1. Introduction
In the past few decades, fractional-order generalizations of various classical integer-order models have been extensively studied [
1,
2,
3]. In many cases, they enable the achievement of better quantitative agreement with experimental data. The non-local character of fractional derivatives over time makes their use appropriate in the modeling of phenomena, where some hereditary mechanisms are present. In such cases, the physical meaning of the order of the fractional derivative is an index of memory [
4].
Regarding the diffusion transport, numerous experimental measurements have reported that the mean squared displacement scales over time as a fractional power law. This deviation from the Markovian description cannot be properly described by the classical diffusion models. Different types of partial differential and integro-differential equations (see, e.g., [
5,
6]) are used to model such anomalous diffusion processes. In particular, the power law dependence of the mean squared displacement on time can be captured by employing time-fractional derivatives.
In general, anomalous diffusion occurs as a part of a more complex process, see for instance, [
7,
8,
9], where reaction–diffusion systems are studied, refs. [
10,
11,
12] for convection–diffusion models, or [
13] for convection-reaction–diffusion systems. Other examples include anomalous diffusion with mass absorption [
14], or the adsorption–desorption process at a boundary [
15,
16,
17,
18]. Additionally, the diffusion itself can have a complex character, e.g., different properties depending on space directions, as in comb-like models [
19]. Different types of fractional derivative operators are used to describe anomalous diffusion [
20]. All of the above studies consider fractional generalizations of the classical integer-order models. In the case of complex multi-component systems, the question of the proper “fractionalization” of a classical model is essential and should be performed in such a way that the fundamental conservation and constitutive laws are satisfied. In this respect, as is mentioned in [
18], the formulation of boundary conditions is important for the proper description of particle transport to and from a bounding interface.
The surfactants play an essential role in a number of man-made and natural processes. When present on an interface, a surfactant reduces the interfacial tension and introduces important mechanical properties, such as surface elasticity. Adsorption of surfactant on an interface is no less important than the process of diffusion for the dynamics of the surfactant distribution, especially on the interface. The first theoretical result on the dynamics of surfactant interfacial layers is the work of Ward and Tordai (1946) [
21]. The Ward–Tordai integral equation is still a basis for most of the theoretical studies that describe the time dependence of the interfacial properties [
22]. Fifteen years later, using the Laplace transform, Hansen [
23] derives the solution of the same problem as an integro-differential equation. Recently, Hristov [
24] pointed out that the Ward–Tordai and Hansen equations are, in fact, fractional integral and differential equations, respectively, of order
. Thus, the famous Ward–Tordai integral equation and the Hansen integro-differential equation, governing the same problem of the diffusion-controlled adsorption of surfactants at an air–liquid interface, are remarkable examples of a fractional-order equation derived from a classical integer-order model.
The present work extends our previous study [
17] on the modeling of surfactant diffusion and the corresponding process of adsorption–desorption by employing fractional derivatives over time. Starting from basic physical concepts (conservation and constitutive laws), a mathematical model is derived, and the behavior of the corresponding solutions is analyzed with the help of computer simulations.
This paper is organized as follows. In
Section 2, the time-fractional modeling of anomalous diffusion is discussed in the case when it is a part of a more complex process, regarding mass conservation. In
Section 3, a time-fractional model of the anomalous mass-transfer of surfactant, soluble in both phases, is derived in the case when the adsorption–desorption process is controlled by diffusion.
Section 4 is devoted to the linear case of the Henry adsorption isotherm and contains a detailed analysis, which is mainly based on the theory of multinomial Mittag–Leffler functions. In
Section 5, a numerical approach is proposed for the general nonlinear case, and some numerical results are presented. A discussion and conclusions can be found in
Section 6.
2. Time-Fractional Diffusion–Adsorption Model
In the present section, we assess a generalization of the classical diffusion equation and the related boundary conditions of adsorption–desorption to anomalous ones. The assessment is based on basic physical principles and laws. The classical model of the mass transfer of species of concentration
C in a fluid medium consists of two parts. The first part is the continuity equation, which, in the case of a concentration problem, guarantees mass conservation. It is written below in a general form, including diffusion, convection, and reaction terms:
where ∇ denotes the gradient vector,
and
are the diffusive and convective fluxes, respectively, and
takes into account the change of concentration due to source or reaction (e.g., chemical or biological).
The second part of the model consists of constitutive equations that describe the different processes under consideration. In the case of the process of diffusion, the constitutive equation for the diffusive flux
is given by Fick’s first law:
where
d is the diffusion coefficient. As a part of the diffusive flux, drift due to an external force can be considered (see, e.g., [
15,
25,
26]). Such is also the electrodiffusion flux in the case of ionic surfactant subjected to an external electromagnetic field (see [
27]).
Convection can be introduced into the model in different ways, for instance, via the convective flux:
Here,
is the fluid velocity, which is external to the diffusion equation.
The first question regarding time fractional diffusion models is how to introduce the fractional operators. In general, there are two main approaches. In the first approach, a fractional derivative
replaces the first time derivative in the left-hand side of the continuity Equation (
1), which implies
Most studies of time-fractional diffusion use this approach, see e.g., [
8,
10,
11,
12,
13,
14,
16,
28]. There are, however, some issues that arise when using this approach. The first is in the case when more than one different processes are considered. Indeed, then (
4) leads to the illogical conclusion that all subprocesses described by the terms in the right-hand side have a fractional evolution of one and the same order,
. Another issue is related to the fact that the continuity equation represents the mass conservation of
C, and a change of (
1) could lead to a violation of the mass conservation law. For instance, if only convective flux is present in the right-hand side of (
1) and the velocity
is not constant, mass conservation would be satisfied only if
is the first order derivative.
In the second approach, a fractional operator is used in the description of a given process (e.g., diffusion or reaction) via the corresponding constitutive equation (e.g., (
2) or g(C,
x,t)). In the case of anomalous diffusion, this is:
see e.g., [
7,
9,
19,
26,
29]. It is worth mentioning that the issues discussed above regarding the first approach are not present here. That is why we use the model of anomalous diffusion via the diffusive flux (
5). Let us note that the two approaches are equivalent when only diffusion is considered, i.e.,
and
(see [
30]).
The second question is: What kind of fractional operator is appropriate for modeling adsorption–desorption at anomalous diffusion? In a mathematical model, all terms of a given equation or boundary condition must have the same physical dimension. In order to guarantee this, in the equation for the anomalous diffusive flux (
5), the dimension of the fractional diffusion coefficient
is chosen to correspond to the dimension of the fractional differential operator. Thus, a requirement on the fractional operator
is to have a well-defined physical dimension.
In the present study, as in [
15], fractional operators of the Riemann–Liouville type are used. The main argument for the choice of Riemann–Liouville operators is the fact that the solution of the classical adsorption–diffusion model is given in the Ward–Tordai equation by the Riemann–Liouville fractional integral of order
.
Recall that the Riemann–Liouville fractional integral
is defined by
The Riemann–Liouville fractional derivative
of order
is:
The operators
and
have dimensions [
] and [
], respectively. In other words, if the time is rescaled as
, then:
By proper choice of the characteristic time
T, the above rescaling is used to render the mathematical model dimensionless and to reduce the number of parameters. In [
20], the authors also consider some non-singular fractional operators for the modeling of anomalous diffusion. They, however, do not have a physical dimension. It is claimed in [
31] that the Caputo–Fabrizio fractional derivative of order
has dimension
. This fractional derivative, however, does not satisfy (
8).
Together with diffusion, adsorption also plays an important role in the process of surfactant transport in multiphase fluid systems. The adsorption process at an interface
is described by the boundary condition:
where
G is the surfactant concentration on the interface
S,
is the inward for
unit vector, which is normal to
S, and
is the flux in the bulk phase
. The boundary condition (
9) represents mass conservation of the surfactant in the vicinity of the interface
S. In the second approach adopted here, the flux
is given by Equation (
5) and the fractional derivative generalization of the classical diffusion–adsorption model reads:
Thus, the time-fractional derivatives in the diffusion equation and adsorption boundary condition are the same (see also [
18]). This means that the flux bulk-interface is the same as that in the continuous phase
, which guarantees mass conservation (see [
15]).
Relatedly, let us point out that the boundary condition on the interface in our previous work [
17] is incorrect.
3. Mathematical Formulation
In this section, the mathematical model of the diffusion-controlled adsorption of surfactant at the liquid–liquid interface is discussed in the one-dimensional case, assuming anomalous diffusion in both liquid phases. The model is based on Equations (
5) and (
10).
Consider a surfactant that is soluble in two liquid phases. The two phases are in contact at an interface. Such a system is, in general, not in equilibrium, and surfactant diffusion processes in the bulk phases and adsorption on the interface take place.
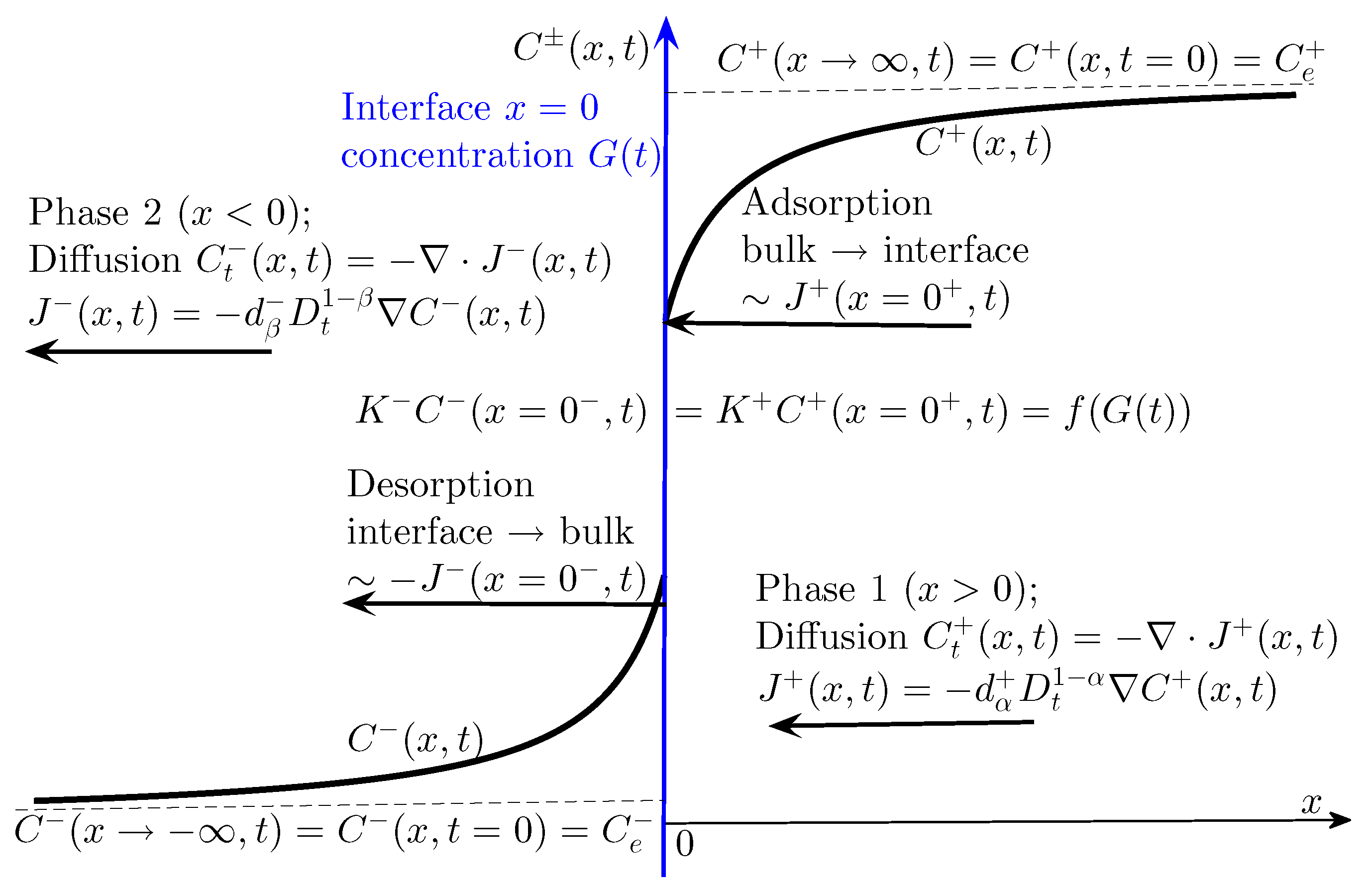
A schematic sketch of the problem for diffusion-controlled adsorption at the liquid–liquid interface is given in
Figure 1, where
and
denote the surfactant concentrations in Phase 1 (
) and Phase 2 (
), respectively;
is the concentration of surfactant on the interface, and
and
are adsorption constants.
The mathematical formulation of the problem for adsorption at the liquid–liquid interface controlled by anomalous diffusion contains two time-fractional equations for the anomalous diffusion of surfactant in the bulk phases:
where
and
are parameters of anomalous diffusion,
, and
,
are diffusion coefficients in the corresponding phases. The above diffusion equations are complemented with the initial conditions of uniform surfactant distribution at
:
where
are the input concentrations of surfactant in the bulk phases. Without loss of generality, we assume
. The boundary conditions are
where
are the subsurface surfactant concentrations. The following identities for the two subsurface surfactant concentrations are satisfied:
where the function
is the so-called adsorption isotherm. Different adsorption isotherms are considered in the literature [
27]. In the present study, three of the most popular models are used. The corresponding definitions of
are given in
Table 1, where the parameter
is the maximum possible surfactant concentration.
The change of the surfactant concentration
on the interface is compensated for by the diffusion fluxes from the bulk phases, resulting in the equation
with initial condition
, where
is the initial surfactant concentration on the interface.
The problem is treated by applying the Laplace transform with respect to time variable
t, where the following notations are used:
The Laplace transform pairs for fractional order integration and differentiation are
which can be derived from the identity
and the relation for the first order derivative
.
To find an equation for the surfactant concentration on the interface
, we first apply the Laplace transform with respect to variable
t to the diffusion Equations (
11) and (
12) by the use of relation (
17). Taking into account the initial conditions (
13), the following identities in the Laplace domain are derived:
These two equations in
x, with
s considered as a parameter, are equipped with boundary conditions for
, derived from conditions (
14):
. Their solutions are
Further, application of the Laplace transform to Equation (
16) yields
which, by the use of relations (
19) and (
20), implies
In order to obtain a fractional differential equation for
in a form convenient for applications, we rewrite (
21) as follows:
Then, the application of the inverse Laplace transform yields, by the use of (
17) and (
18),
where
denotes, as usual, the first derivative of
and
Taking into account identities (
15) for the subsurface surfactant concentrations
, we obtain the following multi-term fractional differential equation for
:
with initial condition
, where
is defined in (
22).
Let us note that the derived Equation (
23) is well defined for parameters
and
in a larger interval, namely
. In this way, we can cover different diffusive regimes, depending on the values of the fractional parameters
and
. Recall that for a fractional parameter
, the case
corresponds to subdiffusion,
cover the superdiffusive regime, and
corresponds to a ballistic diffusion process; see, e.g., [
30]. In the case when
or
in the initial Equations (
11), (
12) and (
16), then the fractional derivative
(resp.
) is replaced by a fractional integral
(resp.
). In view of the above argument, in this work, we consider the whole region
.
Let us point out that, in the case of classical diffusion
, Equation (
23) reduces to a fractional order equation of the form
. In this case, a classical integer-order problem leads to a problem of fractional order for
.
For the presentation of the results and their analysis transformation of the surfactant concentration
G and the time
t is performed next. It renders the governing Equation (
23) dimensionless and leads to a reduction of the parameters of the problem. The transformed variables
and
are, respectively,
In the above transformation,
T is the characteristic anomalous diffusion time and
is the equilibrium surfactant concentration, which is defined based on the parameters in Phase 1 from the equation
For any of the adsorption isotherms given in
Table 1, there is exactly one solution
satisfying (
26). In the present study,
is used as a characteristic surfactant concentration on the interface (note that
).
Using (
8), we apply the transformations (
24) and (
25) to the governing Equation (
23). For simplicity, we restrict our considerations to a practically more interesting case when the surfactant is initially present only in one of the phases (e.g., in Phase 1, i.e.,
). Then, Equation (
23) takes the following dimensionless form:
where the dimensionless group
B and the transformed function
are defined as:
Let us note that for all considered adsorption isotherms the terms
and
depend on one parameter and that is the transformed maximum possible surfactant concentration
. Thus, the parameters of the dimensionless governing Equation (
27) are four:
,
,
B, and
.
The Henry kinetic model plays an important role in the theoretical investigation of surfactant adsorption–desorption. It can serve as a base for a second-order approximation of the nonlinear models, which will be discussed later. That is why the following section is devoted to the derivation of an analytical solution in the case of the Henry adsorption isotherm and a theoretical analysis of its behavior.
5. Numerical Procedure and Results
To derive a dimensionless integral equation for surfactant concentration
on the interface, we apply integration to the dimensionless fractional differential Equation (
27). Taking into account the identity,
we rewrite Equation (
27) in the form
where
It is worth noting that in the case of classical diffusion in the bulk phases,
, the equation governing the surfactant concentration on the liquid–liquid interface, deduced from (
52), reads:
Equations (
52) and (
53) are generalizations of the classical Ward–Tordai equation [
35].
For the numerical solution of the integral Equation (
52), we use a modification of the fractional Adams method [
36,
37], which is based on a predictor–corrector numerical procedure.
A uniform mesh with time step h is used, and by the approximation for is denoted.
The predictor
is calculated by the formula
where
The corrector scheme is:
where
with
One of the challenges of simulating the adsorption–desorption process is the high computational cost, which is due to a combination of several factors. From the figures presented in the case of the Henry kinetic model, it is seen that the computations have to cover a broad time interval, sometimes of order
. At the same time, the high gradients of the solutions at the beginning require very fine time steps, of order
. This, in combination with the convolution type of the fractional operators, requires high computational resources. To overcome this problem in the numerical procedure, different meshes are used in different time regions. Starting at the beginning with a time step of order
, it progressively increases to reach values of order
at the end of the simulations, where the gradients of the solutions tend to zero. To verify the accuracy of the presented numerical procedure, a number of tests and comparisons with the analytical results obtained in the case of the Henry model are performed. Some of them are presented in
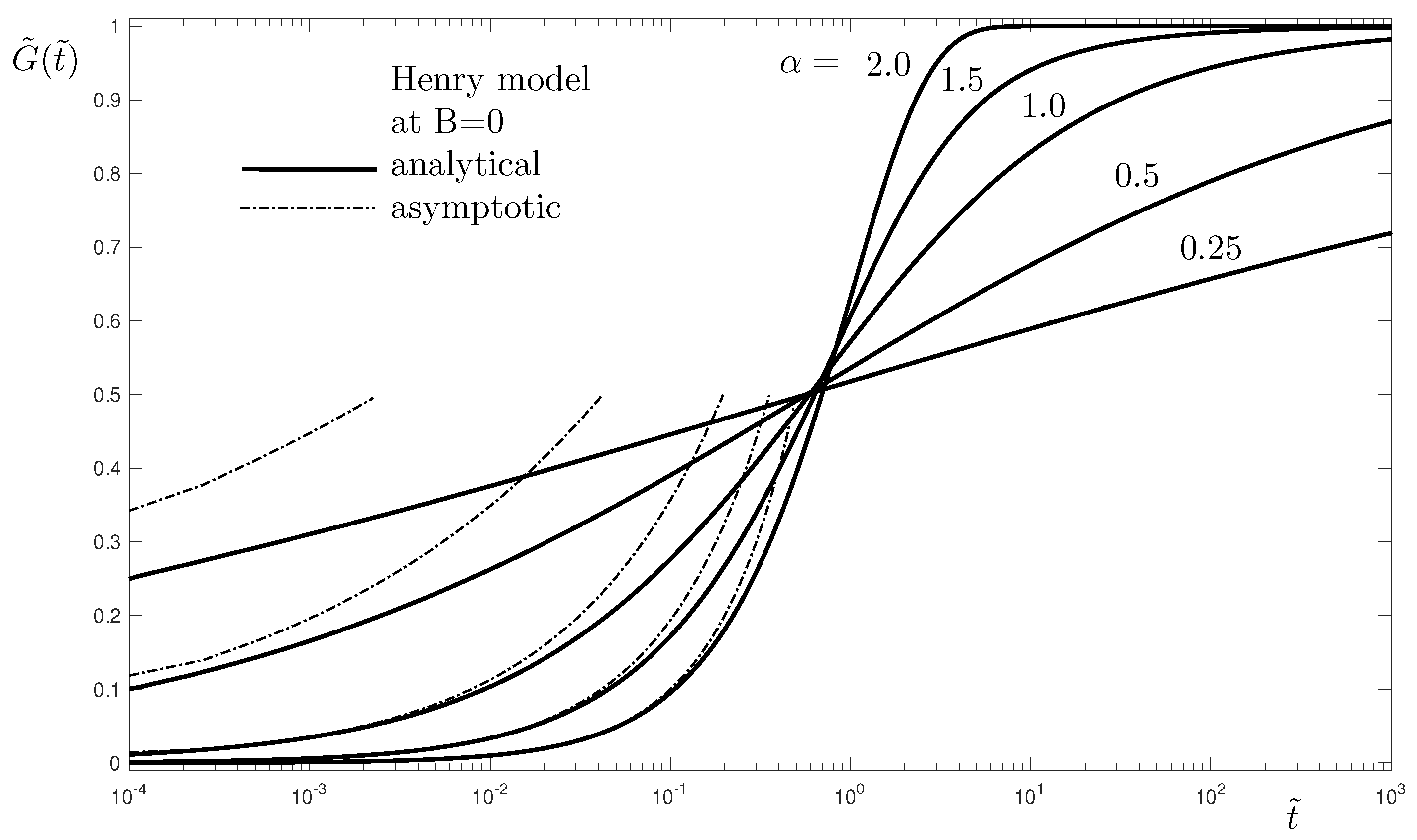
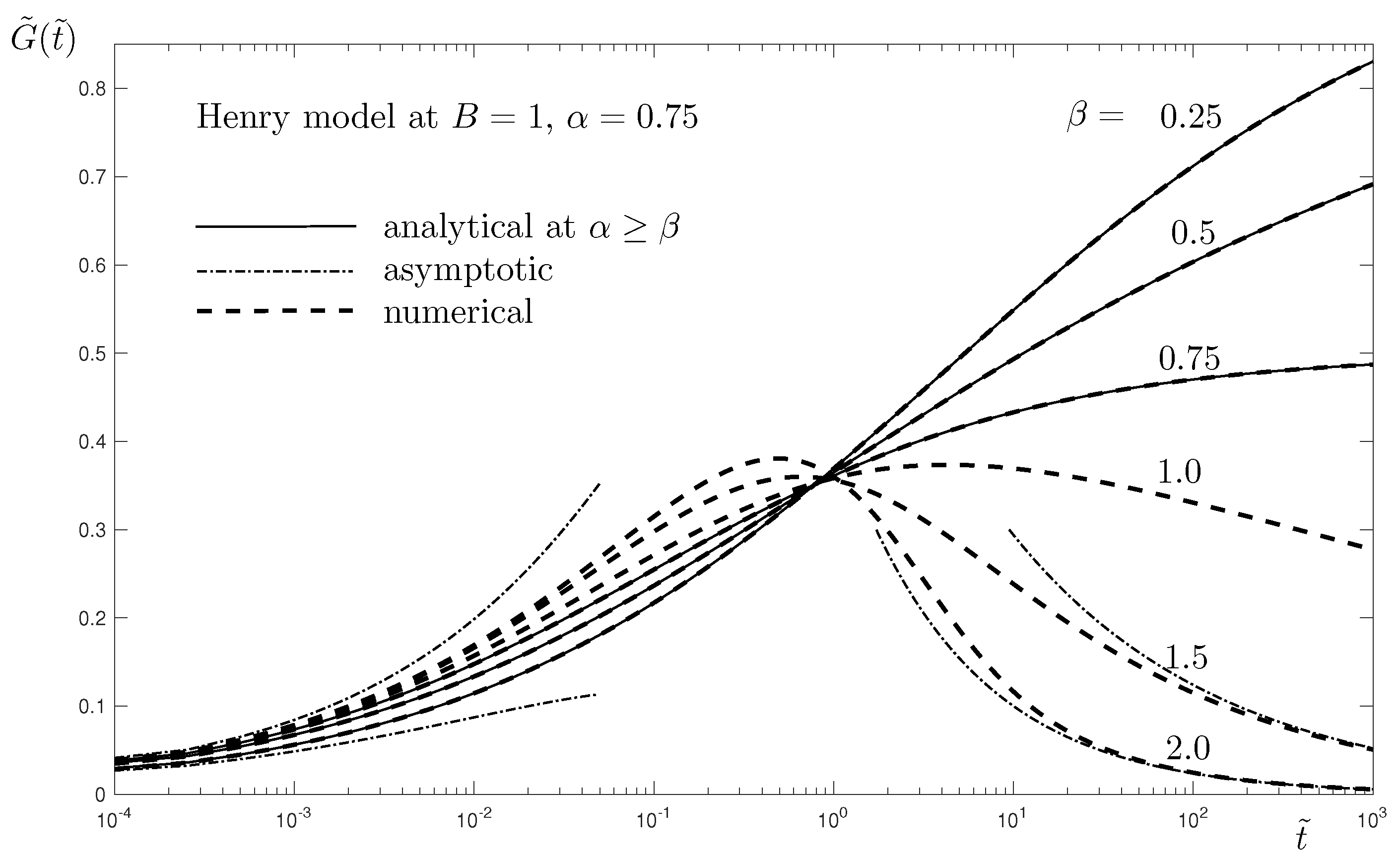
Figure 3, where very good agreement between the numerical and the analytical results is observed.
Until the end of this section, results are presented in the case of the nonlinear adsorption isotherms given in
Table 1.
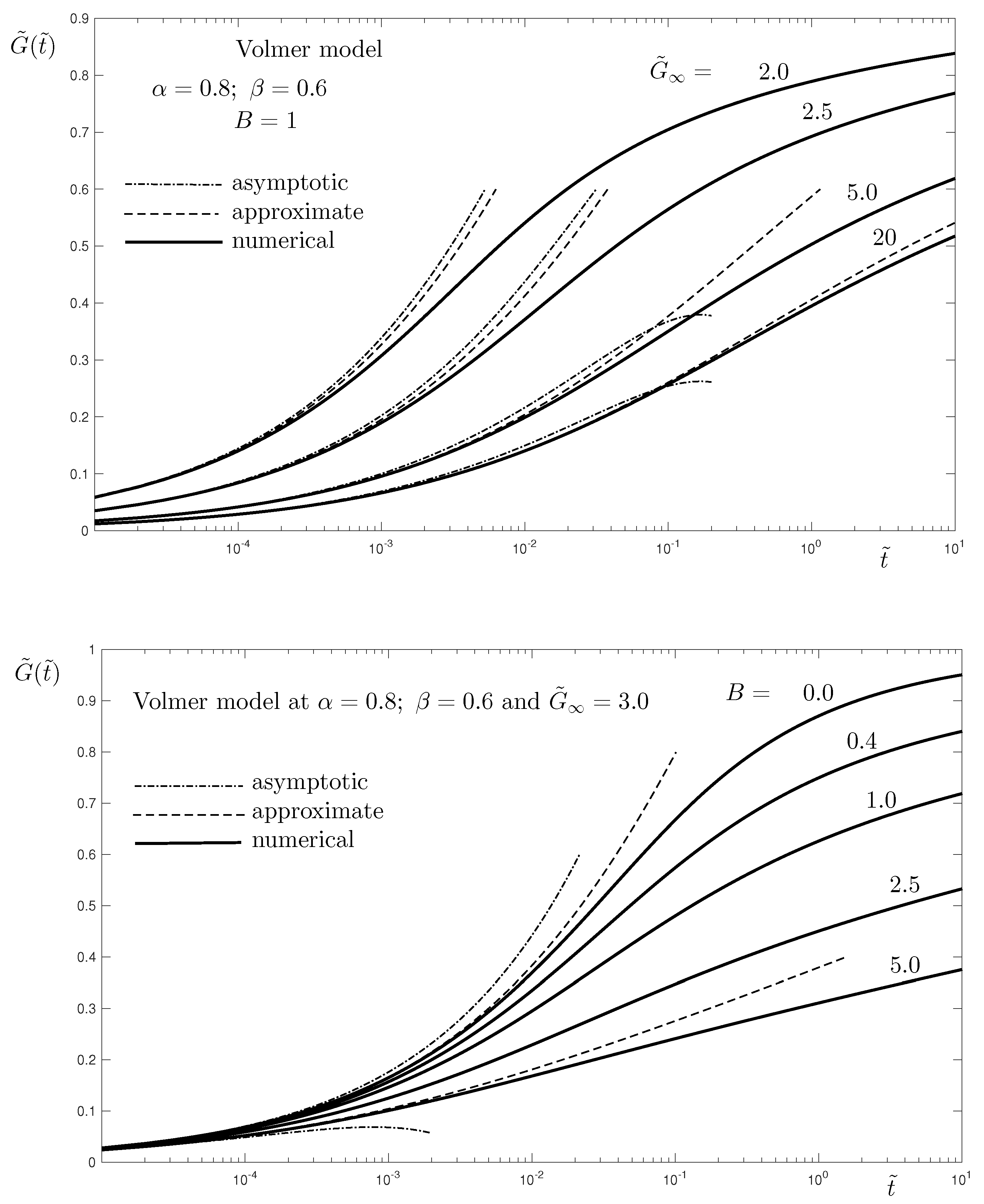
Figure 4 shows the influence of the parameters
(top) and
B (bottom) on the evolution of the surfactant concentration. The parameter
represents the ratio between the maximum possible surfactant concentration on the interface,
, and the equilibrium one,
. The latter is directly dependent on the surfactant concentration in the bulk
, see Equation (
26). Thus, an increase of
can be considered a decrease of the initially available surfactant in the fluids. This explains the slowdown of the adsorption and, respectively, the desorption, with the increase of
. The other parameter,
B, represents the relative influence of the desorption in the total adsorption–desorption process. This is seen from the governing Equation (
27), where the two fractional order terms correspond to the adsorption (
term) and the desorption (
term), respectively. Thus, a larger value of
B means faster desorption, which explains the results in
Figure 4 (bottom);
corresponds to the case without desorption, i.e., surfactant is soluble only in Phase 1. This is achieved by setting
, when the flux interface-Phase 2 (desorption) is absent,
.
Figure 4 shows that the asymptotes (
47) at small
, derived for the linear case of the Henry isotherm, are also applicable for the other models with nonlinear isotherms, see
Table 1. This is due to the fact that the Henry isotherm appears to be an approximation of the nonlinear isotherms. This explains why the asymptotes of the Henry model at
are also asymptotes for the other models. To investigate this further, let us consider the relation between the Henry isotherm and the other isotherms in their transformed forms
. Using Taylor expansion of
, it is easy to see that the adsorption isotherms considered here can be written as:
which means that the Henry adsorption isotherm
is a second-order approximation of the Volmer and Langmuir ones at small
.
Thus, we can approximate the nonlinear model (
27) replacing
with
and get:
The difference between (
57) and the governing equation in the case of the linear Henry model (
51) is in the coefficient
on the right-hand side. It is easy to see that the solution
of the approximating Equation (
57) can be presented via the solution of the Henry model:
where
denotes the solution of (
51), for which analytical solutions are derived in the previous section. Note that the extra parameter is
, and the error of the approximation (
56) is inversely proportional to
. That explains why the approximate solution is better at higher values of
, which is seen in
Figure 4 (top).
The last results presented here are similar to those given in the linear case, see
Figure 3. They are presented in
Figure 5, where the fractional order
in Phase 2 is varied at given values of the other parameters. As discussed regarding the results in the linear case, there is a transition from increasing surfactant concentration at
to more complex behavior at
. At
, the adsorption at smaller fractional order
is faster for small times
, see
Figure 2. At higher
, the desorption at higher fractional order
takes over, leading to a decrease of
. The asymptotic and approximate solutions are presented for
. A much more accurate prediction of the approximate solution (dashed line) is observed.
6. Discussion and Conclusions
A time-fractional mathematical model of anomalous diffusion in combination with surfactant adsorption–desorption on/from an interface is studied in the case when the surfactant is soluble in both phases. This model is considered one-dimensional with a flat interface between two semi-infinite phases. This infinite-phase approach adequately describes most physically important situations that involve adsorption dynamics, as discussed in [
35]. Since the length scale (thickness of the layer at the interface), where adsorption takes place, is much smaller than the typical size of the particles, the approximation of the flat interface is used. The approach presented in this study can be extended to the cases of finite phases and/or spherical interfaces, which are practically interesting.
Applying the Laplace transform technique, an ordinary multi-term fractional differential equation for the surfactant concentration on the interface is derived. The main contribution of the study is the fractional calculus approach to the problem. Using this approach, an analytical solution in the case of the linear adsorption isotherm is obtained, and asymptotes for small and large times are derived. It is shown that the asymptotes are also applicable in the cases of nonlinear adsorption isotherms.
By the proper rescaling of the concentration and time, the governing equation is transformed into a dimensionless one with a smaller number of parameters. A second-order approximation for a small surfactant concentration, which predicts the dynamics of the process at the beginning, is derived. Using the fractional calculus technique, an integral equation containing two fractional integrals is derived, and a predictor–corrector numerical procedure is developed for its numerical solution. It is used for numerical simulations and investigating the influence of the parameters on the adsorption–desorption process. Both sub- and super-diffusion regimes are covered, where the fractional orders are in the interval .
The developed fractional calculus approach could be used for further investigations of other practically interesting regimes of adsorption, for instance, mixed barrier diffusion control.