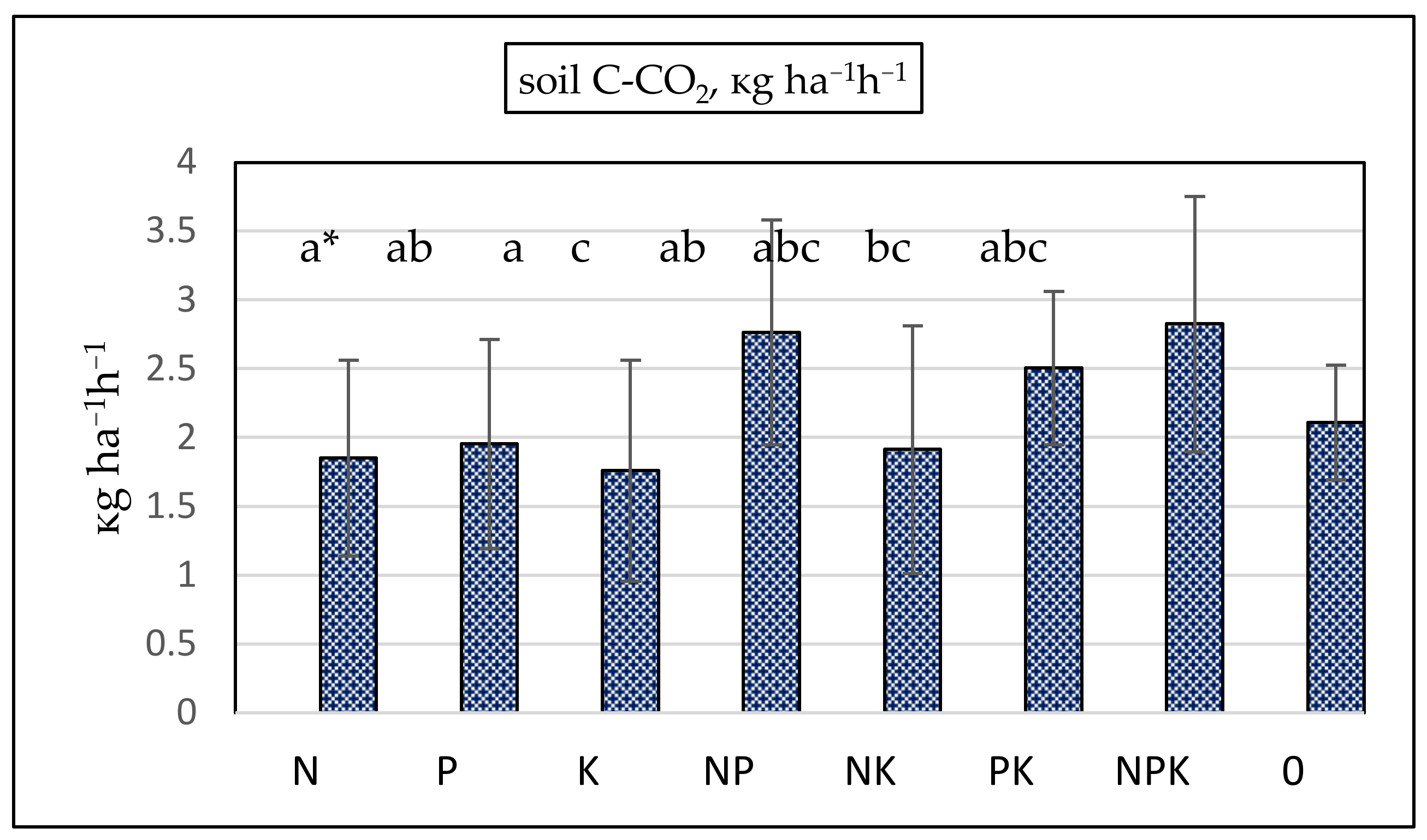
The most intensive CO2 emissions were recorded in the NP and NPK treatments (Figure 5). The highest SOC accumulation was established in these treatments (Table 2). A higher C/N ratio indicated lower mineralization rates and low carbon release in the NPK treatment, but CO2 emissions increased because combined nitrogen and phosphorus fertilization stimulated plants’ carbon assimilation and increased biomass input into the soils, thus stimulating soil heterotrophic respiration, such as root and microbial respiration. The correlation coefficient between SOC and CO2 flows was positive and significant (p < 0.05) in bean, barley, and wheat plots. Soil CO2 fluxes were the most intensive in wheat plots (2.75 kg ha−1 h−1) and lowest in bean plots (1.38 kg ha−1 h−1). Despite the low amount of SOC recorded in the control, CO2 emissions in the absence of fertilization were not the most strongly reduced. CO2 fluxes in the N, P, and K treatments were lower compared to the non-fertilized control.
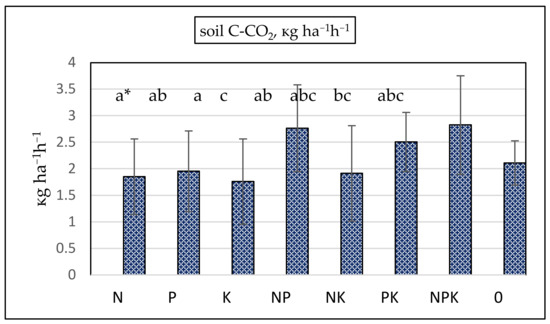
Figure 5.
Soil C-CO2 emissions, kg ha−1 h−1. Vertical bars denote standard deviation. ANOVA: p = 0.0578. * a–c—groups that share the same letter belong to the same homogeneous subset, meaning that their means are statistically similar at the chosen significance level (e.g., p < 0.05).
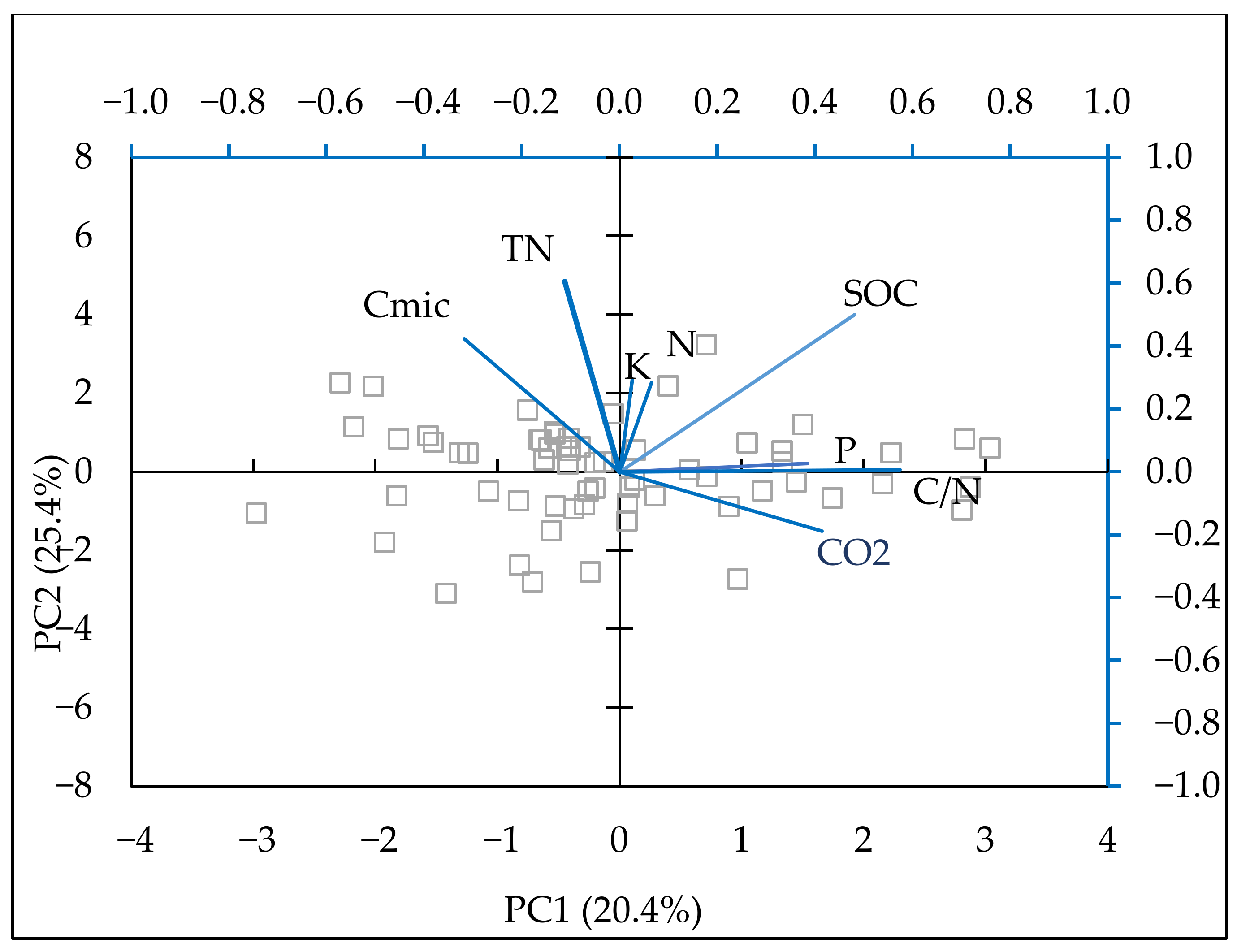
PCA was also conducted (Figure 6) to determine the relationship between soil physicochemical properties, SOC, and greenhouse gas emissions. The model described 73.89% of the variability in the original data. The data were loaded into four principal components (the first two are presented in Table 5), explaining most of the variation between the indicators by fertilization treatment. A positive relationship was observed among the contents of available forms of phosphorus, C/N, SOC, and CO2 emissions. The relationship between mineral nitrogen and CO2 was lower than the impact of phosphorus. Cmic showed a negative relationship with all parameters in these analyses. There was a negative correlation between Cmic and C-CO2 in wheat (r = −0.44, p = 0.09) and maize (r = −0.64, p = 0.007). In bean and barley, these coefficients were also negative (r = −0.12; r = −0.61) but not significant.
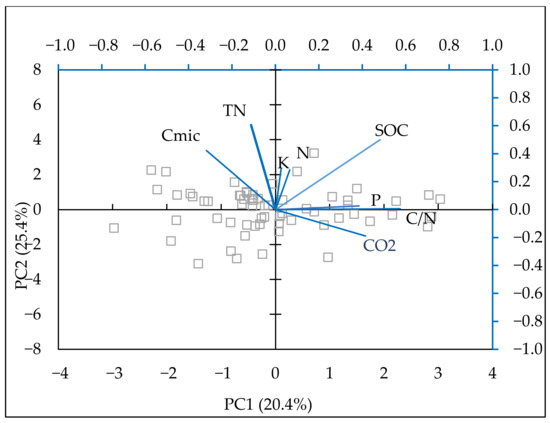
Figure 6.
Biplot of the first two components in the analysis carried out on a dataset including: soil C-CO2 emissions (kg ha−1 h−1), mineral N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), Cmic (mg kg−1) TN (%), C/N ratio.

Table 5.
Variable loadings of the first two components of principal components analysis. Soil C-CO2 emissions (kg ha−1 h−1), mineral N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), Cmic (mg kg−1) TN (%), C/N ratio.
3.3. Effects of Long-Term Mineral Fertilization on Soil Microbiota
Microorganisms are highly sensitive, with their number strongly depending on many factors, and strong variations in quantity. Of all the factors studied, the season had the strongest influence on the number of the studied groups of microorganisms, followed by the growing crop. The influence of the treatments was statistically significant when combining all factors.
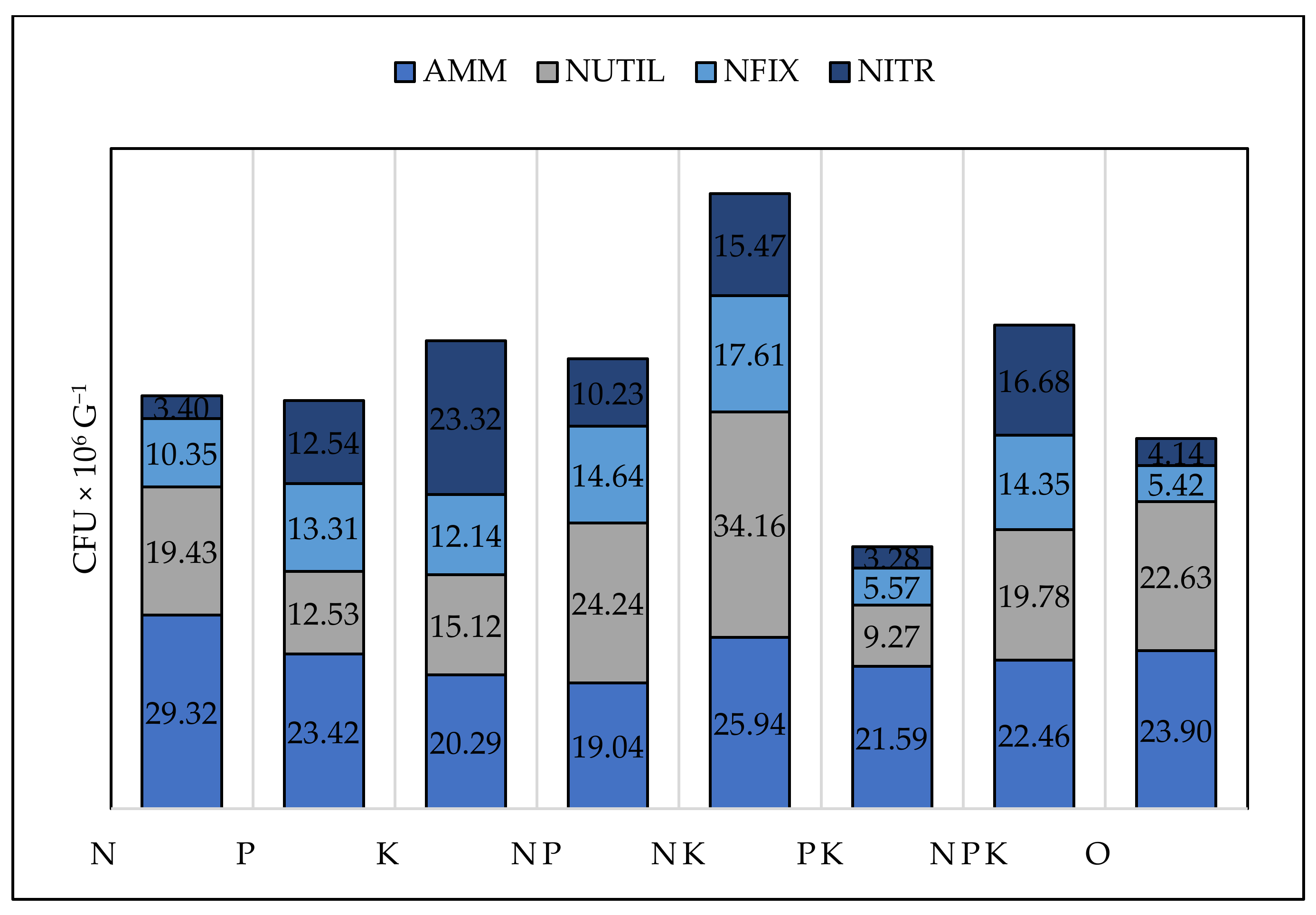
The number of bacteria utilising mineral nitrogen, nitrifying and nitrogen-fixing bacteria were the highest under NP, NK, and NPK treatments (Table 6, Figure 7). The number of nitrogen-fixing and nitrifying bacteria were also high under NK, NPK, and NP treatments, which was not expected but could be explained by the improved soil quality and conditions due to enhanced SOC. The highest amount of nitrifying bacteria was observed under K treatment. The number of nitrogen-fixing (r = 0.53, p = 0.03), nitrifying (r = 0.51, p = 0.04), and assimilating mineral nitrogen (r = 0.14) bacteria, had a positive correlation with the available phosphorus forms in bean plots and a negative correlation with phosphorus in all cereal plots. These three groups of microorganisms had a positive correlation with nitrogen in all crops, most strongly under barley (NFIX, r = 0.51, p = 0.03; NITR, r = 0.62, p = 0.04, NUTIL, r = 0.53, p = 0.05). Nitrogen had a suppressive effect only in maize and wheat under N treatment while, in bean and barley, no such effect was observed, perhaps due to the lower nitrogen dose applied.

Table 6.
Analysis of variance by controlling factors on ammonifying bacteria (CFU × 106 g−1), nitrogen utilizing bacteria (CFU × 106 g−1), nitrogen fixing bacteria, (CFU × 106 g−1), nitrifying bacteria (CFU × 106 g−1), actinomycetes (CFU × 106 g−1), microscopic fungi CFU × 106 g−1).
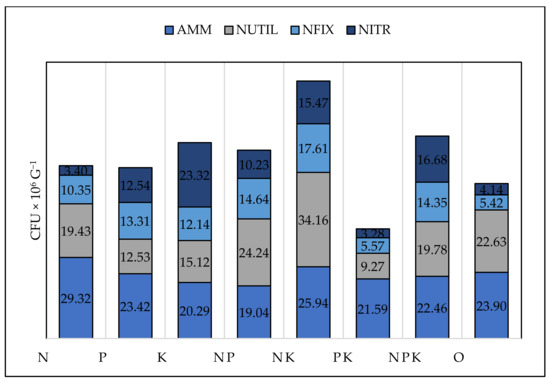
Figure 7.
Number of AMM—ammonifying bacteria (CFU × 106 g−1), NUTIL—nitrogen utilizing bacteria (CFU × 106 g−1), NFIX—nitrogen fixing bacteria, (CFU × 106 g−1), NITR—nitrifying bacteria (CFU × 106 g−1).
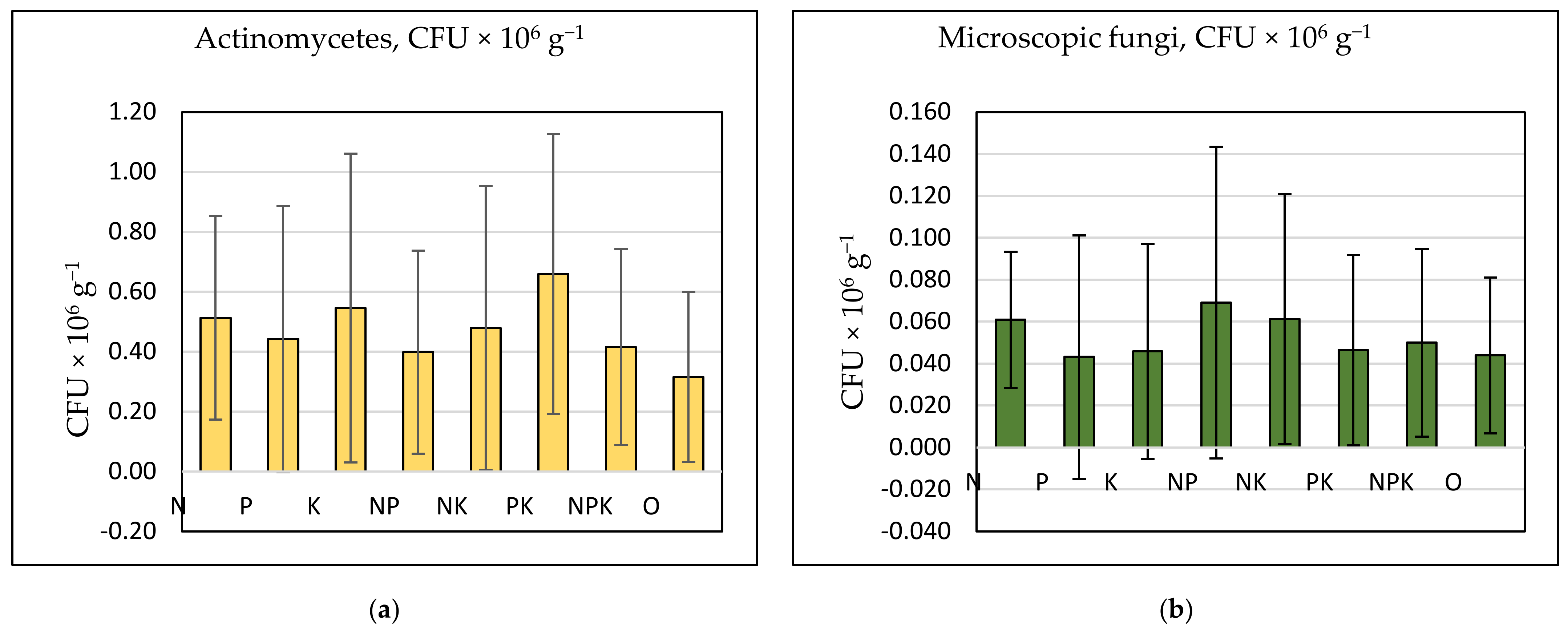
In general, the number of microscopic fungi and actinomycetes were correlated (r = 0.34, p = 0.006), while nitrogen cycle bacteria correlated positively with each other. The quantity of actinomycetes (Figure 8a) was positively affected by potassium fertilization, as the most increased number of actinomycetes was established in K, PK, NK, NPK, and N treatments. The correlation coefficient between the number of actinomycetes and available potassium content was 0.70 (p = 0.068). A higher number of microscopic fungi (Figure 8b) was observed in treatments with a high EC and low pH (N, NP, NK, and NPK), but a positive correlation was not established between N treatment and the number of fungi.
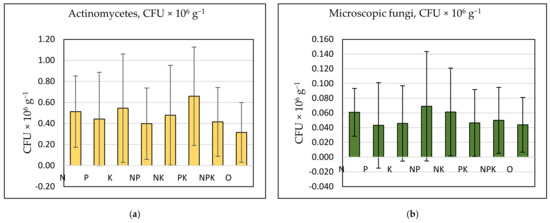
Figure 8.
(a) Abundance of actinomycetes (CFU × 106 g−1) and (b) microscopic fungi (CFU × 106 g−1) by treatment. Vertical bars denote standard deviation.
The ratio of bacteria under different fertilization types showed that ammonifying and mineral nitrogen-utilising bacteria predominated in the non-fertilized control. The trend was similar for the N and PK treatments (Figure 9), indicating enhanced mineralization and a much lower amount of nitrogen-fixing and nitrifying bacteria. The largest shares of NFIX and NUTIL were recorded under K and NPK treatments.
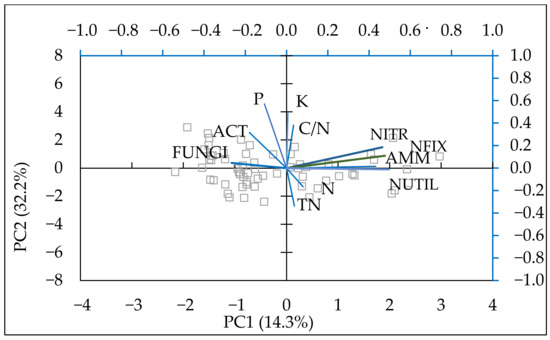
Figure 9.
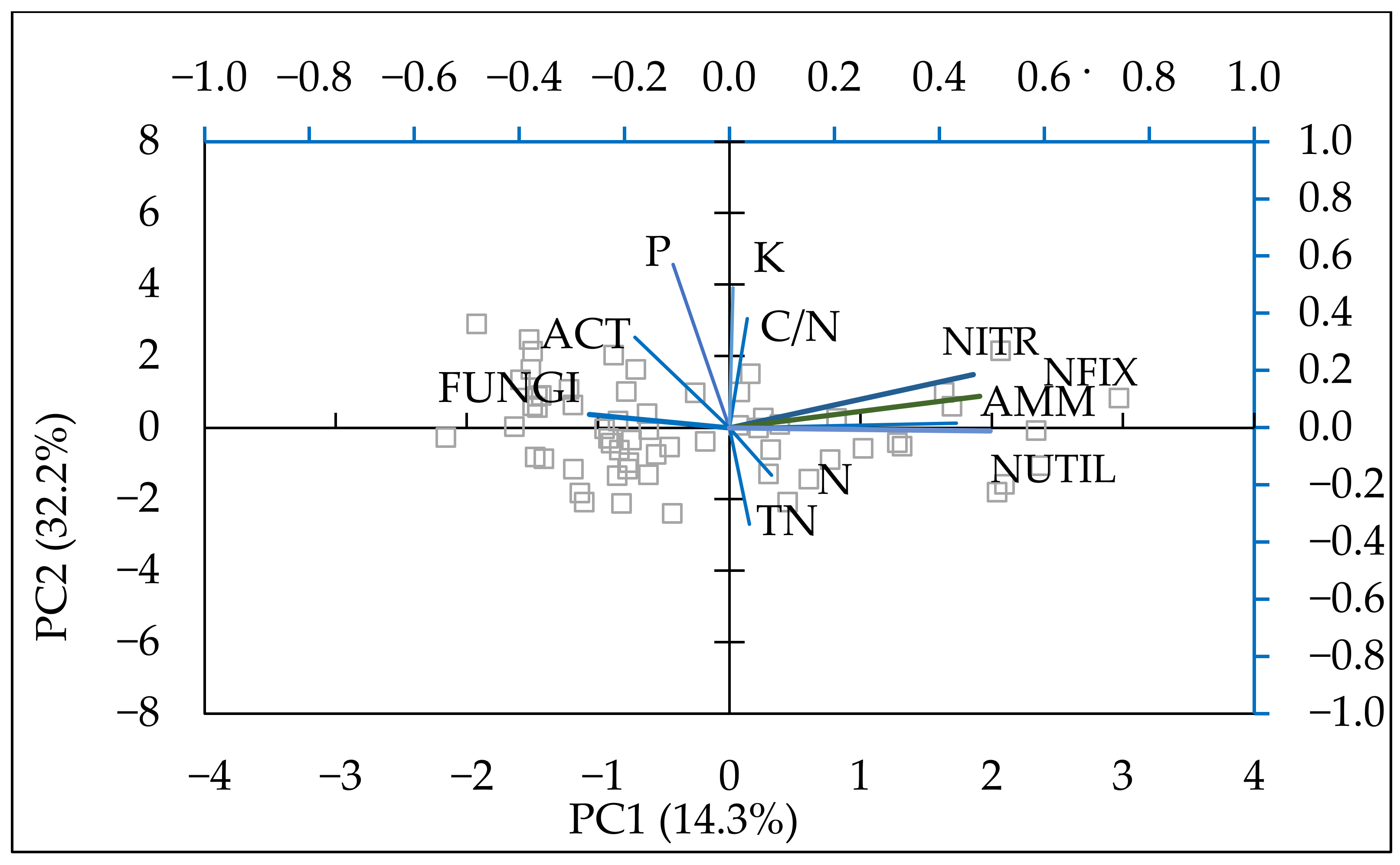
Biplot of the first two components in the analysis carried out on a dataset including: N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), AMM—ammonifying bacteria (CFU × 106 g−1), NUTIL—nitrogen utilizing bacteria (CFU × 106 g−1), NFIX—nitrogen fixing bacteria, (CFU × 106 g−1), NITR—nitrifying bacteria (CFU × 106 g−1), ACT—actinomycetes (CFU × 106 g−1), FUNGI—microscopic fungi CFU × 106 g−1), TN (%), C/N ratio.
PCA was performed on the mineral nitrogen content, available forms of P2O5, K2O, and the main groups of soil microorganisms to establish the connections between nutrient content and soil microbiota. The model described 69.98% of data variability (Table 7). The data were fitted into four principal components that explained most of the variation among variables. On PC1 and PC2, the populations of ammonifying, nitrifying, nitrogen-fixing, and mineral nitrogen-utilising bacteria increased, with the first component showing a slight increase in nitrogen and the second component showing an increase in phosphorus and potassium.

Table 7.
Variable loadings of the first two components of principal components analysis of dataset including: soil mineral N (N-NO3, N-NH4) (mg kg−1), P—available P2O5 (mg 100 g−1), K—available K2O (mg 100 g−1), AMM—ammonifying bacteria (CFU × 106 g−1), NUTIL—nitrogen utilizing bacteria (CFU × 106 g−1), NFIX—nitrogen fixing bacteria, (CFU × 106 g−1), NITR—nitrifying bacteria (CFU × 106 g−1), ACT—actinomycetes (CFU × 106 g−1), FUNGI—microscopic fungi CFU × 106 g−1).
The model described the positive relationship of nitrogen with nitrifying, nitrogen-fixing, ammonifying, and nitrogen-utilising bacteria (Figure 7). A positive effect of potassium was established for NFIX and NUTIL. Both actinomycetes and fungi were affected by the available phosphorus and potassium.
4. Discussion
There are different views on the impact of mineral nitrogen fertilizers on SOC sequestration [37]. Some authors have suggested that nitrogen fertilizers reduce SOC stocks because they accelerate SOC mineralization [38,39], and other reports have suggested that nitrogen fertilizers increase SOC stocks [16,28,40]. A detailed analysis of 84 permanent fertilizer experiments showed that soils fertilized with NPK, N, P, and K increased SOC by 10, 5, 5, and 2%, respectively, and these changes depended on the cropping system, experiment duration, geographic region, and degree of fertilization [41]. In this study, and considering the used soils and site conditions, long-term N treatment resulted in higher SOC levels than the non-fertilized control with 16% and PK treatment with 3%, and had similar effects to K and NK treatments. Nitrogen in NP and NPK treatments resulted in the highest SOC stocks (36%). The increase in organic matter in the variants with nitrogen addition is mainly due to the increase in net primary productivity and the increased import of organic matter into the soil. However, the highest C/N ratios were observed under P and NPK treatments, which were associated with a lower degree of mineralization. The combined effects of nitrogen and phosphorus addition were stronger than that under N and P treatments, and a synergistic effect on net primary production was established, explaining the higher amount of SOC in the treatments with combined fertilization. As a result, there were higher amounts of plant residues and larger carbon inputs into the soil [42]. Another long-term fertilizer experiment found that N fertilization greatly boosted crop yields without altering SOC, while P and K fertilization and their co-application did not significantly alter crop yield or SOC [42]. However, the combined effect of NP and NPK fertilization resulted in a 19–47% higher SOC stock than single N, P, or K fertilization [43,44,45]. Opposite results have been observed in long-term experiments that combined mineral fertilization, which showed a decrease in the carbon pool compared to non-fertilized plots [46].
Variations in the soil’s capacity to store carbon have been attributed to the structure and activity of the soil microbial community, which plays a major role in the carbon cycle [16]. Ecological conditions determining microorganisms’ activities, including their microsite environment, are decisive for metabolism and outputs. For changes in greenhouse gas emissions, they are related to SOC formation and stabilization [47,48,49]. Soil microbial community diversity and distribution responses to fertilization have been widely reported [50,51]. In a long-term experiment on brown soil, the pH and available phosphorus content had the most significant effects on bacterial communities, while mineral nitrogen, pH, and SOC had the strongest effects on fungal diversity [6]. In the current study, an increased number of microscopic fungi was observed in treatments with a high EC and low pH (N, NP, NK, and NPK), which are effects of N fertilization, but a positive correlation was not established between N and the number of fungi. Additionally, in our study, Cmic was positively correlated with the amount of actinomycetes, fungi, and different groups of bacteria, depending on the crop. Microbial biomass carbon was stimulated most strongly by single and combined nitrogen and potassium fertilization (N, K, and NK). In contrast to these findings, long-term N treatment [6] decreased Cmic and the dehydrogenase activity, while combined NP and NPK treatments increased these parameters.
N fertilization boosted all ecosystem C components as well as soil respiration by stimulating both heterotrophic respiration and root respiration [52]. However, many experiments have been conducted to investigate the effects of fertilization on soil CO2 emissions, with controversial results [53]. Fertilization alters the diversity and composition of the soil bacterial communities, which regulate soil CO2 emissions [53]. In previous studies, higher nitrogen application rates resulted in higher greenhouse gas emissions in long-term experiments [54,55], and cumulative CO2 fluxes increased under long-term NP and NPK treatments compared to control and NK treatments [55]. Other studies established a reduction in soil CO2 emissions after nitrogen fertilizer application [16]. The soil CO2 emissions in our experiment were measured under field conditions, and the increase in NP and NPK plots could be the result of the increased root respiration of plants because of higher biomass accumulation or microbial respiration. To distinguish two CO2 flows, by soil heterotrophic respiration or root respiration, additional studies using isotopic tracing or other methods are needed.
5. Conclusions
The strongest influence on SOC formation was the mineral nitrogen content but its combination with phosphorus (NP) and phosphorus and potassium (NPK) were considered here. NP treatment increased soil carbon sequestration by 37% compared to the non-fertilized control, 17% compared to N treatment, and 8% compared to P treatment on Luvic Chernozem soil. Nitrogen fertilizer application most significantly affected soil properties, including pH, EC, and SOC, altering the environment for soil microbial development and influencing the composition of soil microbial communities. The enhancement of total SOC led to a higher intensity of soil respiration and more intense CO2 emissions in the NP and NPK treatments.
Author Contributions
Conceptualization, G.K., G.G., J.P. and H.V.; methodology, G.K., M.K., J.P. and G.G.; software, G.K.; formal analysis, G.G., J.P. and L.T.; resources, G.G.; writing—review and editing, G.K., M.K., H.V. and L.T.; funding acquisition, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge funding of research activities received from the National Science Fund under grant agreement No. KΠ-06-H76/2, 2023 (project Integration of satellite derived and ground-based data for soil water balance components and crop cover into models for assessment of agroecological risks and agricultural practices for their mitigation).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.K.; Chen, X.; Jin, J.; Liu, V.; Wang, G. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agric. Ecosyst. Environ. 2017, 248, 113–122. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Šimanský, V.; Juriga, M.; Jonczak, J.; Uzarowicz, L.; Stapień, W. How relationships between soil organic carbon parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Ullah, S.; Ai, C.; Ding, W.; Jiang, R.; Zhao, S.; Zhang, J.; Zhou, W.; Hou, Y.; He, P. The response of soil fungal diversity and community composition to long-term fertilization. Appl. Soil Ecol. 2019, 140, 35–41. [Google Scholar] [CrossRef]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef]
- Šimanský, V.; Jonczak, J.; Horváthová, J.; Igaz, D.; Aydın, E.; Kováčik, P. Does long-term application of mineral fertilizers improve physical properties and nutrient regime of sandy soils? Soil Tillage Res. 2022, 215, 105224. [Google Scholar] [CrossRef]
- UNEP, United Nations Environmental Programme, Annual Evaluation Report. 2007. Available online: https://www.unep.org/resources/synthesis-reports/unep-annual-evaluation-report-2007 (accessed on 2 June 2008).
- Nasser, H.A.; Mahmoud, M.; Tolba, M.M.; Radwan, A.R.; Gabr, N.M.; ElShamy, A.A.; Yehya, M.S.; Ziemke, A.; Hashem, M.Y. Pros and cons of using green biotechnology to solve food insecurity and achieve sustainable development goals. Euro-Mediterr. J. Environ. Integr. 2021, 6, 29. [Google Scholar] [CrossRef]
- Patrick, M.; Tenywa, J.S.; Ebanyat, P.; Tenywa, M.M.; Mubiru, D.N.; Basamba, T.A.; Leip, A. Soil organic carbon thresholds and nitrogen management in tropical agroecosystems: Concepts and prospects. J. Sust. Dev. 2013, 6, 31–43. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Wang, X.; Song, L. Advances in the Study of NO3—Immobilization by Mic robes in Agricultural Soils. Nitrogen 2024, 5, 927–940. [Google Scholar] [CrossRef]
- Braos, L.B.; Carlos, R.S.; Bettiol, A.C.T.; Bergamasco, M.A.M.; Terçariol, M.C.; Ferreira, M.E.; da Cruz, M.C.P. Soil Carbon and Nitrogen Forms and Their Relationship with Nitrogen Availability Affected by Cover Crop Species and Nitrogen Fertilizer Doses. Nitrogen 2023, 4, 85–101. [Google Scholar] [CrossRef]
- Kong, D.; Liu, N.; Ren, C.; Li, H.; Wang, W.; Li, N.; Ren, G.; Feng, Y.; Yang, G. Effect of Nitrogen Fertilizer on Soil CO2 Emission Depends on Crop Rotation Strategy. Sustainability 2020, 12, 5271. [Google Scholar] [CrossRef]
- Jesmin, T.; Mitchell, D.T.; Mulvaney, R.L. Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil. Nitrogen 2021, 2, 444–460. [Google Scholar] [CrossRef]
- Zang, H.D.; Blagodatskaya, E.; Wang, J.Y.; Xu, X.L.; Kuzyakov, Y. Nitrogen fertilization increases rhizodeposit incorporation into microbial biomass and reduces soil organic matter losses. Biol. Fertil. Soils 2017, 53, 419–429. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Pu, C.; Zhao, X.; Xue, J.F.; Zhang, R.; Nie, Z.J.; Chen, F.; Lal, R.; Zhang, H.L. Tillage effects on carbon footprint and ecosystem services of climate regulation in a winter wheat–summer maize cropping system of the North China Plain. Ecol. Indicat. 2016, 67, 821–829. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C sequestration as a biological negative emission strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- Bradford, M.A.C.; Carey, J.; Atwood, L.; Bossio, D.; Fenichel, E.P.; Gennet, S.; Fargione, J.; Fisher, J.R.B.; Fuller, E.; Kane, D.A.; et al. Soil carbon science for policy and practice. Nat. Sustain. 2019, 2, 1070–1072. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and Principles of Environmentally Sustainable Food and Agriculture Systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Mattila, V.; Dwivedi, P.; Gauri, P.; Ahbab, M. Blockchain for Environmentally Sustainable Economies: Case Study of 5irechain. Int. J. Soc. Sci. Manag. Rev. 2022, 5, 50–62. [Google Scholar] [CrossRef]
- Smith, P.; Soussana, J.F.; Angers, D.; Schipper, L.; Chenu, C.; Rasse, D.P.; Batjes, N.H.; van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to measure, report and verify soil carbon change to realise the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Change Biol. 2019, 26, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Druckman, A.; Jackson, T. The carbon footprint of UK households 1990–2004: A socio-economically disaggregated, quasi-multi-regional input–output model. Ecol. Econ. 2009, 68, 2066–2077. [Google Scholar] [CrossRef]
- Cheng, K.; Pan, G.; Smith, P.; Luo, T.; Li, L.; Zheng, J.; Zhang, X.; Han, X.; Yan, M. Carbon footprint of China’s crop production—An estimation using agro-statistics data over 1993–2007. Agric. Ecosyst. Environ. 2011, 142, 231–237. [Google Scholar] [CrossRef]
- Pathak, H. Impact, adaptation, and mitigation of climate change in Indian agriculture. Environ. Monit. Assess. 2023, 195, 52. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, S.; Xiong, X.; Wang, X.; Sun, Y.; Meng, Z.; Hui, D.; Li, J.; Zhang, D.; Deng, Q. Dynamics of soil microbial communities involved in carbon cycling along three successional forests in southern China. Front. Microbiol. 2024, 14, 1326057. [Google Scholar] [CrossRef]
- Mahal, N.K.; Osterholz, W.R.; Miguez, F.E.; Poffenbarger, H.J.; Sawyer, J.E.; Olk, D.C.; Archontoulis, S.V.; Castellano, M.J. Nitrogen Fertilizer Suppresses Mineralization of Soil Organic carbon in Maize Agroecosystems. Front. Ecol. Evol. 2019, 7, 59. [Google Scholar] [CrossRef]
- Pan, H.; Chen, M.M.; Feng, H.J.; Wei, M.; Song, F.P.; Lou, Y.H.; Cui, X.; Wang, H.; Zhuge, Y. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Till. Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Liu, X.J.; Xiao, L.; Shi, P.; Zhao, B.H. Effects of farmland conversion on the stoichiometry of carbon, nitrogen, and phosphorus in soil aggregates on the Loess Plateau of China. Geoderma 2019, 351, 188–196. [Google Scholar] [CrossRef]
- Filcheva, E.; Tsadilas, C. Influence of cliniptilolite and compost on soil properties. Commun. Soil Sci Plan. 2002, 33, 595–607. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Hoper, H. Substrate-induced respiration. In Microbiological Methods for Assessing Soil Quality, 1st ed.; Bloem, J., Hopkins, D.W., Benedetti, A., Eds.; CABI Publishing: Wallingford, UK, 2008; Chapter 6.3; Available online: https://www.cabi.org/bookshop/book/9780851990989/ (accessed on 1 October 2008).
- Grudeva, V.; Moncheva, P.; Nedeva, S.; Gocheva, B.; Antonova-Nedeva, S.; Naumova, S. Handbook of Microbiology; University edition SU “St. Kl. Ohridski”: Sofia, Bulgaria, 2007. [Google Scholar]
- Bilandžija, D.; Zgorelec, Ž.; Kisić, I. Influence of Tillage Practices and Crop Type on Soil CO2 Emissions. Sustainability 2016, 8, 90. [Google Scholar] [CrossRef]
- Robertson, G.P.; Bruulsema, T.W.; Gehl, R.J.; Kanter, D.; Mauzerall, D.L.; Rotz, C.A. Nitrogen–climate interactions in US agriculture. Biogeochemistry 2013, 114, 41–70. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Russell, A.E.; Cambardella, C.A.; Laird, D.A.; Jaynes, D.B.; Meek, D.W. Nitrogen fertilizer effects on soil carbon balances in Midwestern US agricultural systems. Ecol. Appl. 2009, 19, 1102–1113. [Google Scholar] [CrossRef]
- Poffenbarger, H.J.; Barker, D.W.; Helmers, M.J.; Miguez, F.E.; Olk, D.C.; Sawyer, J.E. Maximum soil organic carbon storage in Midwest US cropping systems when crops are optimally nitrogen-fertilized. PLoS ONE 2017, 12, e0172293. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, B.; Lu, F.; Wang, X.; Zhuang, T.; Zhang, G.; Ouyang, X. Roles of nitrogen, phosphorus, and potassium fertilizers in carbon sequestration in a Chinese agricultural ecosystem. Clim. Change 2017, 142, 587–596. [Google Scholar] [CrossRef]
- Li, W.; Gan, X.; Jiang, Y.; Cao, F.; Lü, X.T.; Ceulemans, T.; Zhao, C. Nitrogen effects on grassland biomass production and biodiversity are stronger than those of phosphorus. Environ. Pollut. 2022, 309, 119720. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Cai, G.; Sauheitl, L.; Xiao, M.; Shibistova, O.; Ge, T.; Guggenberger, G. Meta-analysis on the effects of types and levels of N, P, and K fertilization on organic carbon in cropland soils. Geoderma 2023, 437, 116580. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Liu, X. Long term fertilization effects on crop yield and desalinized soil properties. Agron. J. 2020, 112, 4321–4331. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Xiang, Y.; Cheng, M.; Wen, Y.; Darboux, F. Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China. Sustainability 2022, 14, 5109. [Google Scholar] [CrossRef]
- Li, J.; Shen, F.Y.; Liu, Y.; Yang, Y.C.; Wang, J.; Purahong, W.; Yang, L. Contrasting altitudinal patterns and co-occurrence networks of soil bacterial and fungal communities along soil depths in the cold-temperate montane forests of China. Catena 2022, 209, 105844. [Google Scholar] [CrossRef]
- Liang, C.; Kästner, M.; Rainer Georg, J. Microbial necromass on the rise: The growing focus on its role in soil organic carbon development. Soil Biol. Biochem. 2020, 150, 108000. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.S.; Ma, M.C.; Guan, D.W.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty-four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Chen, K.; Shi, J.; Wang, Y.; Luo, P.; Yang, J.; Wang, Y.; Han, X. Long-term fertilization altered microbial community structure in an aeolian sandy soil in northeast China. Front. Microbiol. 2022, 13, 979759. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhong, Y.; Liu, W.; Shangguan, Z. Asymmetric response of ecosystem carbon components and soil water consumption to nitrogen fertilization in farmland. Agric. Ecosyst. Environ. 2021, 305, 107166. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Li, L.; Effah, Z.; Xie, L.; Luo, Z.; Zhou, Y.; Jiang, Y. Fertilization treatments affect soil CO2 emission through regulating soil bacterial community composition in the semiarid Loess Plateau. Sci. Rep. 2022, 12, 20123. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, H.; Zhang, J.; Huang, J.; Wang, B. Long-Term Application of Organic Manure and Mineral Fertilizer on N2O and CO2 Emissions in a Red Soil from Cultivated Maize-Wheat Rotation in China. Agric. Sci. China 2011, 10, 1748–1757. [Google Scholar] [CrossRef]
- Saeed, Q.; Zhang, A.; Mustafa, A.; Sun, B.; Zhang, S.; Yang, X. Effect of long-term fertilization on greenhouse gas emissions and carbon footprints in northwest China: A field scale investigation using wheat-maize-fallow rotation cycles. J. Clean. Prod. 2022, 332, 130075. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).