Nutrient Availability for Lactuca sativa Cultivated in an Amended Peatland: An Ionic Exchange Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Amendment Description
2.3. Experimental Design
2.4. Ionic Exchange Resin
2.5. Lettuce Crop Management and Yield Evaluation
2.6. Soil Sampling and Analysis
2.7. Data Visualization and Statistics
3. Results
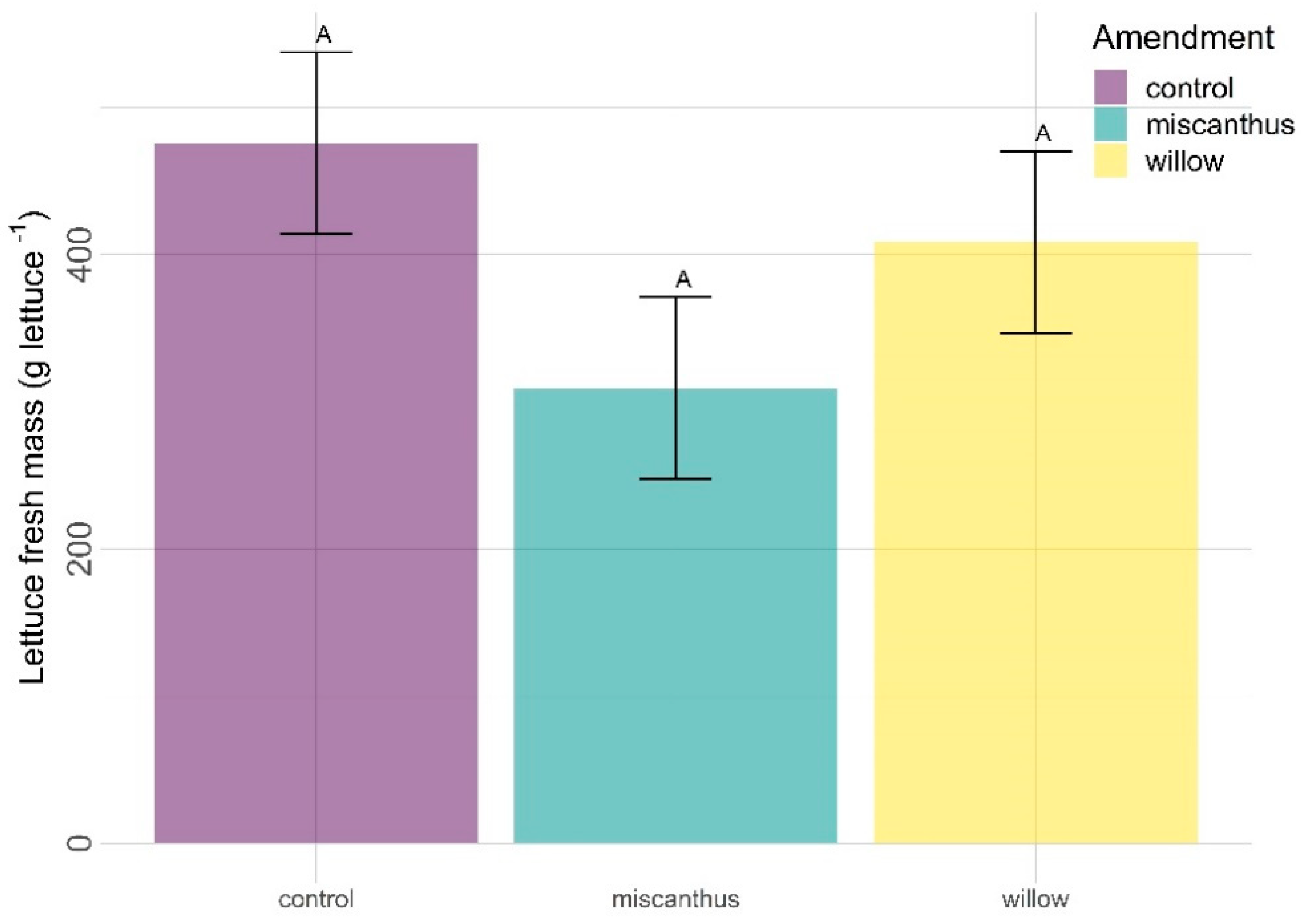
3.1. Yield (Fresh Mass)
3.2. PRS® Probes
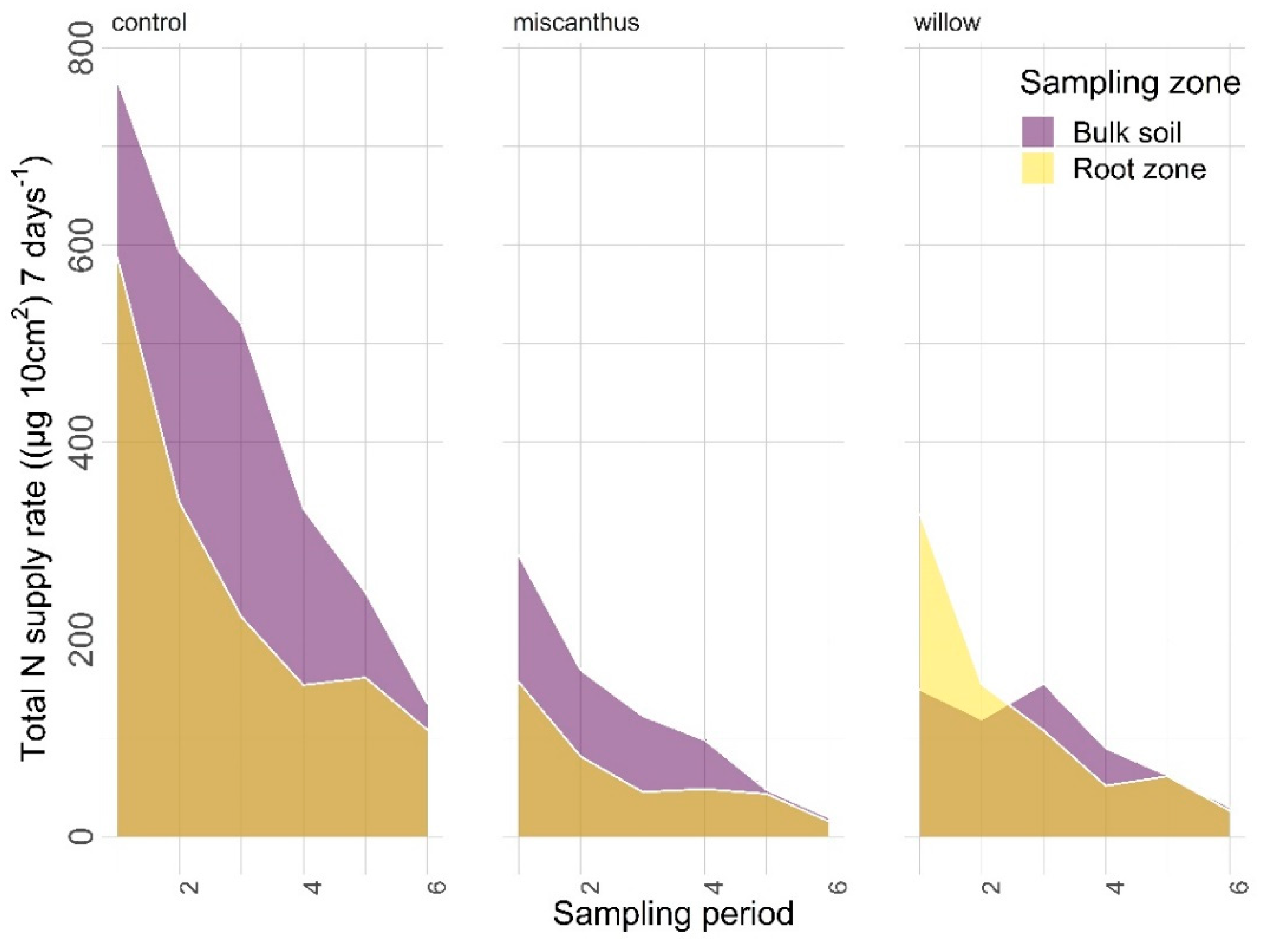
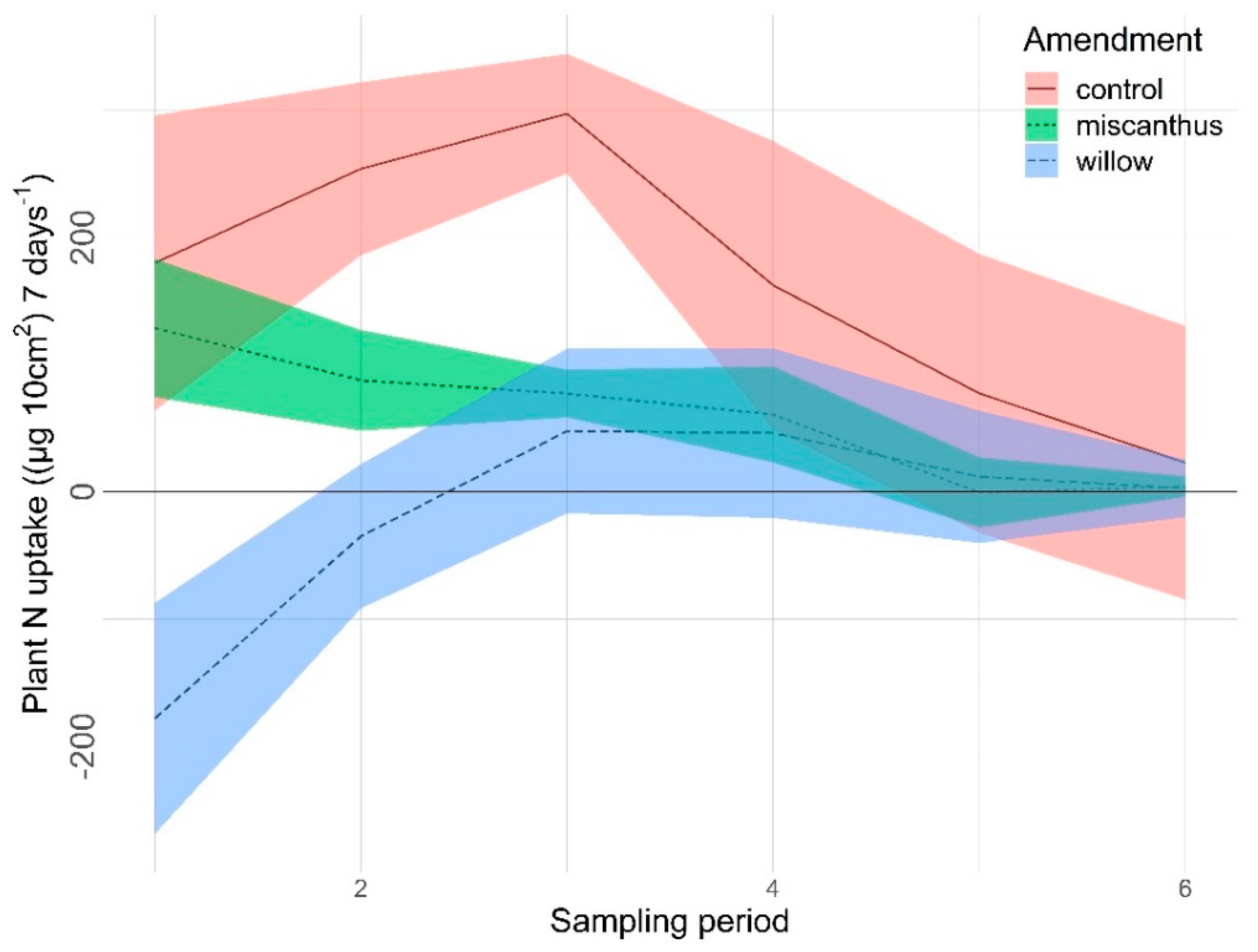
3.2.1. Nitrogen
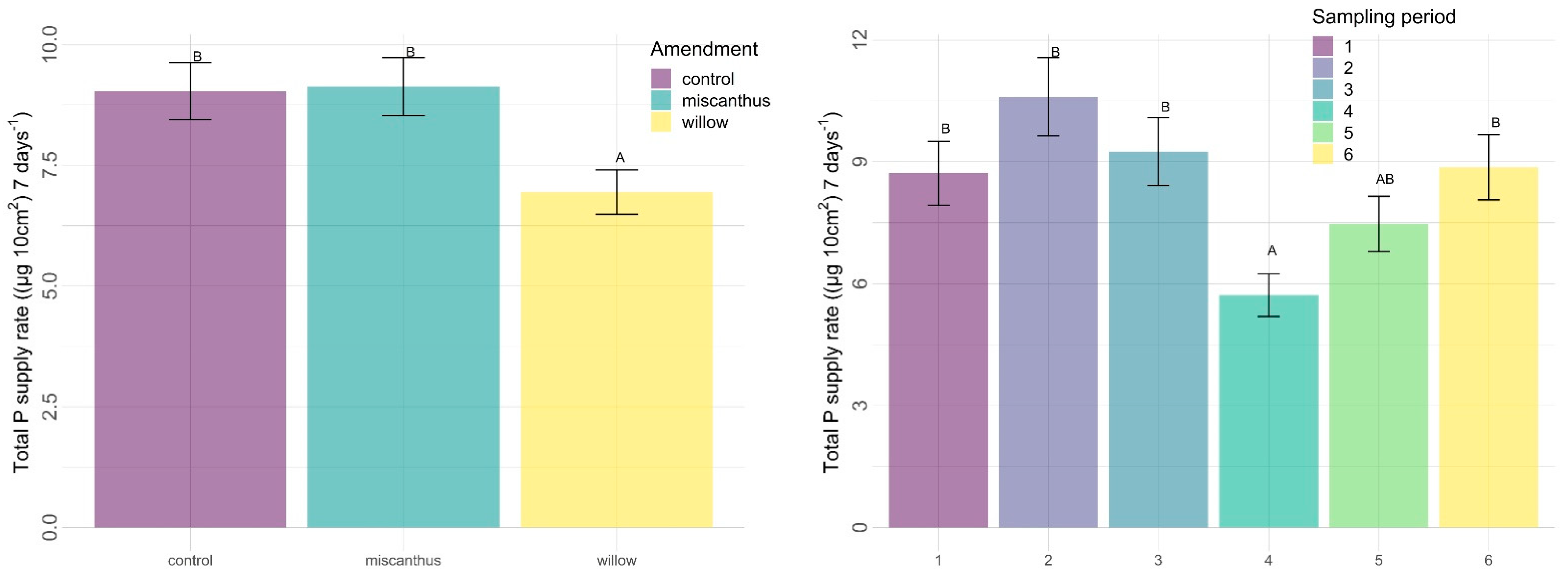
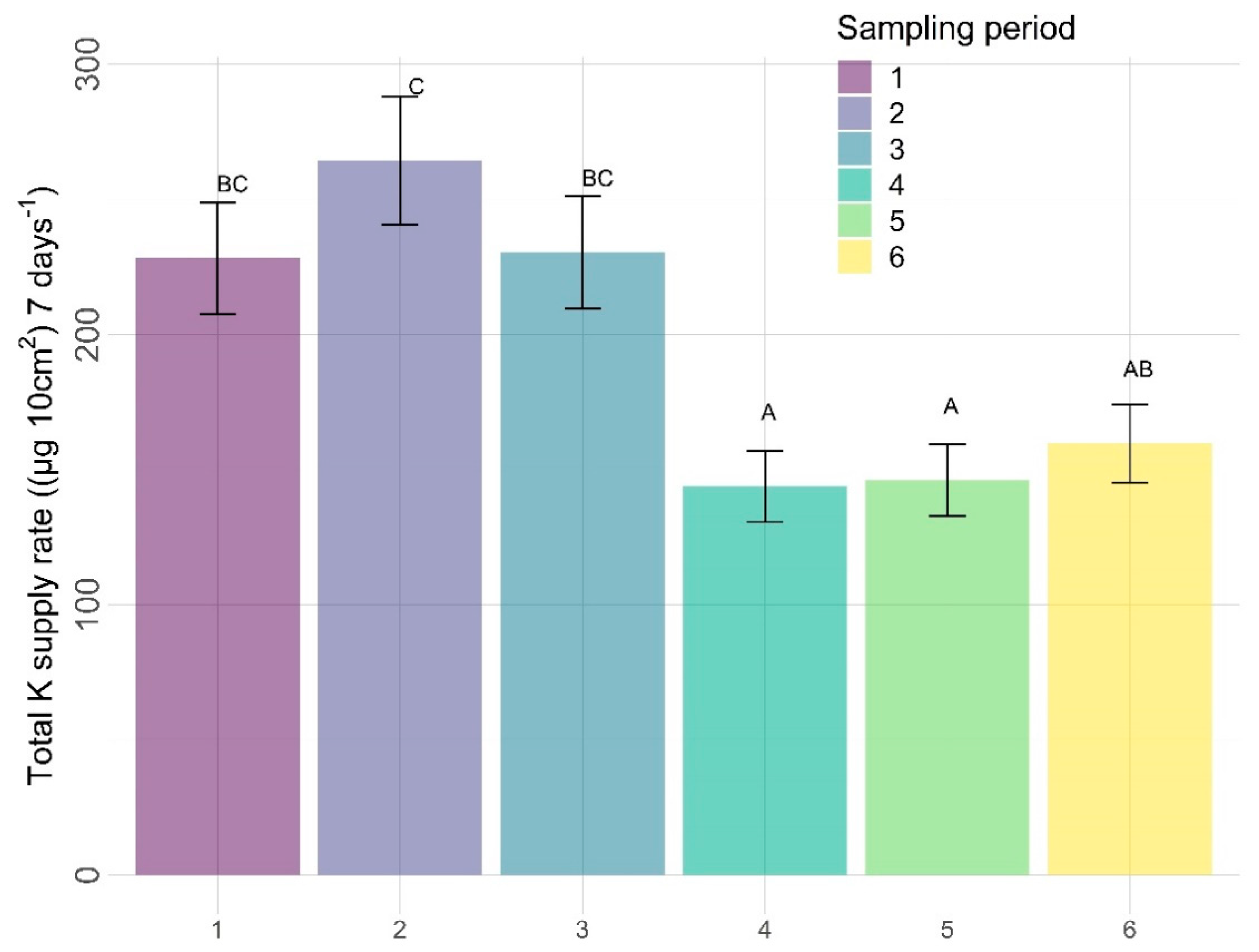
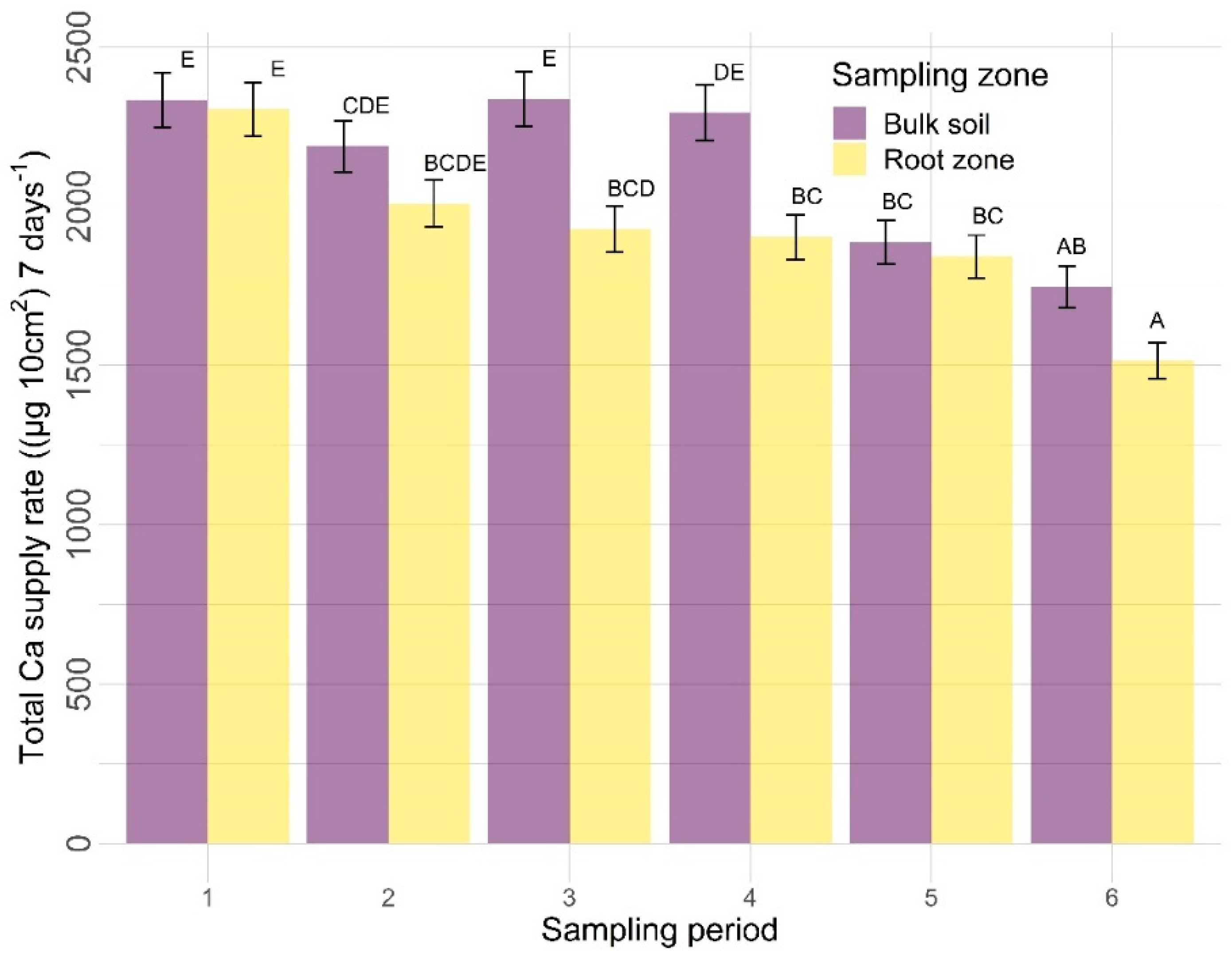
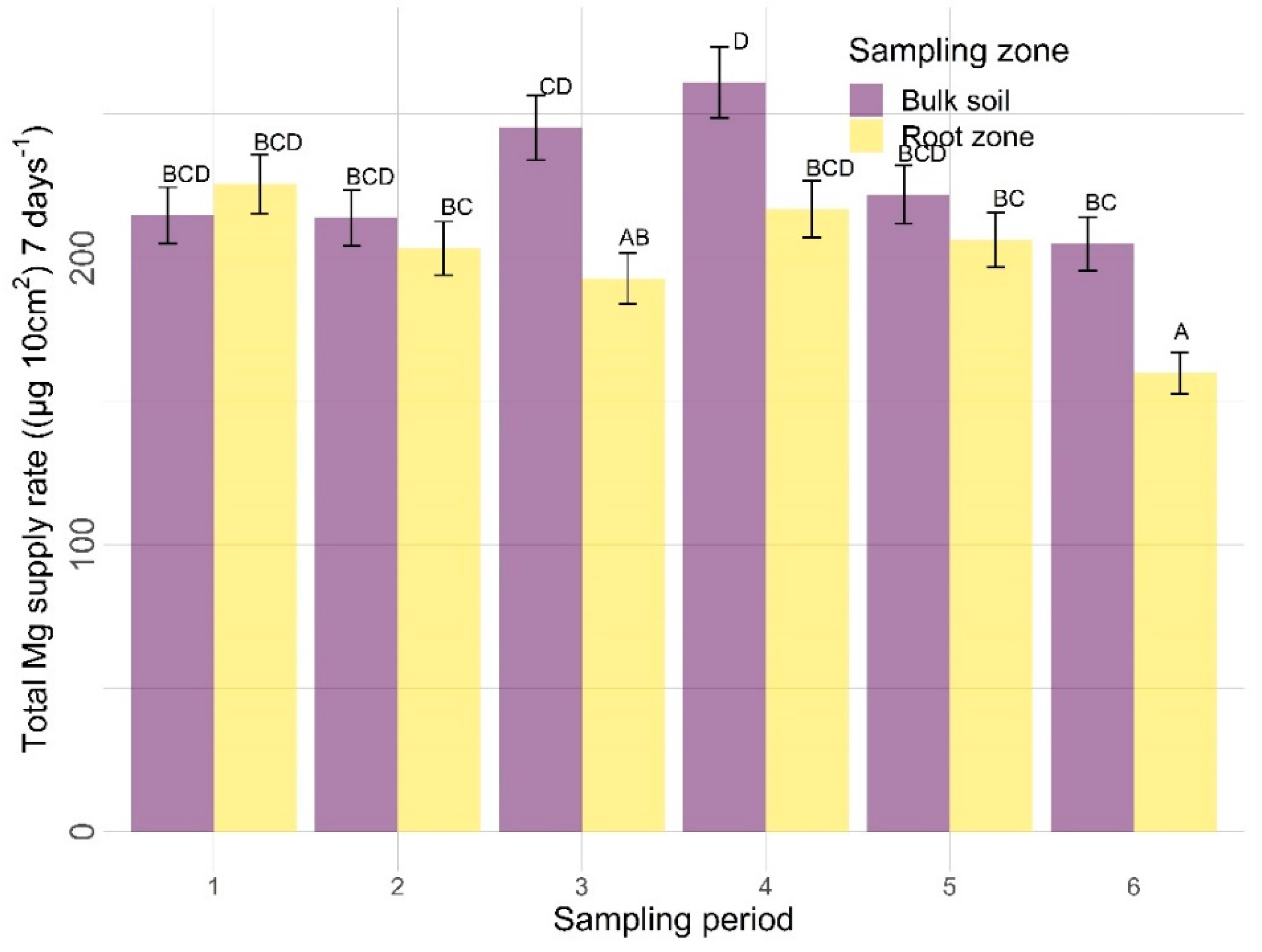
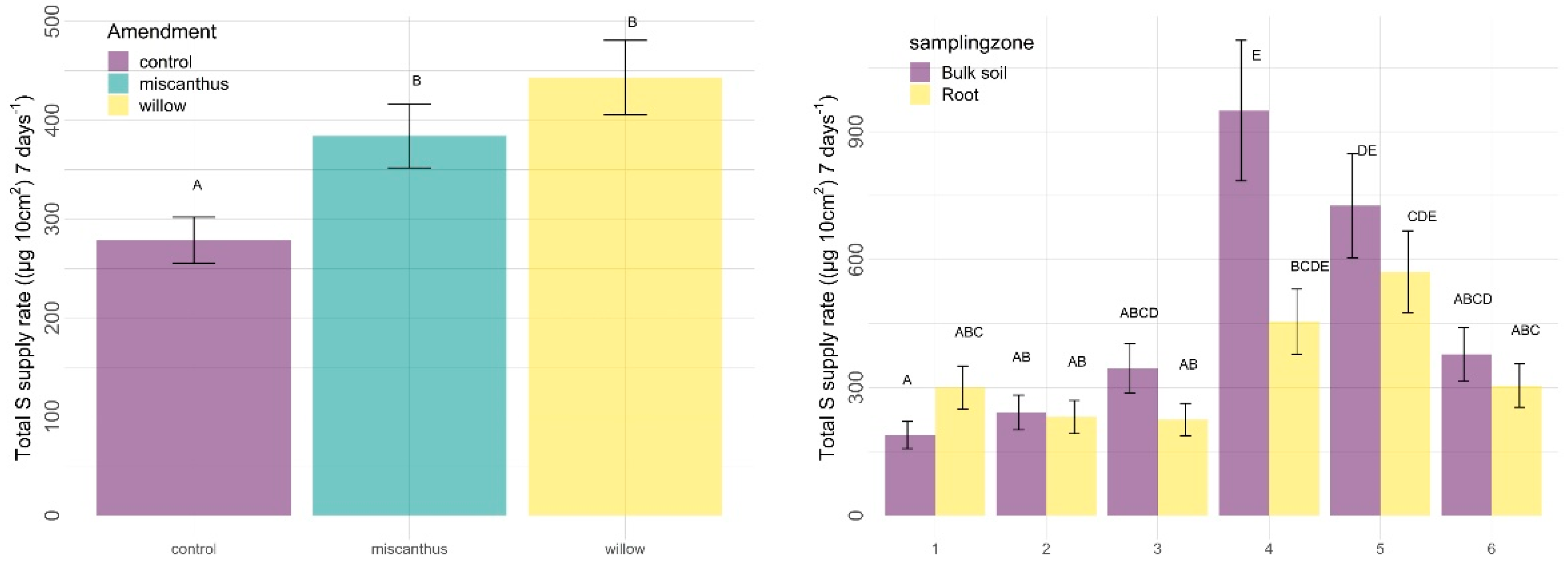
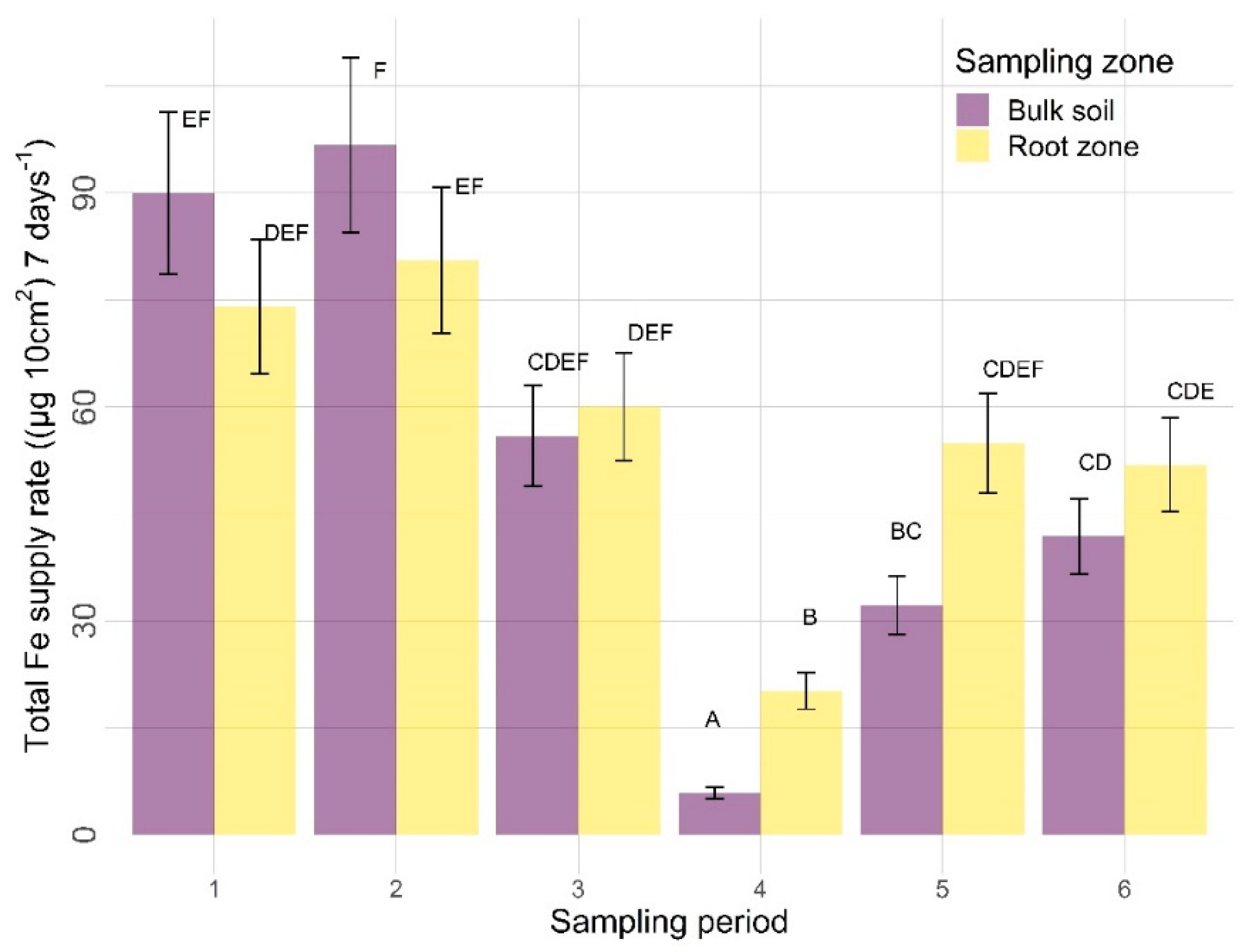
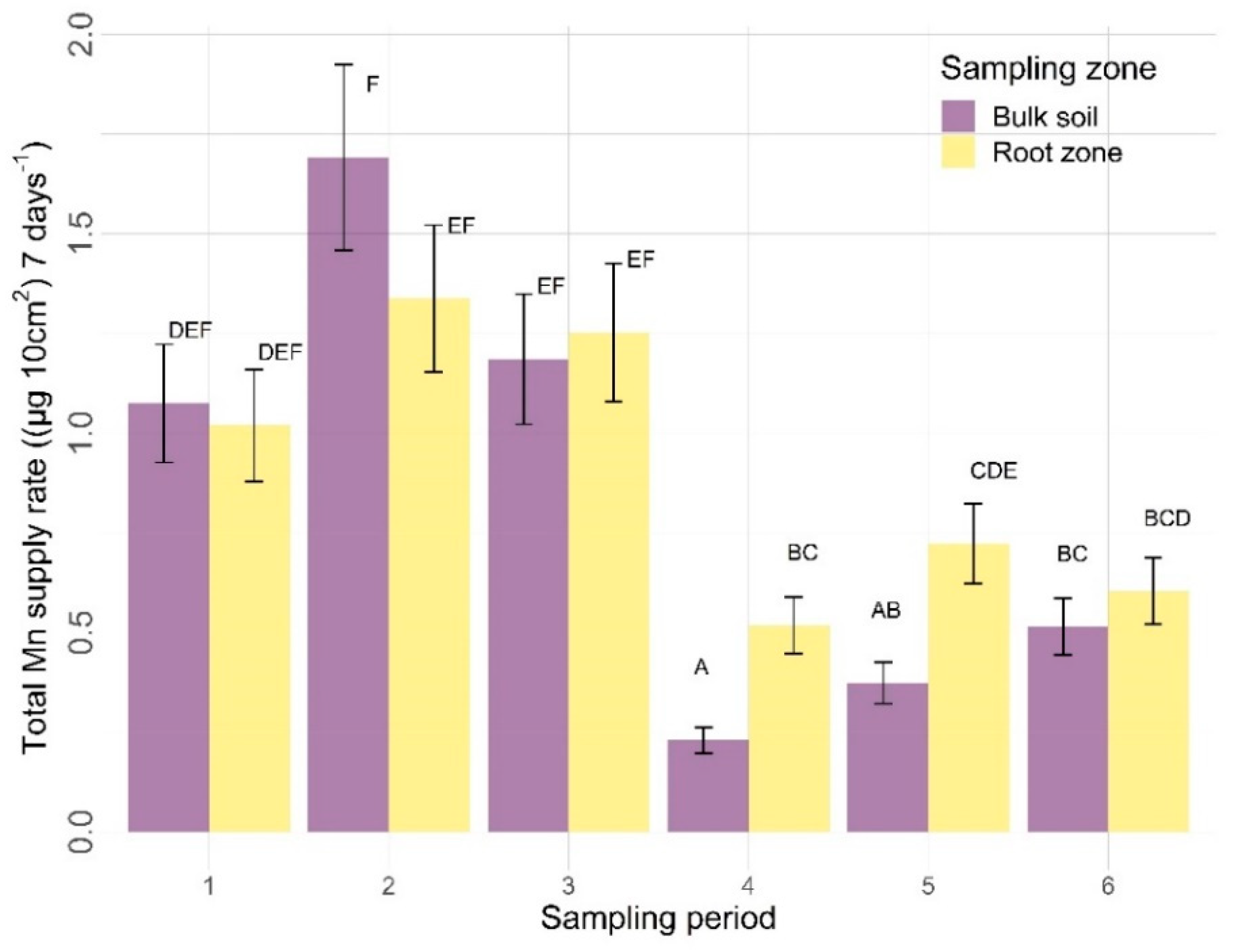
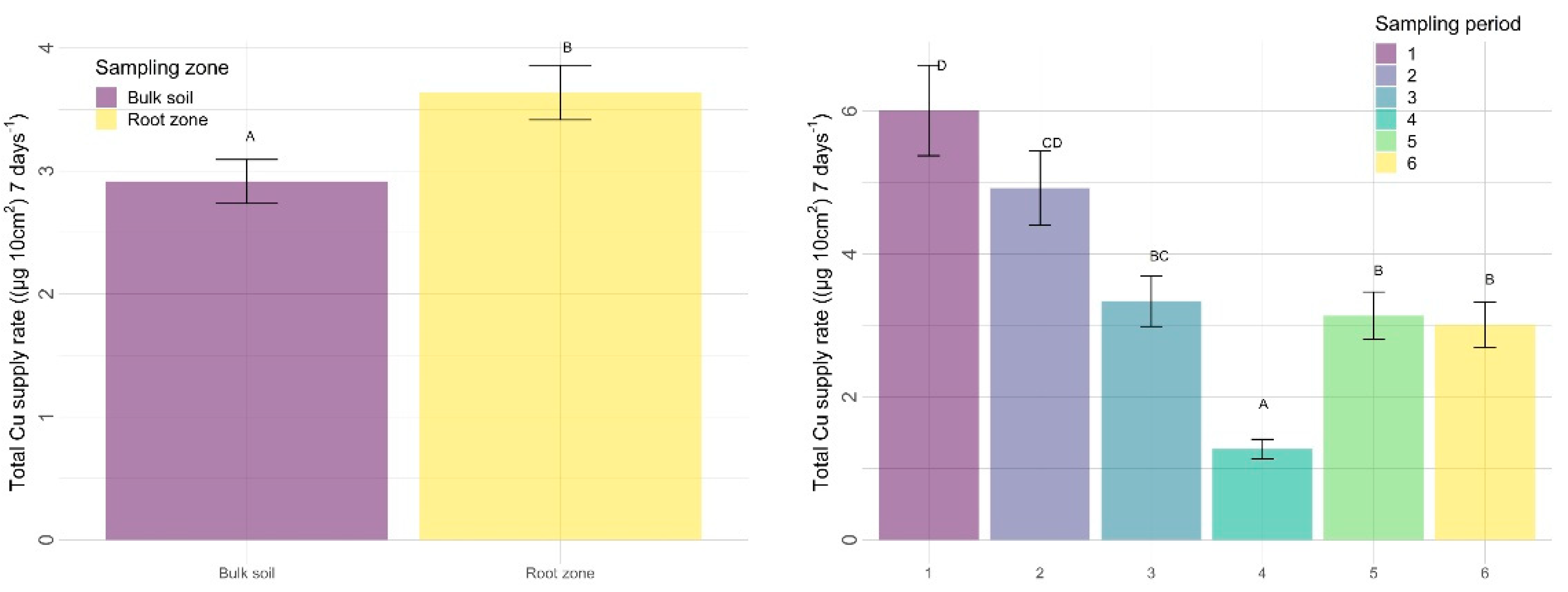
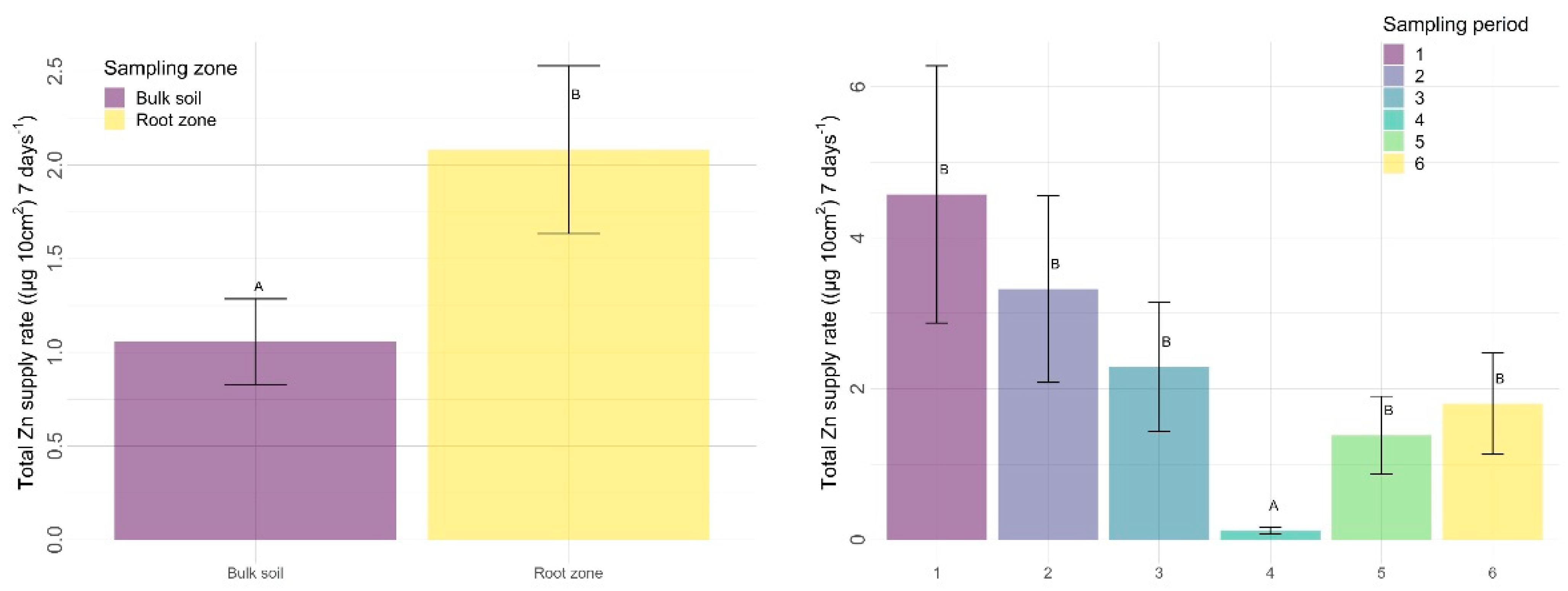
3.2.2. Other Nutrients
3.3. Soil Samples following Lettuce Harvest
4. Discussion
4.1. Nitrogen
4.2. Other Nutrients
4.2.1. Amendments
4.2.2. Sampling Zone
4.2.3. Time
4.3. Retrospective on the Initial Hypotheses
5. Overall Perspective: Found and Missing Pieces of the Proposed Conservation Strategy Puzzle
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bonn, A.; Allott, T.; Evans, M.; Joosten, H.; Stoneman, R. Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Verhoeven, J.T.; Setter, T.L. Agricultural use of wetlands: Opportunities and limitations. Ann. Bot. 2010, 105, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilnicki, P.; Zeitz, J. Organic Soils and Peat Materials for Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2002; pp. 15–32. [Google Scholar]
- Elder, J.W.; Lal, R. Tillage effects on gaseous emissions from an intensively farmed organic soil in North Central Ohio. Soil Tillage Res. 2008, 98, 45–55. [Google Scholar] [CrossRef]
- Kroetsch, D.J.; Geng, X.; Chang, S.X.; Saurette, D.D. Organic soils of Canada: Part 1. Wetland organic soils. Can. J. Soil Sci. 2011, 91, 807–822. [Google Scholar] [CrossRef]
- Kløve, B.; Berglund, K.; Berglund, Ö.; Weldon, S.; Maljanen, M. Future options for cultivated Nordic peat soils: Can land management and rewetting control greenhouse gas emissions? Environ. Sci. Policy 2017, 69, 85–93. [Google Scholar] [CrossRef]
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands: Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use; Food and Agriculture Organization of the United Nations Rome: Rome, Italy, 2012. [Google Scholar]
- Ferré, M.; Muller, A.; Leifeld, J.; Bader, C.; Müller, M.; Engel, S.; Wichmann, S. Sustainable management of cultivated peatlands in Switzerland: Insights, challenges, and opportunities. Land Use Policy 2019, 87, 104019. [Google Scholar] [CrossRef]
- Grootjans, A.P. Paludiculture—Productive Use of Wet Peatlands. Restor. Ecol. 2017, 25, 661–663. [Google Scholar] [CrossRef]
- Joosten, H.; Gaudig, G.; Krawczynski, R.; Tanneberger, F.; Wichmann, S.; Wichtmann, W. 25 Managing Soil Carbon in Europe: Paludicultures as a New Perspective for Peatlands. Soil Carbon Sci. Manag. Policy Mult. Benefits 2014, 71, 297. [Google Scholar]
- Bourdon, K.; Fortin, J.; Dessureault-Rompré, J.; Caron, J. Agricultural peatlands conservation: How does the addition of plant biomass and copper affect soil fertility? Soil Sci. Soc. Am. J. 2021, 85, 1242–1255. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Libbrecht, C.; Caron, J. Biomass crops as a soil amendment in cultivated histosols: Can we reach carbon equilibrium? Soil Sci. Soc. Am. J. 2020, 84, 597–608. [Google Scholar] [CrossRef]
- Parent, L.; Millette, J.; Mehuys, G. Subsidence and erosion of a Histosol. Soil Sci. Soc. Am. J. 1982, 46, 404–408. [Google Scholar] [CrossRef]
- Regina, K.; Sheehy, J.; Myllys, M. Mitigating greenhouse gas fluxes from cultivated organic soils with raised water table. Mitig. Adapt. Strateg. Glob. Change 2015, 20, 1529–1544. [Google Scholar] [CrossRef]
- Lamontagne, L.; Martin, A.; Nolin, M.C. Étude Pédologique du Comté de Napierville (Québec); Laboratoires de pédologie et d'agriculture de précision, Centre de recherche de St-Jean sur le Richelieu, Agriculture et Agroalimentaire Canada: Québec, QC, Canada, 2014. [Google Scholar]
- MAPAQ. Portrait-Diagnostic Sectoriel des Légumes Frais au Québec. 2018. Available online: https://www.mapaq.gouv.qc.ca/fr/Publications/Portraitsectoriellegumesfrais.pdf (accessed on 20 September 2021).
- Rodriguez, A.F.; Gerber, S.; Daroub, S. Modeling soil subsidence in a subtropical drained peatland. The case of the everglades agricultural Area. Ecol. Model. 2019, 415, 108859. [Google Scholar] [CrossRef]
- Grégoire, V. L’apport de Biomasse Végétale et L’amélioration des Propriétés Hydrauliques des sols Organiques Cultivés. Thèse de Maîtrise, Université Laval, Québec, QC, Canada, 2020. [Google Scholar]
- Bourdon, K.; Fortin, J.; Dessureault-Rompré, J.; Caron, J. Can We Slow Down Cultivated Peatland Respiration without Changing Land Use? In Proceedings of the ASA, CSSA and SSSA International Annual Meetings, online, 9–13 November 2020. [Google Scholar]
- Marmier, V. Mineralization of Nitrogen and Release of Dissolved Phosphorus and Carbon in Plant-Amended Organic Soils; Department of Environmental Sciences, ETH Zürich: Zürich, Switzerland, 2021. [Google Scholar]
- Amichev, B.Y.; Hangs, R.D.; Konecsni, S.M.; Stadnyk, C.N.; Volk, T.A.; Bélanger, N.; Vujanovic, V.; Schoenau, J.J.; Moukoumi, J.; Van Rees, K.C. Willow Short-Rotation Production Systems in Canada and Northern United States: A Review. Soil Sci. Soc. Am. J. 2014, 78, S168–S182. [Google Scholar] [CrossRef] [Green Version]
- Erickson, L.E.; Pidlisnyuk, V. (Eds.) Phytotechnology with Biomass Production: Sustainable Management of Contaminated Sites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Klasa, A. Willow biomass production under conditions of low-input agriculture on marginal soils. For. Ecol. Manag. 2011, 262, 1558–1566. [Google Scholar] [CrossRef]
- Emmerling, C.; Pude, R. Introducing Miscanthus to the greening measures of the EU Common Agricultural Policy. Gcb Bioenergy 2017, 9, 274–279. [Google Scholar] [CrossRef]
- Fairchild, D. Ground Water Quality and Agricultural Practices; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Erickson, M. Horticulture production, food safety and organic amendments: Effect on public health. Acta Hortic. 2015, 1076, 201–214. [Google Scholar] [CrossRef]
- Strack, M. Peatlands and Climate Change; IPS, International Peat Society Jyväskylä: Jyväskylä, Finland, 2008; pp. 44–69. [Google Scholar]
- Staff, S.S. Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. Agric. Handb. 1999, 436, 886. [Google Scholar]
- Miller, J.J.; Bremer, E.; Curtis, T. Influence of Organic Amendment and Compaction on Nutrient Dynamics in a Saturated Saline-Sodic Soil from the Riparian Zone. J. Environ. Qual. 2016, 45, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Maynard, D.G. Nitrogen mineralization assessment using PRS™ probes (ion-exchange membranes) and soil extractions in fertilized and unfertilized pine and spruce soils. Can. J. Soil Sci. 2014, 94, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Drohan, P.J.; Merkler, D.J.; Buck, B.J. Suitability of the Plant Root Simulator Probe for Use in the Mojave Desert. Soil Sci. Soc. Am. J. 2005, 69, 1482–1491. [Google Scholar] [CrossRef]
- Qualls, R. Determination of Total Nitrogen and Phosphorus in Water Using Persulfate Oxidation: A Modification for Small Sample Volumes Using the Method of Koroleff (1983). The Biogeochemical Properties of the Retention of Nitrogen, Phosphorus, and Carbon. Ph.D. Thesis, University of Georgia Institute of Ecology, Athens, GA, USA, 1989. [Google Scholar]
- Centre d'Expertise en Analyse Environnementale du Québec. Détermination des métaux et du phosphore assimilables: Méthode par spectrométrie de masse à source ionisante au plasma d’argon. In MA. 200–Mét-Pass. 1.0, Centre d’Expertise en Analyse Environnementale du Québec; Ministère de L’environnement et de la Lutte aux Changements Climatique: Quebec, QC, Canada, 2014; 15p. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Gloutney, A.; Caron, J. Nutrient Availability under Lettuce Grown in Rye Mulch in Histosols. Nitrogen 2020, 1, 12. [Google Scholar] [CrossRef]
- Chopra, M.; Koul, B. Comparative assessment of different types of mulching in various crops: A review. Plant Arch. 2020, 20, 1620–1626. [Google Scholar]
- Kumar, V.; Kumar, M.; Rai, A.P. Mulching in fruit and vegetable crop production under rainfed conditions: A review. J. Soil Water Conserv. 2016, 15, 313. [Google Scholar] [CrossRef]
- Xia, L.; Wang, S.; Yan, X. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice–wheat cropping system in China. Agric. Ecosyst. Environ. 2014, 197, 118–127. [Google Scholar] [CrossRef]
- Song, K.; Yang, J.; Xue, Y.; Lv, W.; Zheng, X.; Pan, J. Influence of tillage practices and straw incorporation on soil aggregates, organic carbon, and crop yields in a rice-wheat rotation system. Sci. Rep. 2016, 6, 36602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, G.; Wang, H.; Lu, D.; Chen, X.; Zhou, J. Effects of Full Straw Incorporation on Soil Fertility and Crop Yield in Rice-Wheat Rotation for Silty Clay Loamy Cropland. Agronomy 2019, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Yu, W.; Ma, Q.; Xu, Y.; Zou, H. Alleviating global warming potential by soil carbon sequestration: A multi-level straw incorporation experiment from a maize cropping system in Northeast China. Soil Tillage Res. 2017, 170, 77–84. [Google Scholar] [CrossRef]
- Zheng, J.; Qu, Y.; Kilasara, M.M.; Mmari, W.N.; Funakawa, S. Nitrate leaching from the critical root zone of maize in two tropical highlands of Tanzania: Effects of fertilizer-nitrogen rate and straw incorporation. Soil Tillage Res. 2019, 194, 104295. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Liang, L.; Zhao, Y.; Yang, B.; Ding, R.; Wang, J.; Nie, J. Changes in soil characteristics and maize yield under straw returning system in dryland farming. Field Crop. Res. 2018, 218, 11–17. [Google Scholar] [CrossRef]
- Garnier, P.; Néel, C.; Aita, C.; Recous, S.; Lafolie, F.; Mary, B. Modelling carbon and nitrogen dynamics in a bare soil with and without straw incorporation. Eur. J. Soil Sci. 2003, 54, 555–568. [Google Scholar] [CrossRef]
- Reichel, R.; Wei, J.; Islam, M.S.; Schmid, C.; Wissel, H.; Schröder, P.; Brüggemann, N. Potential of wheat straw, spruce sawdust, and lignin as high organic carbon soil amendments to improve agricultural nitrogen retention capacity: An incubation study. Front. Plant Sci. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Said-Pullicino, D.; Cucu, M.A.; Sodano, M.; Birk, J.J.; Glaser, B.; Celi, L. Nitrogen immobilization in paddy soils as affected by redox conditions and rice straw incorporation. Geoderma 2014, 228–229, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Fei, C.; Zhang, S.; Wei, W.; Liang, B.; Li, J.; Ding, X. Straw and optimized nitrogen fertilizer decreases phosphorus leaching risks in a long-term greenhouse soil. J. Soils Sediments 2019, 20, 1199–1207. [Google Scholar] [CrossRef]
- Yan, C.; Yan, S.; Hou, Z.; Dong, S.; Gong, Z.; Zhang, Z. Phosphorus adsorption characteristics of soil with rice straw retention in northeast china. Ekoloji 2019, 28, 1671–1678. [Google Scholar]
- Tian, G.; Kang, B.; Brussaard, L. Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions—decomposition and nutrient release. Soil Biol. Biochem. 1992, 24, 1051–1060. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Christie, P. Nitrogen and phosphorus leaching losses from intensively managed paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, Y.; Ladha, J.; Singh, B.; Singh, J.; Singh, G.; Pathak, H. Yield and Phosphorus Transformations in a Rice-Wheat System with Crop Residue and Phosphorus Management. Soil Sci. Soc. Am. J. 2007, 71, 1500–1507. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Zhang, H.; Tian, G. The influence of no-till coupled with straw return on soil phosphorus speciation in a two-year rice-fallow practice. Soil Tillage Res. 2019, 195, 104389. [Google Scholar] [CrossRef]
- Guérin, J.; Parent, L.; Abdelhafid, R. Agri-environmental Thresholds using Mehlich III Soil Phosphorus Saturation Index for Vegetables in Histosols. J. Environ. Qual. 2007, 36, 975–982. [Google Scholar] [CrossRef]
- Eriksen, J. Gross sulphur mineralisation–immobilisation turnover in soil amended with plant residues. Soil Biol. Biochem. 2005, 37, 2216–2224. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Neumann, G.; Bott, S.; Ohler, M.; Mock, H.-P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Astolfi, S.; Lehto, N.; Robinson, B.; Brunetto, G.; Terzano, R.; Cesco, S. Nutrient availability in the rhizosphere: A review. Acta Hortic. 2018, 1217, 13–28. [Google Scholar] [CrossRef]



| Characteristics | Miscanthus | Willow |
|---|---|---|
| C (%) | 45.3 | 46.4 |
| N (%) | 0.198 | 0.343 |
| P (%) | 0.048 | 0.031 |
| C/N | 228 | 135 |
| C/P | 242 | 381 |
| Hemicellulose (%) | 29.8 | 10.9 |
| Cellulose (%) | 23.0 | 32.2 |
| Lignin (%) | 34.8 | 35.5 |
| Lignin/N | 176 | 103 |
| Burial Date | Retrieval Date | Period |
|---|---|---|
| 2019-07-10 | 2019-07-17 | 1 |
| 2019-07-17 | 2019-07-24 | 2 |
| 2019-07-24 | 2019-07-31 | 3 |
| 2019-07-31 | 2019-08-07 | 4 |
| 2019-08-07 | 2019-08-14 | 5 |
| 2019-08-14 | 2019-08-19 | 6 |
| Amendments | Sampling Zone | Time | Amendment X Sampling Zone | Amendment X Time | Sampling Zone X Time | |
|---|---|---|---|---|---|---|
| Lettuce yield | 0.175 | NA | NA | NA | NA | NA |
| N | <0.0001 | 0.0012 | <0.0001 | 0.0134 | 0.9938 | 0.5338 |
| P | 0.0027 | 0.4923 | 0.0001 | 0.2515 | 0.4876 | 0.4075 |
| K | 0.4556 | 0.8435 | <0.0001 | 0.1581 | 0.9557 | 0.5026 |
| Ca | 0.6404 | <0.0001 | <0.0001 | 0.3570 | 0.0148 | 0.0489 |
| Mg | 0.0906 | <0.0001 | <0.0001 | 0.8260 | 0.0165 | 0.0058 |
| S | 0.0005 | 0.0412 | <0.0001 | 0.3465 | 0.7285 | 0.0173 |
| Fe | 0.4555 | 0.0002 | <0.0001 | 0.1651 | 0.8025 | <0.0001 |
| Mn | 0.3166 | 0.0039 | <0.0001 | 0.0541 | 0.8456 | 0.0008 |
| Cu | 0.3731 | 0.0110 | <0.0001 | 0.1131 | 0.9251 | 0.1421 |
| Zn | 0.9325 | 0.0275 | <0.0001 | 0.2657 | 1.0000 | 0.1650 |
| Control | Miscanthus | Willow | p-Values | |
|---|---|---|---|---|
| Mineral N | 60.4 ± (7.5) | 58.1 ± (10.0) | 62.4 ± (6.2) | 0.933 |
| Soluble organic N | 70.1± (9.6) | 21.0± (3.2) | 30.1± (6.2) | 0.005 |
| Active C | 10 604.0 ± (172.0) | 10 779.0 ± (189.4) | 12 430.7 ± (1487.2) | 0.331 |
| C | 45.9 ± (0.4) | 46.5 ± (0.2) | 46.6 ± (0.4) | 0.331 |
| N | 1.9 ± (0.0) | 2.0 ± (0.0) | 2.0 ± (0.0) | 0.617 |
| C/N | 23.7 ± (0.1) | 23.9± (0.1) | 23.5 ± (0.2) | 0.164 |
| P | 314.7 ± (39.3) | 219.7 ± (25.3) | 201.7 ± (35.5) | 0.110 |
| K | 1 91.2 ± (66.4) | 324.3 ± (19.4) | 328.0 ± (33.3) | 0.120 |
| Ca | 14 033.3 ± (550.7) | 13 891.0 ± (497.6) | 13 886.7 ± (456.6) | 0.970 |
| Mg | 1 107.3 ± (34.9) | 1 135.0 ± (62.5) | 1 138.3 ± (15.1) | 0.970 |
| Fe | 900.0 ± (40.3) | 754.7 ± (9.9) | 831.7 ± (38.9) | 0.055 |
| Mn | 16.3 ± (1.6) | 17.4 ± (0.8) | 21.5 ± (1.9) | 0.106 |
| Cu | 26.8 ± (3.4) | 26.6 ± (1.7) | 37.8 ± (3.2) | 0.055 |
| Zn | 22.2 ± (3.3) | 27.3 ± (2.9) | 34.6 ± (3.9) | 0.105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessureault-Rompré, J.; Gloutney, A.; Caron, J. Nutrient Availability for Lactuca sativa Cultivated in an Amended Peatland: An Ionic Exchange Study. Nitrogen 2022, 3, 26-42. https://doi.org/10.3390/nitrogen3010002
Dessureault-Rompré J, Gloutney A, Caron J. Nutrient Availability for Lactuca sativa Cultivated in an Amended Peatland: An Ionic Exchange Study. Nitrogen. 2022; 3(1):26-42. https://doi.org/10.3390/nitrogen3010002
Chicago/Turabian StyleDessureault-Rompré, Jacynthe, Alexis Gloutney, and Jean Caron. 2022. "Nutrient Availability for Lactuca sativa Cultivated in an Amended Peatland: An Ionic Exchange Study" Nitrogen 3, no. 1: 26-42. https://doi.org/10.3390/nitrogen3010002
APA StyleDessureault-Rompré, J., Gloutney, A., & Caron, J. (2022). Nutrient Availability for Lactuca sativa Cultivated in an Amended Peatland: An Ionic Exchange Study. Nitrogen, 3(1), 26-42. https://doi.org/10.3390/nitrogen3010002






