On Fractional Order Model of Tumor Growth with Cancer Stem Cell
Abstract
1. Introduction
2. Basic Definitions
3. The Fractional Model
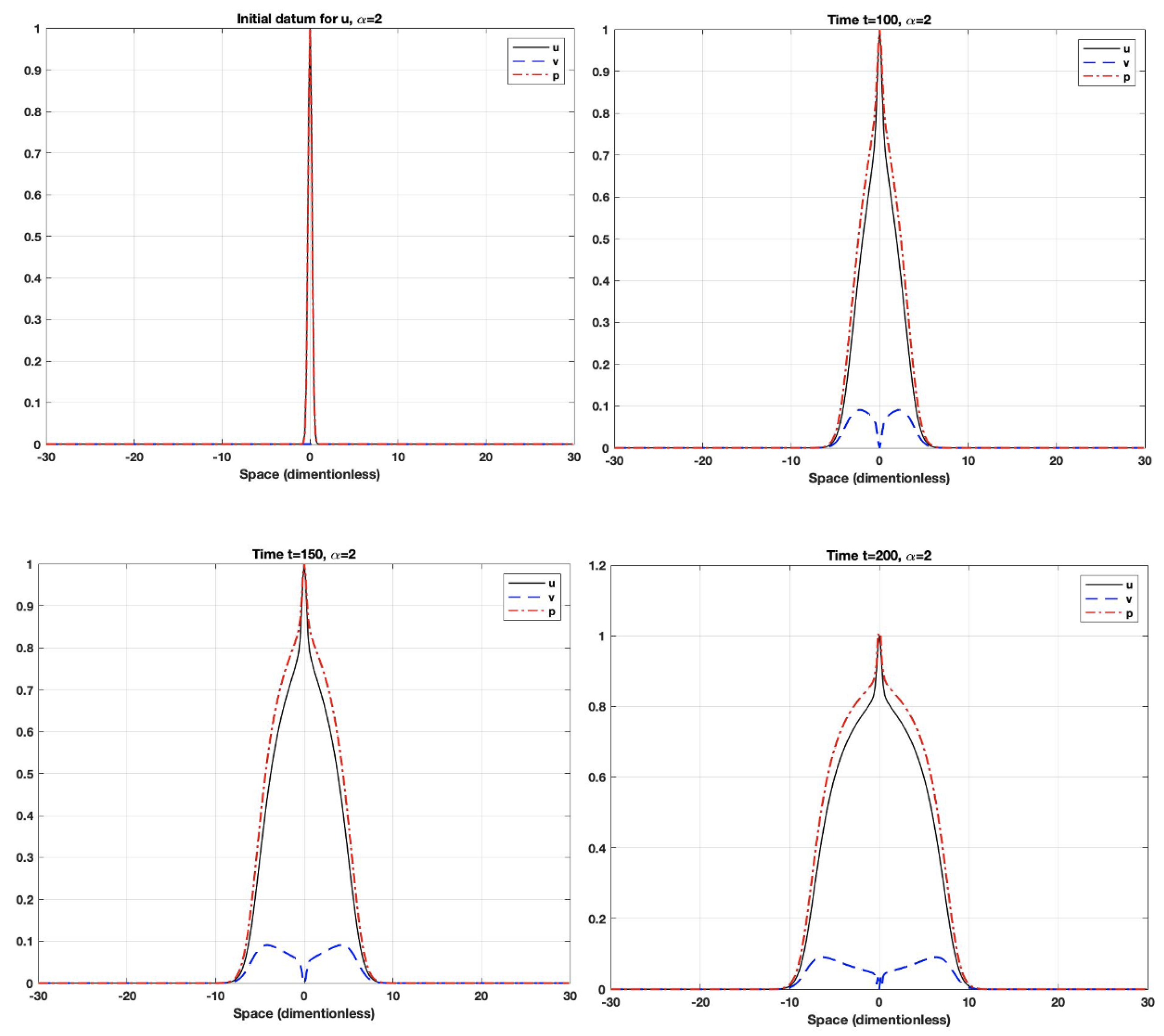
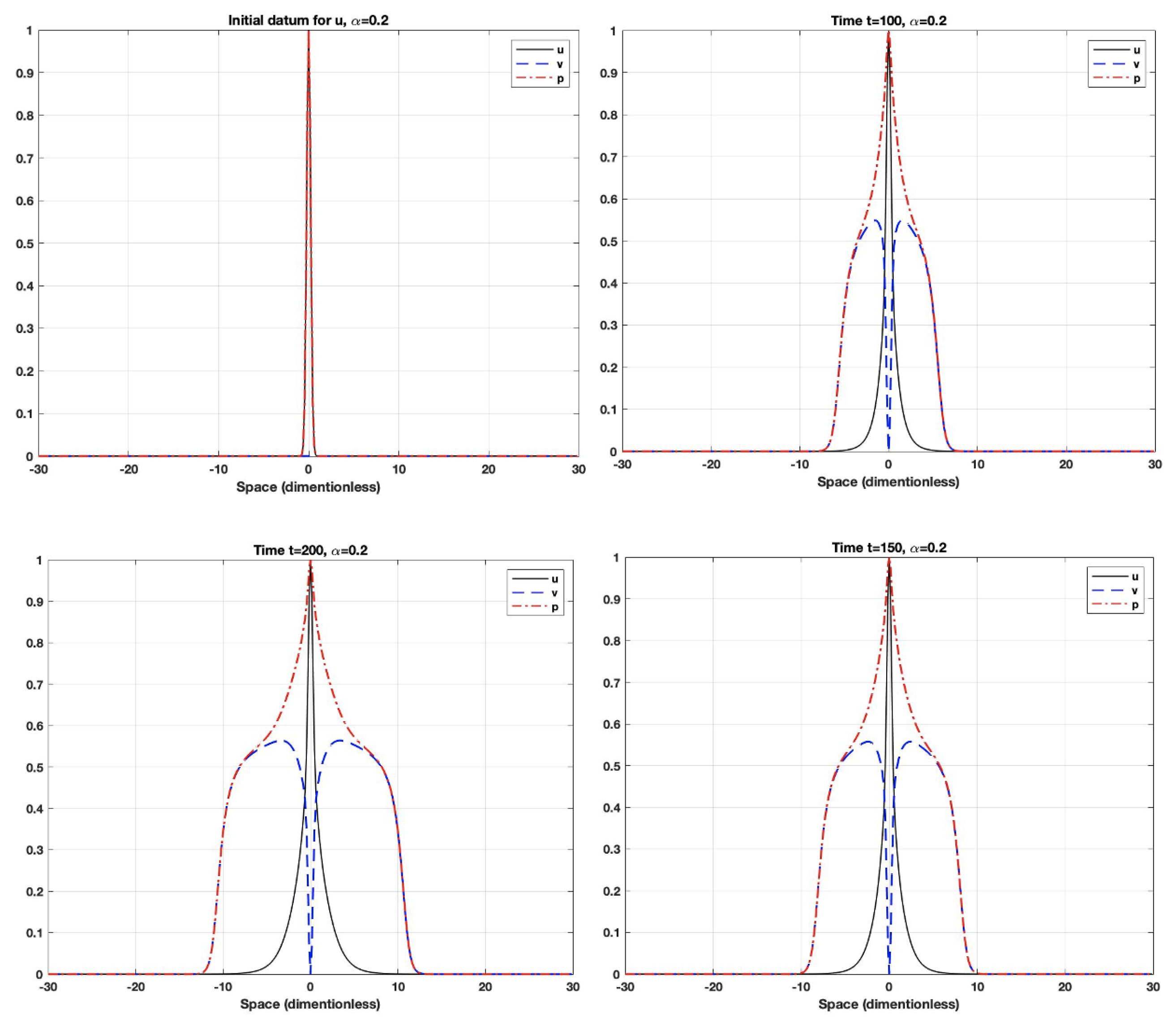
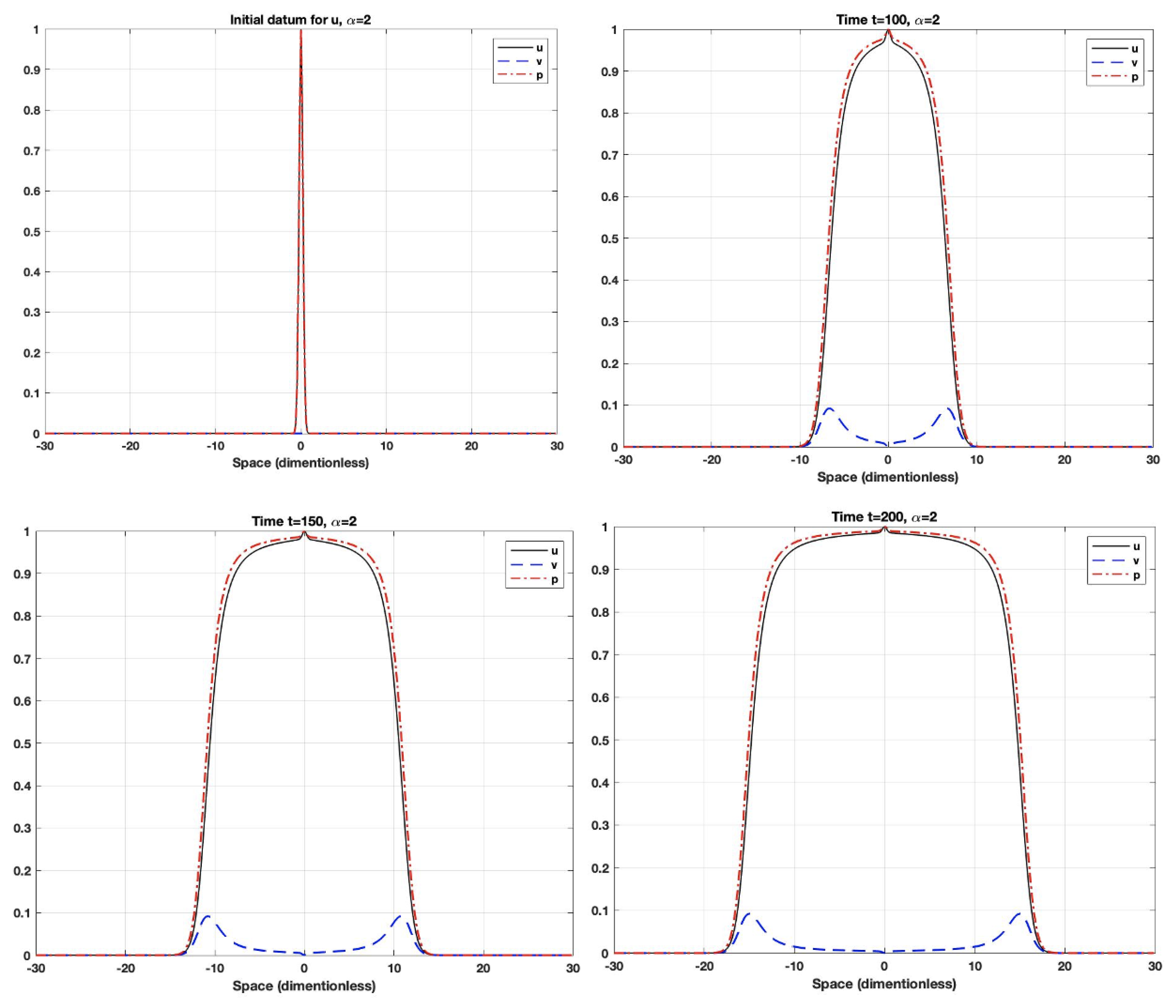
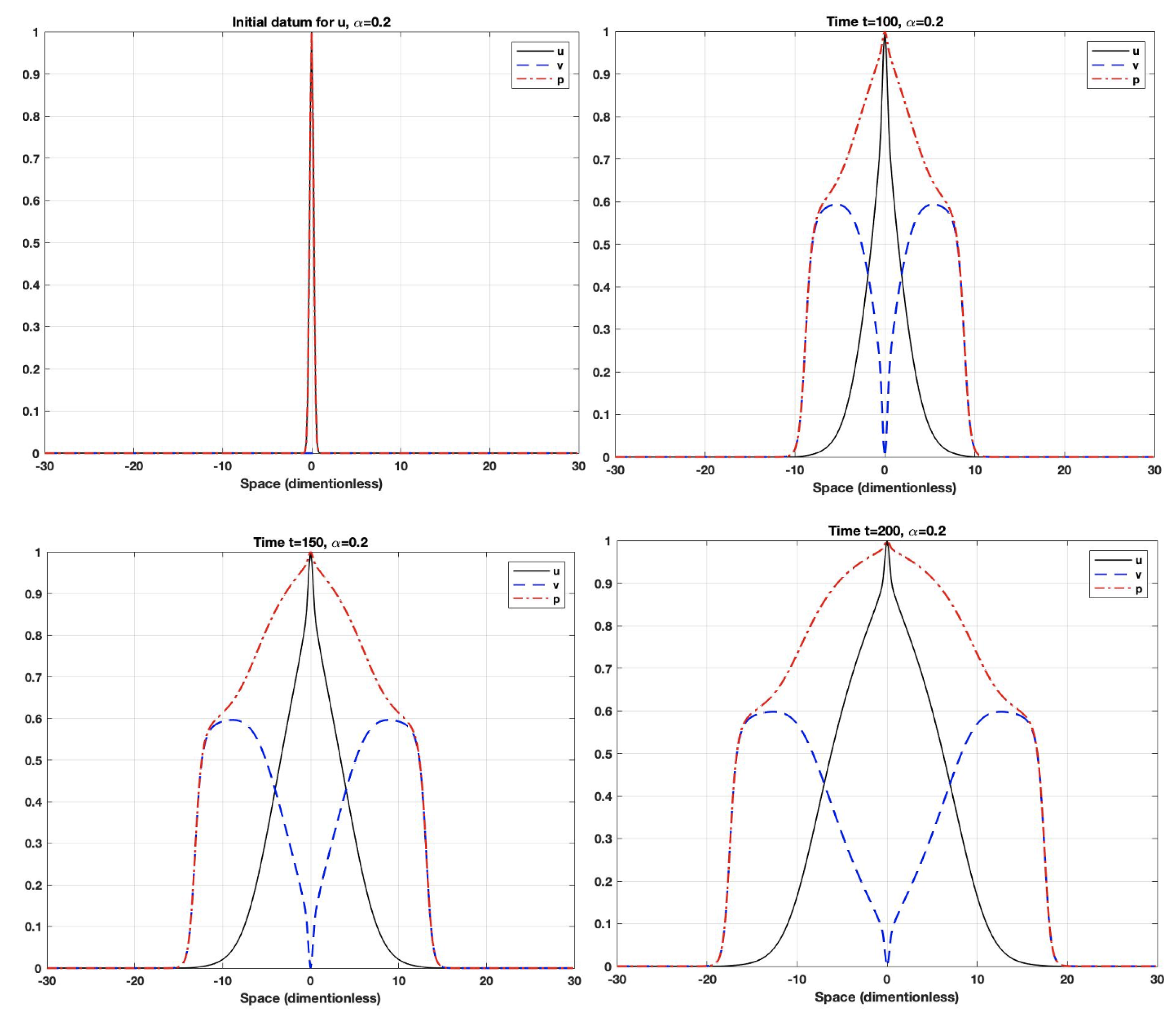
- The fractional derivative is a long-term operator, which means that the system response at any time will be affected by all previous responses. Additionally, the fractional-order parameter could control the dependence on the previous response (i.e., memory). When the order is small, the system uses the previous cases more often than it does when the order gets closer to one. As the order gets closer to one, the system will have a shorter memory dependence.
- Using fractional-order parameters enhances the system performance by increasing the degree of freedom. This allows the system to extend to a larger area.
- The term shows the probability density of the tumour cell (CSCs or NSCCs) that a mother cell located at y generates a daughter cell at the position x. As cell distribution only takes place inside the domain , the kernel of integral equals zero for all . Note that , since it is impossible to distribute more than one cell per cell cycle. Let , because this integration represents the total distribution of the cells in the whole domain. Furthermore, stands for a monotonically decreasing function (with the variables x and y). Due to the fact that the probability increases when the cells are close to each other and decreases when the cells are far apart. Thus, the distribution kernel depends on the total cell population at x, i.e., , causes the volume effect, which describes the more value of p is at node , the lower probability of the cell generation is at that one node [4]. Therefore, the integral kernel can be considered separable:in which . It should be noticed that is a non-increasing, non-negative and strictly decreasing when . denotes continuous Lipschitz function in including , . We suppose that function shows the distance between the two nodal points x and y, that is, . Furthermore, we can consider the arguments of with “small” variances. An obvious example is the Gaussian distribution kernel
- The terms and denote the density of CSCs and CCs, respectively and also shows the density of tumour cells.
4. Existence and Uniqueness Analysis
- (1)
- if and , ∀ then and when .
- (2)
- Suppose that and , ∀, then (i) if when then, when . (ii) if when then, when .
- (3)
- if when then, when .
- (4)
- if when then, when .
- (1)
- (2)
- To prove recalling previous part we note that w is non-negative, so for all . Therefore, one can conclude that when and hence w is non-decreasing, so we have ∀. As previous part guarantees , we deduce and for by definition. This implies that and ∀.To show , let represent first time, so that . From the first part, we conclude that:because of , we obtain:By assumption of the continuous Lipschitz on F and , in the next step, we conclude that w cannot reach the equilibrium point i.e., , in a finite time.
- (3)
- The previous part demonstrates that for . Consider is the first time so that , so implies that which leads to contradiction.
- (4)
- We proceed with a similar argument as adopted in part (3).
5. Numerical Simulations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enderling, H.; Anderson, A.R.; Chaplain, M.A.; Beheshti, A.; Hlatky, L.; Hahnfeldt, P. Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res. 2009, 69, 8814–8821. [Google Scholar] [CrossRef] [PubMed]
- Hillen, T.; Enderling, H.; Hahnfeldt, P. The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull. Math. Biol. 2013, 75, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Borsi, I.; Fasano, A.; Primicerio, M.; Hillen, T. A non-local model for cancer stem cells and the tumour growth paradox. Math. Med. Biol. J. IMA 2017, 34, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Mancini, A.; Primicerio, M. Tumours with cancer stem cells: A PDE model. Math. Biosci. 2016, 272, 76–80. [Google Scholar] [CrossRef]
- Maddalena, L. Analysis of an integro-differential system modeling tumor growth. Appl. Math. Comput. 2014, 245, 152–157. [Google Scholar] [CrossRef]
- Stiehl, T.; Marciniak-Czochra, A. Mathematical modeling of leukemogenesis and cancer stem cell dynamics. Math. Model. Nat. Phenom. 2012, 7, 166–202. [Google Scholar] [CrossRef]
- Maddalena, L.; Ragni, S. Existence of solutions and numerical approximation of a non-local tumor growth model. Math. Med. Biol.: J. IMA 2020, 37, 58–82. [Google Scholar] [CrossRef]
- Akgül, A.; Sajid Iqbal, M.; Fatima, U.; Ahmed, N.; Iqbal, Z.; Raza, A.; Rafiq, M.; Rehman, M.A.U. Optimal existence of fractional order computer virus epidemic model and numerical simulations. Math. Methods Appl. Sci. 2021, 44, 10673–10685. [Google Scholar] [CrossRef]
- Akgül, A.; Fatima, U.; Iqbal, M.S.; Ahmed, N.; Raza, A.; Iqbal, Z.; Rafiq, M. A fractal fractional model for computer virus dynamics. Chaos Solitons Fractals 2021, 147, 110947. [Google Scholar] [CrossRef]
- Dayan, F.; Ahmed, N.; Rafiq, M.; Akgül, A.; Raza, A.; Ahmad, M.O.; Jarad, F. Construction and numerical analysis of a fuzzy non-standard computational method for the solution of an SEIQR model of COVID-19 dynamics. AIMS Math. 2022, 7, 8449–8470. [Google Scholar] [CrossRef]
- Iqbal, Z.; Rehman, M.A.u.; Imran, M.; Ahmed, N.; Fatima, U.; Akgül, A.; Rafiq, M.; Raza, A.; Djuraev, A.A.; Jarad, F. A finite difference scheme to solve a fractional order epidemic model of computer virus. AIMS Math. 2023, 8, 2337–2359. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations. Math. Sci. Eng. 1999, 198, 41–117. [Google Scholar]
- He, J.H. Fractal calculus and its geometrical explanation. Results Phys. 2018, 10, 272–276. [Google Scholar] [CrossRef]
- El-Sayed, A.; El-Mesiry, A.; El-Saka, H. On the fractional-order logistic equation. Appl. Math. Lett. 2007, 20, 817–823. [Google Scholar] [CrossRef]
- Qiao, L.; Qiu, W.; Xu, D. Error analysis of fast L1 ADI finite difference/compact difference schemes for the fractional telegraph equation in three dimensions. Math. Comput. Simul. 2023, 205, 205–231. [Google Scholar] [CrossRef]
- Qiao, L.; Guo, J.; Qiu, W. Fast BDF2 ADI methods for the multi-dimensional tempered fractional integrodifferential equation of parabolic type. Comput. Math. Appl. 2022, 123, 89–104. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, D.; Yang, X.; Zhang, H. The efficient ADI Galerkin finite element methods for the three-dimensional nonlocal evolution problem arising in viscoelastic mechanics. Discret. Contin. Dyn. Syst. B 2023, 28, 3079–3106. [Google Scholar] [CrossRef]
- Akram, T.; Abbas, M.; Ali, A. A numerical study on time fractional Fisher equation using an extended cubic B-spline approximation. J. Math. Comput. Sci. 2021, 22, 85–96. [Google Scholar] [CrossRef]
- Luo, M.; Qiu, W.; Nikan, O.; Avazzadeh, Z. Second-order accurate, robust and efficient ADI Galerkin technique for the three-dimensional nonlocal heat model arising in viscoelasticity. Appl. Math. Comput. 2023, 440, 127655. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, K.; Dohare, R. Caputo fractional order derivative model of Zika virus transmission dynamics. J. Math. Comput. Sci. 2023, 2023, 145–157. [Google Scholar] [CrossRef]
- Oderinu, R.A.; Owolabi, J.A.; Taiwo, M. Approximate solutions of linear time-fractional differential equations. J. Math. Comput. Sci. 2023, 29, 60–72. [Google Scholar] [CrossRef]
- Cao, Y.; Nikan, O.; Avazzadeh, Z. A localized meshless technique for solving 2D nonlinear integro-differential equation with multi-term kernels. Appl. Numer. Math. 2023, 183, 140–156. [Google Scholar] [CrossRef]
- Nikan, O.; Molavi-Arabshai, S.M.; Jafari, H. Numerical simulation of the nonlinear fractional regularized long-wave model arising in ion acoustic plasma waves. Discret. Contin. Dyn. Syst. S 2021, 14, 3685. [Google Scholar] [CrossRef]
- Caputo, M. Linear models of dissipation whose Q is almost frequency independent—II. Geophys. J. Int. 1967, 13, 529–539. [Google Scholar] [CrossRef]
- Atangana, A.; Kilicman, A. Analytical solutions of the space-time fractional derivative of advection dispersion equation. Math. Probl. Eng. 2013, 2013, 853127. [Google Scholar] [CrossRef]
- Diethelm, K. Monotonicity of functions and sign changes of their Caputo derivatives. Fract. Calc. Appl. Anal. 2016, 19, 561–566. [Google Scholar] [CrossRef]
- Herrmann, R. Fractional Calculus: An Introduction for Physicists; World Scientific: Singapore, 2011. [Google Scholar]
- Gómez-Aguilar, J.; Rosales-García, J.; Bernal-Alvarado, J.; Córdova-Fraga, T.; Guzmán-Cabrera, R. Fractional mechanical oscillators. Rev. Mex. Física 2012, 58, 348–352. [Google Scholar]
- Ye, H.; Gao, J.; Ding, Y. A generalized Gronwall inequality and its application to a fractional differential equation. J. Math. Anal. Appl. 2007, 328, 1075–1081. [Google Scholar] [CrossRef]
- Nieto, J.J. Maximum principles for fractional differential equations derived from Mittag–Leffler functions. Appl. Math. Lett. 2010, 23, 1248–1251. [Google Scholar] [CrossRef]
- Garrappa, R. Numerical solution of fractional differential equations: A survey and a software tutorial. Mathematics 2018, 6, 16. [Google Scholar] [CrossRef]

| Parameter | Value | Description |
|---|---|---|
| fractional order of time derivative | ||
| fraction of symmetrical mitosis for CSCs | ||
| mortality rate of NSCCs | ||
| diffusion coefficients of NSCCs | ||
| diffusion coefficients of CSCs | ||
| republication rate of NSCCs | ||
| republication rate of CSCs | ||
| auxiliary parameter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliasghari, G.; Mesgarani, H.; Nikan, O.; Avazzadeh, Z. On Fractional Order Model of Tumor Growth with Cancer Stem Cell. Fractal Fract. 2023, 7, 27. https://doi.org/10.3390/fractalfract7010027
Aliasghari G, Mesgarani H, Nikan O, Avazzadeh Z. On Fractional Order Model of Tumor Growth with Cancer Stem Cell. Fractal and Fractional. 2023; 7(1):27. https://doi.org/10.3390/fractalfract7010027
Chicago/Turabian StyleAliasghari, Ghazaleh, Hamid Mesgarani, Omid Nikan, and Zakieh Avazzadeh. 2023. "On Fractional Order Model of Tumor Growth with Cancer Stem Cell" Fractal and Fractional 7, no. 1: 27. https://doi.org/10.3390/fractalfract7010027
APA StyleAliasghari, G., Mesgarani, H., Nikan, O., & Avazzadeh, Z. (2023). On Fractional Order Model of Tumor Growth with Cancer Stem Cell. Fractal and Fractional, 7(1), 27. https://doi.org/10.3390/fractalfract7010027








