West Nile Virus Lineage 2 Overwintering in Italy
Abstract
:1. Introduction
2. Materials and Methods
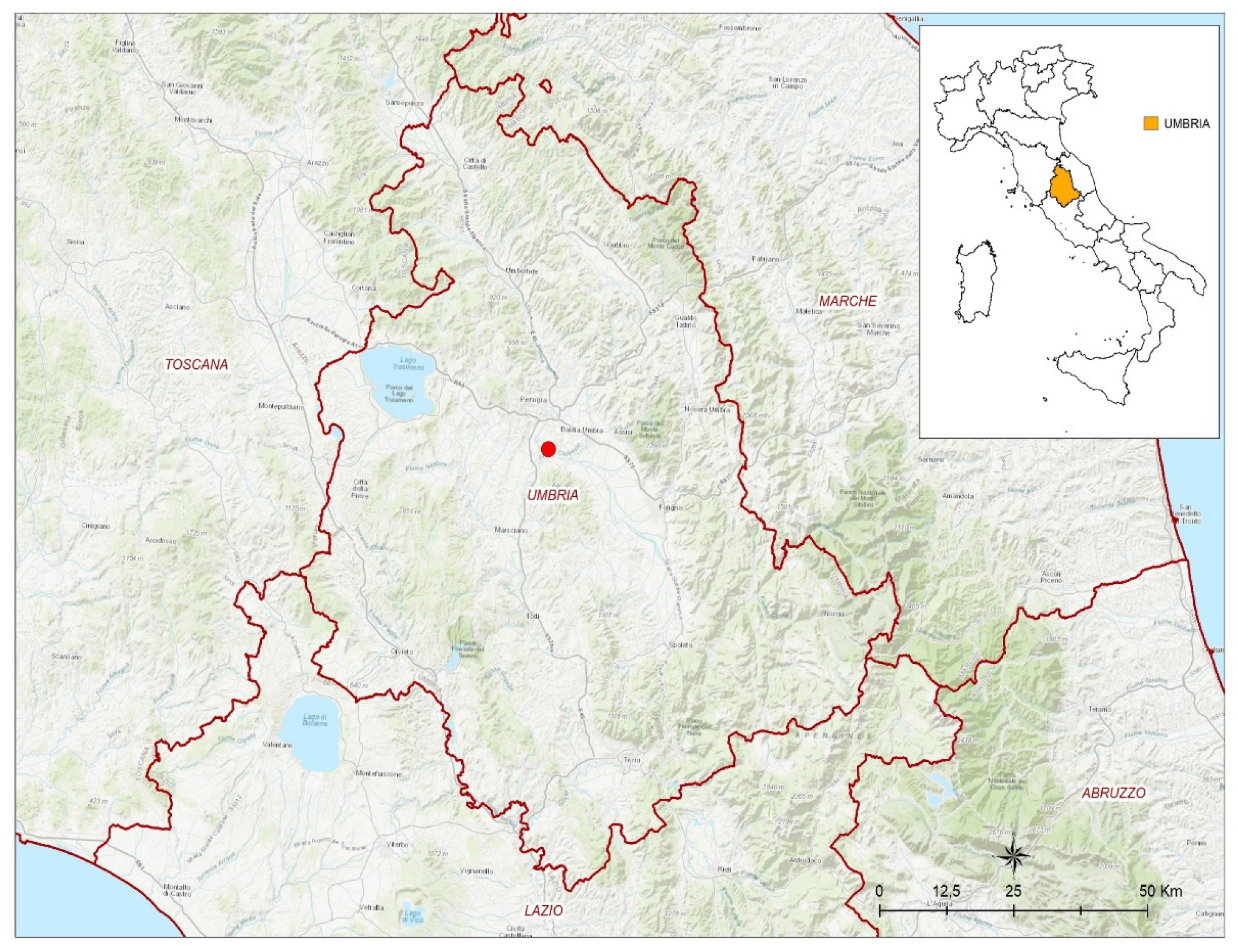
2.1. Case Report
2.2. Laboratory Analyses
2.2.1. Real-Time PCR for WNV and USUV
2.2.2. Illumina and Sanger Sequencing
2.2.3. Phylogenetic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Ndione, M.H.D.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile Virus in Africa: An Underestimated Threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef]
- Popescu, C.P.; Florescu, S.A.; Ruta, S.M. West Nile Virus in Central Europe—Pandora’s Box Is Wide Open! Travel Med. Infect. Dis. 2020, 37, 101864. [Google Scholar] [CrossRef]
- Mancuso, E.; Cecere, J.G.; Iapaolo, F.; Di Gennaro, A.; Sacchi, M.; Savini, G.; Spina, F.; Monaco, F. West Nile and Usutu Virus Introduction via Migratory Birds: A Retrospective Analysis in Italy. Viruses 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Byas, A.D.; Ebel, G.D. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisen, W.K.; Hahn, D.C. Comparison of Immune Responses of Brown-Headed Cowbird and Related Blackbirds to West Nile and Other Mosquito-Borne Encephalitis Viruses. J. Wildl. Dis. 2007, 43, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; Freire, C.C.d.M.; Loucoubar, C.; Zanotto, P.M.d.A.; Faye, O.; Sall, A.A. Biological and Phylogenetic Characteristics of West African Lineages of West Nile Virus. PLoS Negl. Trop Dis. 2017, 11, e0006078. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Ivanics, É.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Beck, C.; Leparc Goffart, I.; Franke, F.; Gonzalez, G.; Dumarest, M.; Lowenski, S.; Blanchard, Y.; Lucas, P.; de Lamballerie, X.; Grard, G.; et al. Contrasted Epidemiological Patterns of West Nile Virus Lineages 1 and 2 Infections in France from 2015 to 2019. Pathogens 2020, 9, 908. [Google Scholar] [CrossRef]
- West Nile Virus Infection. Available online: https://www.ecdc.europa.eu/en/west-nile-virus-infection (accessed on 3 May 2022).
- Bakonyi, T.; Haussig, J.M. West Nile Virus Keeps on Moving up in Europe. Euro Surveill 2020, 25, 2001938. [Google Scholar] [CrossRef]
- Rizzo, C.; Napoli, C.; Venturi, G.; Pupella, S.; Lombardini, L.; Calistri, P.; Monaco, F.; Cagarelli, R.; Angelini, P.; Bellini, R.; et al. West Nile Virus Transmission: Results from the Integrated Surveillance System in Italy, 2008 to 2015. Eurosurveillance 2016, 21, 30340. [Google Scholar] [CrossRef] [Green Version]
- Cantile, C.; Di Guardo, G.; Eleni, C.; Arispici, M. Clinical and Neuropathological Features of West Nile Virus Equine Encephalomyelitis in Italy. Equine Vet. J. 2000, 32, 31–35. [Google Scholar] [CrossRef]
- Monaco, F.; Lelli, R.; Teodori, L.; Pinoni, C.; Di Gennaro, A.; Polci, A.; Calistri, P.; Savini, G. Re-Emergence of West Nile Virus in Italy. Zoonoses Public Health 2010, 57, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Giglia, G.; Mencattelli, G.; Lepri, E.; Agliani, G.; Gobbi, M.; Gröne, A.; van den Brand, J.M.A.; Savini, G.; Mandara, M.T. West Nile Virus and Usutu Virus in Wild Birds from Rescue Centers, a Post-Mortem Monitoring Study from Central Italy. bioRxiv 2022. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A Novel Quantitative Multiplex Real-Time RT-PCR for the Simultaneous Detection and Differentiation of West Nile Virus Lineages 1 and 2, and of Usutu Virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real Time PCR Assay for Detection of All Known Lineages of West Nile Virus. J. Virol. Methods 2016, 236, 266–270. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A Rapid and Specific Real-Time RT-PCR Assay to Identify Usutu Virus in Human Plasma, Serum, and Cerebrospinal Fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Mencattelli, G.; Iapaolo, F.; Monaco, F.; Fusco, G.; de Martinis, C.; Portanti, O.; Di Gennaro, A.; Curini, V.; Polci, A.; Berjaoui, S.; et al. West Nile Virus Lineage 1 in Italy: Newly Introduced or a Re-Occurrence of a Previously Circulating Strain? Viruses 2022, 14, 64. [Google Scholar] [CrossRef]
- Marcacci, M.; De Luca, E.; Zaccaria, G.; Di Tommaso, M.; Mangone, I.; Aste, G.; Savini, G.; Boari, A.; Lorusso, A. Genome Characterization of Feline Morbillivirus from Italy. J. Virol. Methods 2016, 234, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Cito, F.; Pasquale, A.D.; Cammà, C.; Cito, P. The Italian Information System for the Collection and Analysis of Complete Genome Sequence of Pathogens Isolated from Animal, Food and Environment. Int. J. Infect. Dis. 2018, 73, 296–297. [Google Scholar] [CrossRef]
- Aprea, G.; Scattolini, S.; D’Angelantonio, D.; Chiaverini, A.; Di Lollo, V.; Olivieri, S.; Marcacci, M.; Mangone, I.; Salucci, S.; Antoci, S.; et al. Whole Genome Sequencing Characterization of HEV3-e and HEV3-f Subtypes among the Wild Boar Population in the Abruzzo Region, Italy: First Report. Microorganisms 2020, 8, 1393. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Grubaugh, N.D.; Gangavarapu, K.; Quick, J.; Matteson, N.L.; De Jesus, J.G.; Main, B.J.; Tan, A.L.; Paul, L.M.; Brackney, D.E.; Grewal, S.; et al. An Amplicon-Based Sequencing Framework for Accurately Measuring Intrahost Virus Diversity Using PrimalSeq and IVar. Genome Biol. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, M.; Kulshrestha, S.; Puri, A. Genome Sequencing. Methods Mol. Biol. 2017, 1525, 3–33. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Aguilera-Sepúlveda, P.; Napp, S.; Llorente, F.; Solano-Manrique, C.; Molina-López, R.; Obón, E.; Solé, A.; Jiménez-Clavero, M.Á.; Fernández-Pinero, J.; Busquets, N. West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017–2020). Viruses 2022, 14, 569. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Balzarini, F. Epidemiology of West Nile Virus Infections in Humans, Italy, 2012-2020: A Summary of Available Evidences. Trop Med. Infect. Dis. 2021, 6, 61. [Google Scholar] [CrossRef]
- Peterson, R.; Mountfort, G.; Hollom, P.A.; Pandolfi, M.; Frugis, S. Guida Degli Uccelli d’Europa. Atlante Illustrato a Colori. Libri—Amazon.It. Available online: https://www.amazon.it/uccelli-dEuropa-Atlante-illustrato-colori/dp/8874130473 (accessed on 28 April 2022).
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Kratz, G.E.; Bates, R.; Scherpelz, J.A.; Bowen, R.A.; Komar, N. Clinical Evaluation and Outcomes of Naturally Acquired West Nile Virus Infection in Raptors. J. Zoo Wildl. Med. 2009, 40, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, A.E.; Van Kruiningen, H.J.; French, R.A.; Anderson, J.F.; Andreadis, T.G.; Kumar, A.; West, A.B. Recovery and Identification of West Nile Virus from a Hawk in Winter. J. Clin. Microbiol. 2000, 38, 3110–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, H.S.; Van Wettere, A.J.; McFarlane, L.; Shearn-Bochsler, V.; Dickson, S.L.; Baker, J.; Hatch, G.; Cavender, K.; Long, R.; Bodenstein, B. West Nile Virus Transmission in Winter: The 2013 Great Salt Lake Bald Eagle and Eared Grebes Mortality Event. PLoS Curr. 2014, 6, 3110. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.; Gould, D.; Bowen, R.; Komar, N. Natural and Experimental West Nile Virus Infection in Five Raptor Species. J. Wildl. Dis. 2006, 42, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Montecino-Latorre, D.; Barker, C.M. Overwintering of West Nile Virus in a Bird Community with a Communal Crow Roost. Sci. Rep. 2018, 8, 6088. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.S.; Vineyard, M.P.; Woods, L.W.; Reisen, W.K. Dynamics of West Nile Virus Persistence in House Sparrows (Passer Domesticus). PLoS Negl. Trop. Dis. 2012, 6, e1860. [Google Scholar] [CrossRef] [Green Version]
- Reisen, W.K.; Fang, Y.; Lothrop, H.D.; Martinez, V.M.; Wilson, J.; Oconnor, P.; Carney, R.; Cahoon-Young, B.; Shafii, M.; Brault, A.C. Overwintering of West Nile Virus in Southern California. J. Med. Entomol. 2006, 43, 344–355. [Google Scholar] [CrossRef]
- Conte, A.; Candeloro, L.; Ippoliti, C.; Monaco, F.; Massis, F.D.; Bruno, R.; Sabatino, D.D.; Danzetta, M.L.; Benjelloun, A.; Belkadi, B.; et al. Spatio-Temporal Identification of Areas Suitable for West Nile Disease in the Mediterranean Basin and Central Europe. PLoS ONE 2015, 10, e0146024. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Langevin, S.A.; Brault, A.C.; Woods, L.; Carroll, B.D.; Reisen, W.K. Detection of Persistent West Nile Virus RNA in Experimentally and Naturally Infected Avian Hosts. Am. J. Trop Med. Hyg. 2012, 87, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Tesh, R.B.; Siirin, M.; Guzman, H.; Travassos da Rosa, A.P.A.; Wu, X.; Duan, T.; Lei, H.; Nunes, M.R.; Xiao, S.-Y. Persistent West Nile Virus Infection in the Golden Hamster: Studies on Its Mechanism and Possible Implications for Other Flavivirus Infections. J. Infect. Dis. 2005, 192, 287–295. [Google Scholar] [CrossRef]
- Appler, K.K.; Brown, A.N.; Stewart, B.S.; Behr, M.J.; Demarest, V.L.; Wong, S.J.; Bernard, K.A. Persistence of West Nile Virus in the Central Nervous System and Periphery of Mice. PLoS ONE 2010, 5, e10649. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; van de Peppel, L.J.J.; van Vliet, A.J.H.; Westenberg, M.; Ibañez-Justicia, A.; Stroo, A.; Buijs, J.A.; Visser, T.M.; Koenraadt, C.J.M. Winter Activity and Aboveground Hybridization Between the Two Biotypes of the West Nile Virus Vector Culex Pipiens. Vector Borne Zoonotic Dis. 2015, 15, 619–626. [Google Scholar] [CrossRef]
- Di Luca, M.; Toma, L.; Boccolini, D.; Severini, F.; La Rosa, G.; Minelli, G.; Bongiorno, G.; Montarsi, F.; Arnoldi, D.; Capelli, G.; et al. Ecological Distribution and CQ11 Genetic Structure of Culex Pipiens Complex (Diptera: Culicidae) in Italy. PLoS ONE 2016, 11, e0146476. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Amadon, D. Eagles, Hawks and Falcons of the World; Wellfleet: Secaucus, NJ, USA, 1989; ISBN 978-1-55521-472-2. [Google Scholar]
- Bollettino_WND_2021_19 (1).Pdf—Adobe Cloud Storage. Available online: https://acrobat.adobe.com/link/file/?x_api_client_id=chrome_extension_viewer&uri=urn%3Aaaid%3Asc%3AUS%3A9868069d-ee54-4d0a-9351-4da5a42ffa6b&filetype=application%2Fpdf&size=1302218 (accessed on 25 July 2022).
- Bollettino_WND_2022_3.Pdf—Adobe Cloud Storage. Available online: https://acrobat.adobe.com/link/file/?x_api_client_id=chrome_extension_viewer&uri=urn%3Aaaid%3Asc%3AUS%3A359a79f6-a813-4fdd-90b8-b52904f3ca1b&filetype=application%2Fpdf&size=1468985 (accessed on 25 July 2022).
- Riccò, M. Epidemiology of Tick-Borne Encephalitis in North-Eastern Italy (2017–2020): International Insights from National Notification Reports. Acta Biomed. 2021, 92, e2021229. [Google Scholar] [CrossRef]
- Toscana_2020.Pdf—Adobe Cloud Storage. Available online: https://acrobat.adobe.com/link/file/?x_api_client_id=chrome_extension_viewer&uri=urn%3Aaaid%3Asc%3AUS%3A35ca9c99-25e9-406b-b502-28c485af700&filetype=application%2Fpdf&size=344881 (accessed on 25 July 2022).
- Mancini Mosquito Species Involved in the Circulation of West Nile and Usutu Viruses in Italy. Vet. Ital. 2017, 53, 97–110. [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencattelli, G.; Iapaolo, F.; Polci, A.; Marcacci, M.; Di Gennaro, A.; Teodori, L.; Curini, V.; Di Lollo, V.; Secondini, B.; Scialabba, S.; et al. West Nile Virus Lineage 2 Overwintering in Italy. Trop. Med. Infect. Dis. 2022, 7, 160. https://doi.org/10.3390/tropicalmed7080160
Mencattelli G, Iapaolo F, Polci A, Marcacci M, Di Gennaro A, Teodori L, Curini V, Di Lollo V, Secondini B, Scialabba S, et al. West Nile Virus Lineage 2 Overwintering in Italy. Tropical Medicine and Infectious Disease. 2022; 7(8):160. https://doi.org/10.3390/tropicalmed7080160
Chicago/Turabian StyleMencattelli, Giulia, Federica Iapaolo, Andrea Polci, Maurilia Marcacci, Annapia Di Gennaro, Liana Teodori, Valentina Curini, Valeria Di Lollo, Barbara Secondini, Silvia Scialabba, and et al. 2022. "West Nile Virus Lineage 2 Overwintering in Italy" Tropical Medicine and Infectious Disease 7, no. 8: 160. https://doi.org/10.3390/tropicalmed7080160
APA StyleMencattelli, G., Iapaolo, F., Polci, A., Marcacci, M., Di Gennaro, A., Teodori, L., Curini, V., Di Lollo, V., Secondini, B., Scialabba, S., Gobbi, M., Manuali, E., Cammà, C., Rosà, R., Rizzoli, A., Monaco, F., & Savini, G. (2022). West Nile Virus Lineage 2 Overwintering in Italy. Tropical Medicine and Infectious Disease, 7(8), 160. https://doi.org/10.3390/tropicalmed7080160










