A Fractional Analysis of Hyperthermia Therapy on Breast Cancer in a Porous Medium along with Radiative Microwave Heating
Abstract
1. Introduction
- Thermal ablation or high-temperature hyperthermia, i.e., temperature is greater than or equal to 46 °C for 4 to 6 min, (T ≥ 46 °C for 4–6 min).
- Comfortable-temperature hyperthermia, i.e., temperature is between 41° and 46° for 15 to 60 min, (41° < T < 46° for 15–60 min).
- Hyperthermia at a low temperature for a long time, i.e., temperature is less than or equal to 41° for 6 to 72 min, (T ≤ 41 °C for 6–72 min).
2. Mathematical Modeling and Solution
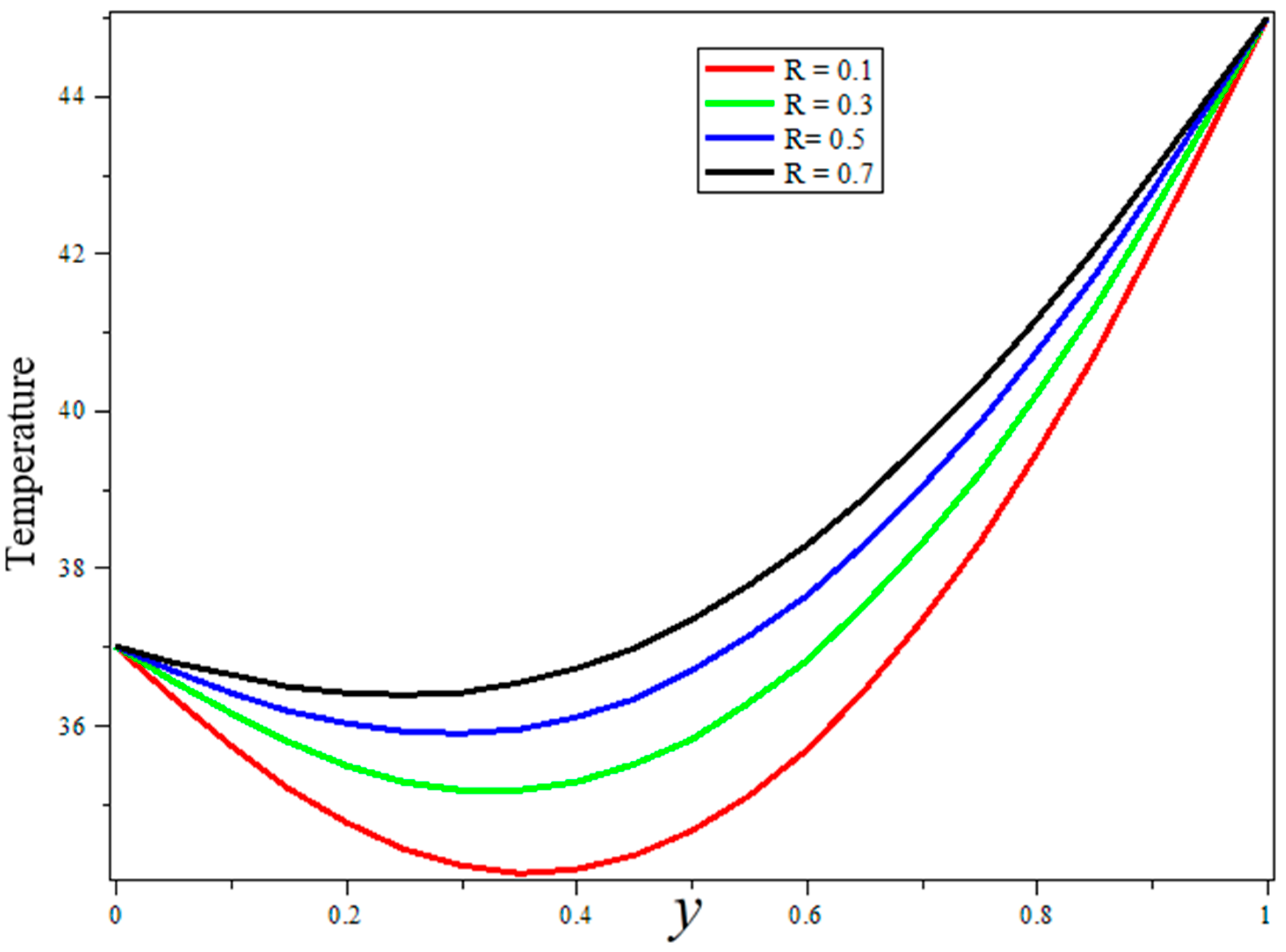
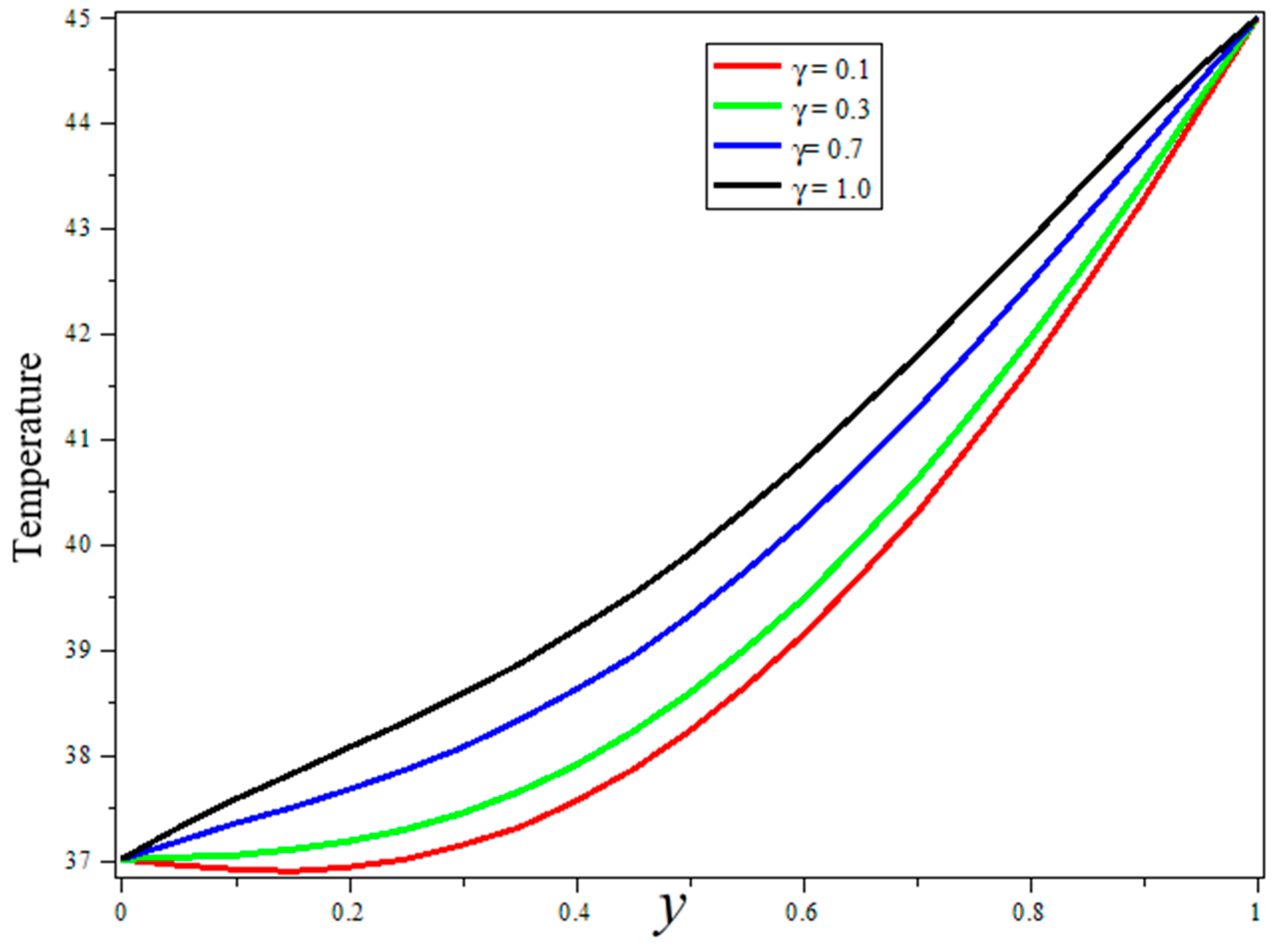
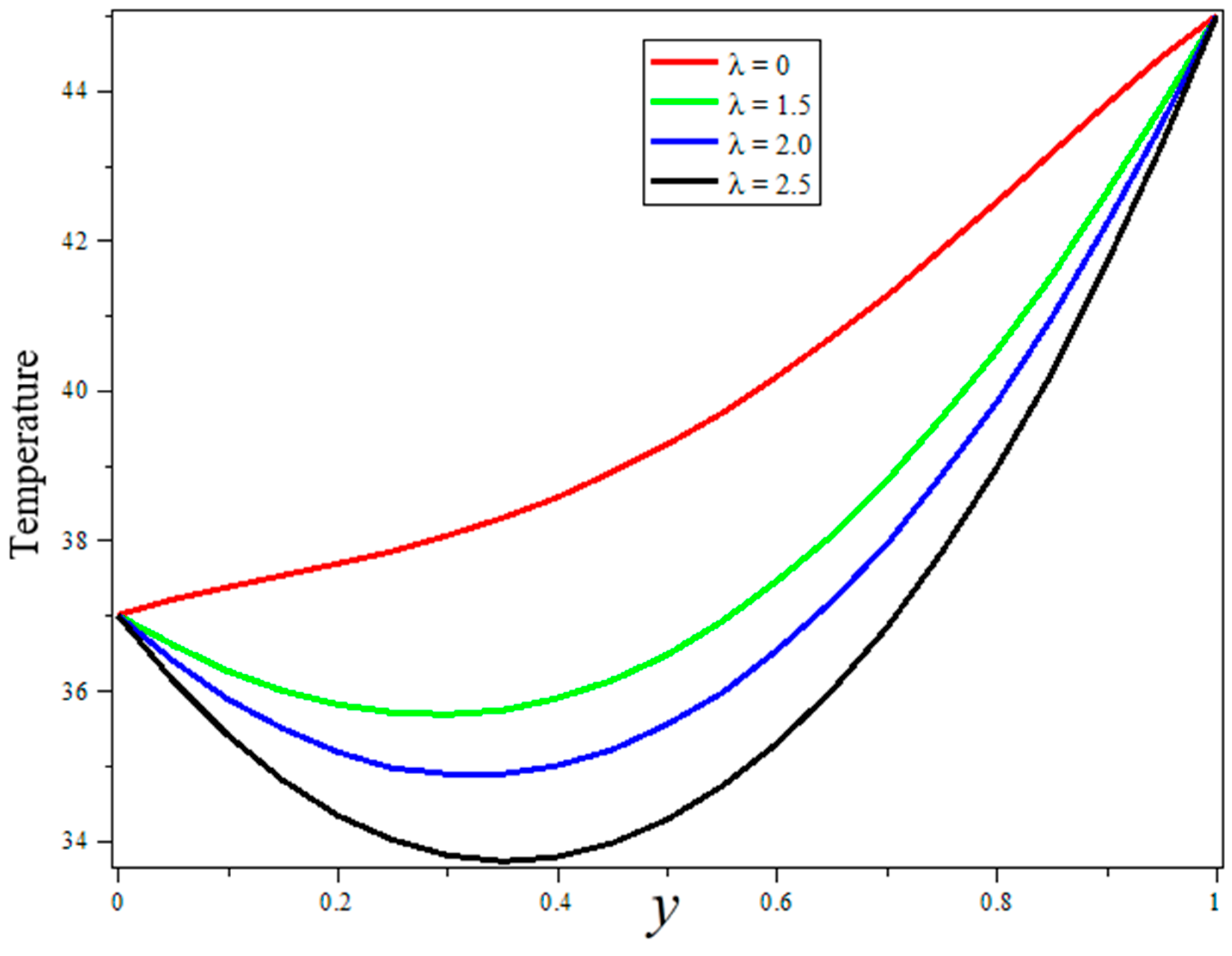
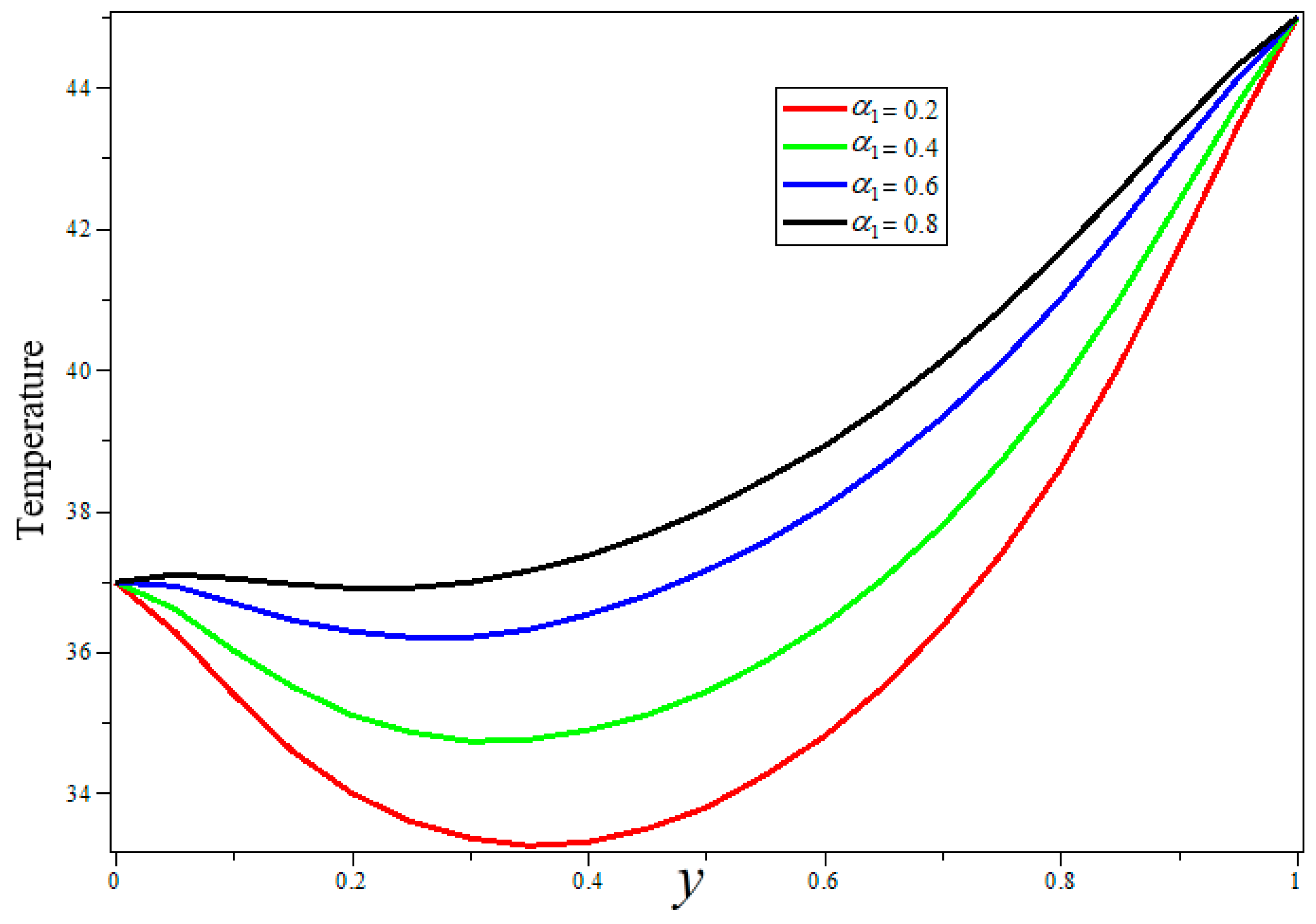
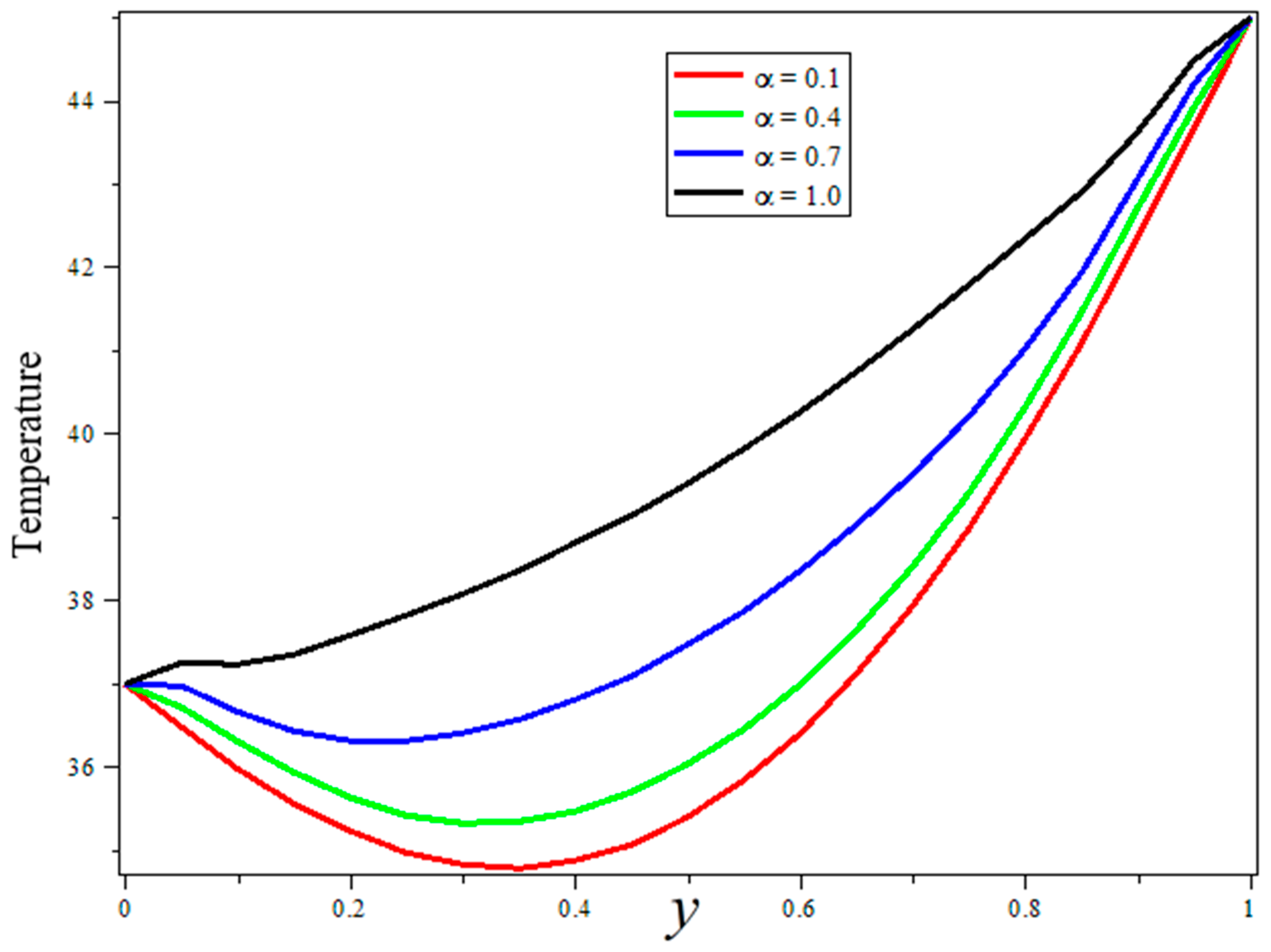
3. Results and Discussion
4. Conclusions
- The fractional model is more suitable for data fitting and memory effect.
- The solution obtained by using Durbin’s and Zakian’s numerical techniques coincides perfectly.
- Blood thermal conductivity, blood perfusion, and radiation parameters are shown to have good agreements for a temperature boost during treatment.
- Porosity and heat sources can be used for better temperature control during treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Density of blood | Stefan Boltzmann | ||
| Blood-specific heat at a constant pressure | Porosity of the tissue | ||
| Temperature profile | Permeability of the porous medium | ||
| Blood thermal conductivity | Radiation parameters | ||
| Blood volumetric perfusion rate | Fractional derivative parameter | ||
| Specific heat of tissue | Vascularization parameter | ||
| Body-heating coefficient | Source-of-heat parameter | ||
| Electric field | Thermal conductivity parameter | ||
| Porosity parameter | Mean absorption coefficient |
References
- Gurmu, E.D.; Koya, P.R. Impact of chemotherapy treatment of SITR compartmentalization and modeling of human papilloma virus (HPV). IOSR J. Math. (IOSR–JM) 2019, 15, 17–29. [Google Scholar]
- Oke, S.I.; Matadi, M.B.; Xulu, S.S. Optimal control analysis of a mathematical model for breast cancer. Math. Comput. Appl. 2018, 23, 21. [Google Scholar]
- Chestnov, O. World Health Organization Global Action Plan for the Prevention and Control of Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Patel, M.; Nagl, S. The Role of Model Integration in Complex Systems Modelling: An Example from Cancer Biology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Allen, B.G.; Bhatia, S.K.; Anderson, C.M.; Eichenberger-Gilmore, J.M.; Sibenaller, Z.A.; Mapuskar, K.A.; Schoenfeld, J.D.; Buatti, J.; Spitz, D.R.; Fath, M.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014, 2, 963–970. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Liu, J.; Jiye, A.A.; Zhou, F.; Wang, G. Multi-dimensional roles of ketone bodies in cancer biology: Opportunities for cancer therapy. Pharmacol. Res. 2019, 150, 104500. [Google Scholar] [CrossRef]
- Gilbert, D.L.; Pyzik, P.L.; Freeman, J.M. The ketogenic diet: Seizure control correlates better with serum β-hydroxybutyrate than with urine ketones. J. Child Neurol. 2000, 15, 787–790. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I.; Berezovskaya, F. Cancer immunoediting: A process driven by metabolic competition as a predator–prey–shared resource type model. J. Theor. Biol. 2015, 380, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models; Chapman and Hall/CRC: New York, NY, USA, 2007. [Google Scholar]
- Pontryagin, L.S. Mathematical Theory of Optimal Processes; CRC Press: California, CA, USA, 1987. [Google Scholar]
- De Pillis, L.G.; Radunskaya, A. A mathematical tumor model with immune resistance and drug therapy: An optimal control approach. Comput. Math. Methods Med. 2001, 3, 79–100. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Stamatakos, G.S.; Uzunoglu, N.K.; Nikita, K.S.; Marioli, A. A four-dimensional simulation model of tumour response to radiotherapy in vivo: Parametric validation considering radiosensitivity, genetic profile and fractionation. J. Theor. Biol. 2004, 230, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nani, F.; Freedman, H.I. A mathematical model of cancer treatment by immunotherapy. Math. Biosci. 2000, 163, 159–199. [Google Scholar] [CrossRef]
- Xu, F.; Lu, T.J.; Seffen, K.A. Biothermomechanics of skin tissues. J. Mech. Phys. Solids 2008, 56, 1852–1884. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Nabil, M.; Zunino, P. A computational study of cancer hyperthermia based on vascular magnetic nanoconstructs. R. Soc. Open Sci. 2016, 3, 160287. [Google Scholar] [CrossRef]
- Bhowmik, A.; Singh, R.; Repaka, R.; Mishra, S.C. Conventional and newly developed bioheat transport models in vascularized tissues: A review. J. Therm. Biol. 2013, 38, 107–125. [Google Scholar] [CrossRef]
- Hill, J.M.; Pincombe, A.H. Some similarity temperature profiles for the microwave heating of a half-space. ANZIAM J. 1992, 33, 290–320. [Google Scholar] [CrossRef]
- Strohbein, J.W.; Trembly, B.S.; Douple, E.B. Blood flow effects on the temperature distributions from an invasive microwave antenna array used in cancer therapy. IEEE Trans. Biomed. Eng. 1982, BME-29, 649–661. [Google Scholar] [CrossRef]
- Foster, K.R.; Kritikos, H.N.; Schwan, H.P. Effect of surface cooling and blood flow on the microwave heating of tissue. IEEE Trans. Biomed. Eng. 1978, BME-25, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Marchant, T.R.; Liu, B. On the heating of a two-dimensional slab in a microwave cavity: Aperture effects. ANZIAM J. 2001, 43, 137–148. [Google Scholar] [CrossRef][Green Version]
- Kritikos, H.N.; Poster, K.R.; Schwan, H.P. Temperature profiles in spheres due to electromagnetic heating. J. Microw. Power 1981, 16, 327–344. [Google Scholar] [CrossRef] [PubMed]
- El-dabe, N.T.; Mohamed, M.A.; El-Sayed, A.F. Effects of microwave heating on the thermal states of biological tissues. Afr. J. Biotechnol. 2003, 2, 453–459. [Google Scholar]
- Oke, S.I.; Salawu, S.O.; Matadi, M.B.; Animasaun, I.L. Radiative Microwave Heating of Hyperthermia Therapy on Breast Cancer in a Porous Medium. 2018. Available online: http://eprints.lmu.edu.ng/2407/ (accessed on 10 December 2021).
- Popoola, A.O.; Ayeni, O.B. A note on the multiplicity of solutions of a boundary value problem arising from the theory of microwave heating of cancerous tumor. Int. Inst. Sci. Technol. Educ. J. 2013, 3, 113–116. [Google Scholar]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Salawu, S.O.; Oke, S.I. Inherent irreversibility of exothermic chemical reactive third-grade poiseuille flow of a variable viscosity with convective cooling. J. Appl. Comput. Mech. 2018, 4, 167–174. [Google Scholar]
- Gupta, P.K.; Singh, J.; Rai, K.N. A numerical study on heat transfer in tissues during hyperthermia. Math. Comput. Model. 2013, 57, 1018–1037. [Google Scholar] [CrossRef]
- Salawu, S.O.; Dada, M.S. Radiative heat transfer of variable viscosity and thermal conductivity effects on inclined magnetic field with dissipation in a non-Darcy medium. J. Niger. Math. Soc. 2016, 35, 93–106. [Google Scholar] [CrossRef]
- Saqib, M.; Khan, I.; Shafie, S. Shape effect in magnetohydrodynamic free convection flow of sodium alginate-ferrimagnetic nanofluid. J. Therm. Sci. Eng. Appl. 2019, 11, 041019. [Google Scholar] [CrossRef]

| y | Durbin’s Algorithm | Zakian’s Algorithm |
|---|---|---|
| 0 | 37 | 37 |
| 0.1 | 36.76483 | 36.76488 |
| 0.2 | 36.68123 | 36.68129 |
| 0.3 | 36.74372 | 36.74376 |
| 0.4 | 36.96405 | 36.96411 |
| 0.5 | 37.37310 | 37.37315 |
| 0.6 | 38.02321 | 38.02327 |
| 0.7 | 38.99283 | 38.99284 |
| 0.8 | 40.39212 | 40.39214 |
| 0.9 | 42.36186 | 42.36189 |
| 1 | 45 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, D.; Rahman, A.u.; Kumam, P.; Watthayu, W. A Fractional Analysis of Hyperthermia Therapy on Breast Cancer in a Porous Medium along with Radiative Microwave Heating. Fractal Fract. 2022, 6, 82. https://doi.org/10.3390/fractalfract6020082
Khan D, Rahman Au, Kumam P, Watthayu W. A Fractional Analysis of Hyperthermia Therapy on Breast Cancer in a Porous Medium along with Radiative Microwave Heating. Fractal and Fractional. 2022; 6(2):82. https://doi.org/10.3390/fractalfract6020082
Chicago/Turabian StyleKhan, Dolat, Ata ur Rahman, Poom Kumam, and Wiboonsak Watthayu. 2022. "A Fractional Analysis of Hyperthermia Therapy on Breast Cancer in a Porous Medium along with Radiative Microwave Heating" Fractal and Fractional 6, no. 2: 82. https://doi.org/10.3390/fractalfract6020082
APA StyleKhan, D., Rahman, A. u., Kumam, P., & Watthayu, W. (2022). A Fractional Analysis of Hyperthermia Therapy on Breast Cancer in a Porous Medium along with Radiative Microwave Heating. Fractal and Fractional, 6(2), 82. https://doi.org/10.3390/fractalfract6020082







