Abstract
The prevalence of bacteremia caused by carbapenem-non-susceptible Acinetobacter baumannii (CNSAB) continues to increase, and it is associated with a high mortality rate. Early recognition of infection and mortality determinants risk factors is necessary for adequate antibiotic administration. We aimed to determine the risk factors and outcomes of CNSAB bacteremia in Indonesia. A multicenter case-control study was conducted in three referral hospitals in Indonesia. Data were collected retrospectively from January 2019 to December 2021. Cases were defined as patients with bacteremia where CNSAB was isolated from the blood, while the controls were patients with bacteremia caused by carbapenem-susceptible A. baumannii (CSAB). Risk factors for bacteremia and mortality associated with CNSAB bacteremia were determined using univariates analysis (chi-squared and Student’s t-test or Mann–Whitney test) and multivariate logistic regression analysis. A total of 144 bacteremia patients were included, of whom 72 patients were for each case and control group. The final model of multivariate regression analysis revealed that bacteremia source from the lower respiratory tract (adjusted odds ratio (aOR): 3.24; 95% CI: 1.58–6.63, p = 0.001) and the use of central venous catheter (aOR: 2.56; 95% CI: 1.27–5.18; p = 0.009) were independent risk factors for CNSAB bacteremia. Charlson Comorbidity Index ≥ 4 (aOR: 28.56; 95% CI: 3.06–265.90, p = 0.003) and Pitt Bacteremia Score ≥ 4 (aOR: 6.44; 95% CI: 1.17–35.38; p = 0.032) were independent risk factors for mortality due to CNSAB bacteremia. Only high Pitt Bacteremia Score was an independent risk factor for mortality of CSAB bacteremia. In conclusion, we identified the risk factors for CNSAB-associated bacteremia and the risk factors for death, which are relevant for empiric therapy and infection control prevention, as well as prognosis evaluation of patients with bloodstream infections.
1. Introduction
Acinetobacter baumannii (A. baumannii) is a Gram-negative coccobacillus that is a significant cause of hospital-associated infections worldwide, particularly lower respiratory tract infections and bacteremia [1,2]. A. baumannii can accumulate various antibiotic resistance mechanisms to create multidrug-resistant strains, including resistance to carbapenem antibiotics [1,3]. A. baumannii is a member of the ESKAPE pathogen, which is a group of pathogens responsible for the majority of nosocomial infections and are capable of escaping the biocidal action of antimicrobial agents [4]. Bacteremia caused by multidrug-resistant A. baumannii is associated with a high mortality rate [5,6,7,8,9,10,11]. According to a meta-analysis, the mortality rate from bacteremia caused by carbapenem-resistant A. baumannii also known as carbapenem-non-susceptible A. baumannii (CNSAB), is 33% [11]. The limited antibiotic therapeutic options for bacteremia caused by CNSAB make managing this infection challenging [12]. The prevalence of bacteremia caused by CNSAB is increasing globally, including in Indonesia [13]. According to the Indonesian National Surveillance on Antimicrobial Resistance data in 2020, 61% of A. baumannii isolates from blood specimens were confirmed as carbapenem-resistant [14].
Several studies have demonstrated that inappropriate empirical antibiotic therapy is one of the factors associated with increase mortality due to bacteremia caused by CNSAB [5,8,9,15,16,17]. Therefore, early recognition of risk factors of CNSAB bacteremia is necessary to provide adequate antibiotics. Previous studies have identified some of the risk factors associated with CNSAB bacteremia such as old age, male, history of previous use of antibiotics, history of previous chemotherapy or radiotherapy, history of colonization by A. baumannii, history of prolonged hospital stay, and use of invasive devices such as central venous catheters, ventilators, or drainage catheters [6,7,10,15,16,18,19,20,21,22,23,24]. However, the risk factors, and outcomes of CNSAB bacteremia in developing countries, including Indonesia, are limited. Since factors associated with clinical characteristics, risk factors, and outcomes of CNSAB bacteremia could be affected by a variety of factors, including ethnic, geographic, environmental, and economic factors, CNSAB prevalence, and differences in clinical practice and antibiotic prescribing habits [6], studies from developing countries are essential. Therefore, this study sought insights into CNSAB bacteremia from a developing country. The aims of this study were (1) to determine the risk factors for CNSAB bacteremia and (2) to determine the outcomes of CNSAB bacteremia and its associated determinants in Indonesia.
2. Methods
2.1. Study Design, Setting and Patients
A multicenter case-control study was conducted in three province referral hospitals located on two major islands in Indonesia: Arifin Achmad Hospital (600-bed hospital) in Pekanbaru (Sumatra Island); Dr. Soetomo Hospital (1500-bed hospital) in Surabaya (Java Island), and Dr. Saiful Anwar Hospital (800-bed hospital) in Malang (Java Island).
Cases were defined as bacteremia patients from whom CNSAB was isolated from their blood. Controls were patients with bacteremia from whom carbapenem-susceptible A. baumannii (CSAB) was isolated from the blood specimen. Samples were collected retrospectively from January 2019 to December 2021, with the ratio of cases and controls being 1:1. Controls were matched with the cases based on the time of hospital admission (i.e., the control and case were hospitalized at approximately the same time), age, and length of hospital stay. Patients with the absence of medical records or inadequate data and discharged against medical advice were excluded. The flowchart of the enrolled cases and controls is described in Figure 1. A case report form (CRF) was prepared and used to collect the data from the medical records and the hospital databases.
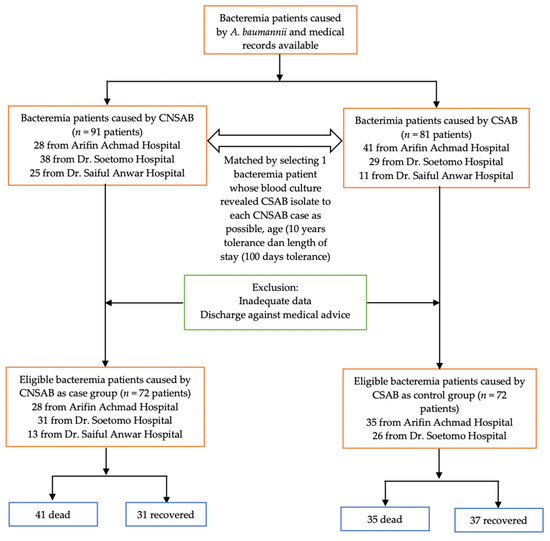
Figure 1.
Flowchart of the sample selection.
2.2. Blood Culture and Antibiotic Resistance Test
The automated Bact/Alert (bioMérieux, Marcy l’Etoile, France) or automated BACTEC system (Becton Dickinson, Franklin Lakes, NJ, USA) were used to culture the blood samples. The automated Vitek 2 System (Biomerieux, Marcy l’Etoile, France) or Phoenix (BD, Franklin Lakes, NJ, USA) were used for identification and antibiotic resistance tests in all study sites. Vitek 2 System was used in Arifin Achmad Hospital and Dr. Saiful Anwar Hospital, whereas Dr. Soetomo Hospital used Phoenix for identification and susceptibility testing. The interpretation of antibiotic resistance tests followed the Clinical and Laboratory Standards Institute (CLSI) in all hospitals. To confirm the carbapenemase production of CNSAB isolates, a molecular examination to identify the carbapenemase genes was conducted. All of the tested isolates carried carbapenemase genes; the results have been published elsewhere [25].
2.3. Study Variables and Definitions
There were two response variables assessed in this study: (1) type of bacteremia based on the antibiotic resistance profile of A. baumannii (CNSAB bacteremia vs. CSAB bacteremia) and (2) the outcome of bacteremia caused by CNSAB (recovered vs. death). CNSAB was defined if the antibiotic sensitivity test showed resistance results to one of the carbapenem antibiotics (meropenem, imipenem, or doripenem), while CSAB was defined as samples with sensitive A. baumannii to all carbapenem antibiotics. The outcome of CNSAB bacteremia was classified as recovered or death. Death refers to hospital mortality.
Several risk factors for CNSAB bacteremia were collected in this study, including age, sex, type of ward, whether the patient was referred from another hospital, the length of onset and hospital stay, days to mortality, type or origin of the isolates, source of bacteremia, severity of sepsis, and comorbid factors. In addition, data on surgical history, antibiotic use history, immunosuppressant medication history within three months, and hospitalization history within the last 12 months were also collected.
The length of onset was the duration from the date of hospitalization to the date of bacteremia onset, and the length of stay was defined as the duration from the date of hospitalization to the date of hospital discharge. Days to mortality was the duration from the date of hospitalization to the date of death. Type of A. baumannii isolate was classified as hospital isolate if the date of onset of bacteremia occurred more than two days after hospital admission or if the patient was referred from another hospital where the patient had been treated for two days, while community isolate was defined if the date of bacteremia onset occurred ≤2 days after hospital admission [26].
Sources of bacteremia were grouped into primary and secondary. Primary bacteremia is not secondary to an infection at another body site. Secondary bacteremia could be an infection from the upper and lower respiratory tract, urinary tract, skin and soft tissue, reproduction, surgical wounds, gastrointestinal tract, and central nervous system. For statistical purposes, bacteremia originating from the lower respiratory tract, the dominant source, was also compared to bacteremia due to other sources.
The severity of sepsis was assessed using a Pitt Bacteremia Score as previously proposed [27]. The scores were calculated at the onset of bacteremia and included the indicator of temperature, blood pressure, consciousness, presence of cardiac arrest, and use of mechanical ventilation [27]. In addition, the level of consciousness (alert, stupor, disorientation, coma, or sedation) was assessed, and for the statistical purpose, it was divided into two groups: alert and disturbance (stupor, disorientation, coma, or sedation). The Pitt Bacteremia Score ranges from 0 to 14 points, with a score ≥ 4 commonly used as an indicator of critical illness and increased mortality rate [28].
The patient’s comorbid factor was calculated using the Charlson Comorbidity Index, which includes age, myocardial infarction, chronic heart failure, peripheral vascular disease, cerebrovascular accident, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer, hemiplegia, chronic kidney disease, leukemia, lymphoma, HIV/AIDS, solid tumors, diabetes mellitus, and liver disorders [29]. In this study, the Charlson Comorbidity Index was categorized into two groups: <4 and ≥4, based on the previous study [30].
2.4. Statistical Analysis
Demographic data, type of ward, whether the patient was referred from another hospital, length of onset, length of hospital stay, days to mortality, type of the isolates, source of bacteremia, the severity of sepsis, comorbid factors, surgical history, antibiotic use history, immunosuppressant medication history within three months, and hospitalization history were included in the risk factors analysis.
The univariate analyses (Chi-squared test and Student’s t-test or Mann–Whitney test) were performed based on the type of data to determine the risk factors associated with the bacteremia caused by CNSAB and determinants associated with CNSAB mortality. All variables with p ≤ 0.25 within the univariate analysis were included in the multivariate logistic regression analysis. The final multivariate analysis model used the backward elimination approach, in which variables that had the least significant effect on the model were removed. The crude odds ratio (OR) and adjusted OR (aOR) were calculated during univariate and multivariate, respectively, together with the 95% confidence intervals (95% CIs). The significance was assessed using p < 0.05. All statistical analyses were conducted using Stata version 16 software (College Station, TX: Stata Corp LLC, TX, USA).
3. Results
3.1. Risk Factors Associated with CNSAB Bacteremia
In this study, there was an equal number of bacteremia patients in the CNSAB (case group) and CSAB (control group): 72 patients for each group were included. There was no significant difference in the distribution of cases and controls based on site, island, and year of sampling (see Table S1 Supplementary files). The comparison of demographics and risk factors between CNSAB and CSAB bacteremia are presented in Table 1.

Table 1.
Characteristics of respondents and potential risk factors for bacteremia due to CNSAB and CSAB (n = 144).
Univariate analysis revealed that intensive care unit (ICU) admission (OR: 2.35; 95% CI: 1.06–5.19), bacteremia source from the lower respiratory tract (OR: 3.49; 95% CI: 1.73–7.03), use of mechanical ventilation (OR 2.00; 95 CI: 1.02–3.92), having diabetes mellitus (OR: 3.94; 95% CI: 1.47–10.58), and use of central venous catheter (OR: 2.80; 95% CI: 1.42–5.52) were associated with the risk of CNSAB bacteremia (Table 1).
Multivariate logistic regression analysis was performed to assess the independent factors of CNSAB bacteremia by including variables with a p ≤ 0.25 in univariate analyses. The variables included in the multivariate analysis were sex, type of ward, bacteremia source, the presence of hypotension, use of mechanical ventilation, presence of diabetes mellitus, central venous catheter use, and the history of previous antibiotic use. The final model revealed that the source of bacteremia originating from the lower respiratory tract (aOR: 3.24; 95% CI: 1.58–6.63, p = 0.001) and the use of central venous catheter (aOR: 2.56; 95% CI: 1.27–518, p = 0.09) were the independent risk factors for bacteremia caused by CNSAB (Table 2).

Table 2.
Final model of multivariate analysis showing the risk factors of bacteremia due to CNSAB (n = 144).
3.2. Risk Factors Associated with the Mortality of CNSAB and CSAB Bacteremia
Our study analyzed the mortality risk factors of both CNSAB and CSAB bacteremia. The risk factors were slightly different between CNSAB and CSAB bacteremia. In CNSAB bacteremia, univariate analysis found that older age (p = 0.018), bacteremia source from the lower respiratory tract (OR: 3.07; 95% CI: 1.03–9.11), high Pitt Bacteremia Score (p < 0.001), Pitt Bacteremia Score ≥ 4 (OR: 16.18; 95% CI: 4.20–62.38), hypotension (OR: 5.97; 95% CI: 1.56–22.95), cardiac arrest (p = 0.009), consciousness disturbance (OR: 14.54; 95% CI: 4.21–50.19), high Charlson Comorbidity Index (p < 0.001), or Charlson Comorbidity Index ≥ 4 (OR: 9.28; 95% CI: 1.94–44.35) were associated with death (Table 3).

Table 3.
Risk factors for mortality of bacteremia caused by CNSAB (n = 72).
In CSAB bacteremia, the risk factors of mortality were being admitted to the ICU (OR: 3.05; 95% CI: 1.06–8.74), high Pitt Bacteremia Score (p < 0.001), Pitt Bacteremia Score ≥ 4 (OR: 16.00; 95% CI: 4.85–52.82), fever (OR: 3.34; 95% CI: 1.03–10.79), hypotension (OR: 7.00; 95% CI: 1.41–34.76), using a mechanical ventilator (OR: 5.91; 95% CI: 2.14–16.34), cardiac arrest (p < 0.001), having a disturbance of consciousness (OR: 14.46; 95% CI: 4.63–45.22), using central venous catheter (OR: 4.05; 95% CI: 1.41–11.64), and having a history of using antibiotics previously (OR: 3.54; 95% CI: 1.34–9.34) (Table 4).

Table 4.
Risk factors for mortality of bacteremia caused by CSAB (n = 72).
Multivariate logistic regression analysis was carried out to assess the independent determinants of death due to CNSAB and CSAB bacteremia by including variables with a p < 0.25 in univariate analysis. In the final model, two independent risk factors for mortality of bacteremia due to CNSAB were identified: the Pitt Bacteremia Score ≥ 4 (aOR: 13.29; 95% CI: 3.31–53.33, p < 0.001) and Charlson Comorbidity Index ≥ 4 (aOR: 6.44; 95% CI: 1.17–35.38, p = 0.032). Meanwhile, in CSAB bacteremia, only one independent factor associated with death, which was having a high Pitt Bacteremia Score (aOR: 1.87; 95% CI: 1.41–2.47, p < 0.001) (Table 5).

Table 5.
The final model of multivariate analysis showing determinant of death of bacteremia due to CNSAB (n = 41) and CSAB (n = 35).
The patients with CNSAB bacteremia with the Pitt Bacteremia Score ≥ 4 were at approximately 13.29 times higher risk of death than patients with index less than 4; aOR: 13.29; 95% CI: 3.31–53.33 with p < 0.001. In addition, those with the Charlson Comorbidity Index ≥4 had higher odds of mortality than those with a score less than 4 with OR: 6.44; 95% CI: 1.17–35.38 and p = 0.032 (Table 5).
4. Discussion
To the best of our knowledge, this is the first multicenter case-control study in Indonesia to investigate the risk factors for CNSAB bacteremia and determinants of CNSAB bacteremia mortality. Previous studies have identified several risk factors related to bacteremia caused by multidrug-resistant A. baumannii, including CNSAB [6,7,10,15,16,18,19,20,21,22,23,24]. In the present study, ICU admission, infection source of lower respiratory tract infection, ventilator or central venous catheter use, and the presence of diabetes mellitus were found to be more common in bacteremia caused by CNSAB than CSAB. Patients admitted to the ICU are in severe condition, have already been exposed to multiple antibiotics, and have a compromised immune system. This condition makes the patients are susceptible to cross-transmission infection from health workers or other patients and invasive procedures after a lengthy hospital stay [9,10,12,14,16].
A. baumannii is able to survive in the environment and medical equipment for long period. Bacteremia occurs when the bacteria entering the patient’s exposed skin or mucosal barriers during invasive procedures [10]. The use of mechanical ventilator and central venous catheter indicates that the patient is in severe condition and is at risk of suffering from ventilator-associated pneumonia and central line-associated bloodstream infection, which are commonly caused by multidrug-resistant bacteria, including CNSAB [5,6,7,12,15,23]. Using ventilators and central venous catheters, both risk factors for bacteremia caused by CNSAB, emphasizes the importance of strictly adhering to the ventilator and central venous catheter bundle in the ICU [31,32,33].
One of the clinical manifestations of A. baumannii infection is lower respiratory tract infection [17]. In our study, the most frequent source of infection from bacteremia due to A. baumannii was lower respiratory tract infection. Interestingly, the lower respiratory tract infection was more frequently the source of bacteremia due to CNSAB than CSAB and was an independent risk factor in multivariate analysis. Similar findings also have been reported previously [10,17]. These could provide insight to medical practitioners to be more aware of the possible CNSAB bacteremia among patients with lower respiratory tract infections.
In the present study, diabetes was found more common in bacteremia caused by CNSAB than CSAB. Diabetes is a significant public health concern globally, and Indonesia is the country with the fifth-most prevalent diabetes among individuals, with 19.5 million cases [34]. A study in Saudi Arabia found that A. baumannii isolated from diabetic patients harbored a significantly higher frequency to have carbapenem resistance [21]. Many studies have indicated that diabetic patients have a higher risk of antibiotic resistance than non-diabetic patients [18,19,20]. Due to diabetic-associated complications and decreased immunity, diabetic patients are more susceptible to repeated bacterial infections and antibiotic exposure [20], particularly in countries with relatively less tight antibiotic use, such as Indonesia. These could cause infection-associated bacteria in diabetic patients to be more resistant, including A. baumannii [20]. Therefore, blood sugar monitoring, diagnosis, and prompt and aggressive antibiotic therapy are critically needed in diabetic patients with infections, in line with the implementation of national antibiotic usage policies to reduce antibiotic resistance rates [20].
The prognosis of bacteremia patients caused by CNSAB depends on the severity of the disease and the patient’s immunological status. Previous studies found that high Pitt Bacteremia Score and Acute Physiology and Chronic Health Evaluation II (APACHE II), the presence of septic shock, multi-organ failure, pneumonia, ICU admission, use of ventilator and central venous catheter, increased leukocyte count, and low albumin levels were all associated with disease severity of CNSAB bacteremia [2,5,8,9,10,11,12,14,17]. Meanwhile, the patient’s immunological status factor included old age, the presence of comorbid factors such as liver disease, chronic kidney disease, hypertension, diabetes, malignancy, post-transplantation, and receiving immunosuppressant therapy [2,11,14,23]. In our study, some of those mentioned factors were more common in patients dying of CNSAB bacteremia; high Pitt Bacteremia Score and high Charlson Comorbidity Index (score ≥ 4) were identified as independent risk factors for mortality of CNSAB bacteremia in the final model. Pitt Bacteremia Score is easy to use. In addition, studies found that it could predict the clinical outcomes in bloodstream infections due to Pseudomonas aeruginosa, Enterobacter, Klebsiella, and multidrug-resistant A. baumannii [2,14]. These data suggest that the comorbidities of the patients with CNSAB infection must be assessed during infection to predict mortality risk. One simple way to assess the patient’s comorbidities is by using the Charlson Comorbidity Index, an instrument to measure the burden of comorbidity consisting of 19 categories related to chronic health problems [35]. Its calculation is only based on anamnesis, making it is relatively rapid, simple, and inexpensive.
Study Limitations
There are some limitations of this study that need to be discussed. The matching tolerance for the length of stay between case and control was quite large (up to 100 days) due to the limited number of eligible controls. This study did not assess the antibiotic therapy of the patients, either empirical or definitive, as a factor influencing the mortality of the patients. Several studies have demonstrated that inappropriate empiric antibiotic therapy is an independent factor influencing mortality of CNSAB bacteremia [2,8,11,16,23,24]. The history of antibiotic use was also not differentiated by class of antibiotics in the present study because the information on antibiotic types is unavailable. Studies have revealed that using meropenem was the most prevalent history of antibiotic use associated with the incidence of bacteremia caused by multidrug-resistant A. baumannii [9,12,13,14,15,17,23]. Furthermore, piperacillin/tazobactam, cefoperazone/sulbactam, fourth-generation cephalosporins, aminoglycosides, linezolid, and colistin are risk factors for multidrug-resistant A. baumannii [9,13,16,17,23]. Therefore, a prospective study assessing the association between the type of antibiotic use and the mortality of CNSAB bacteremia needs to be conducted in the near future.
5. Conclusions
Our data suggest that the infection source of lower respiratory tract infections and central venous catheter use are independent risk factors for CNSAB bacteremia. High Pitt Bacteremia Score and Charlson Comorbidity Index (score ≥ 4) became independent determinants for death due to bacteremia caused by CNSAB. The determinants are slightly different in CSAB bacteremia, of which only Pitt Bacteremia Score ≥ 4 was the independent risk factor for mortality. Understanding these risk variables is essential for determining empiric therapy and infection control preventive priorities and assessing the patient’s prognosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed7080161/s1, Table S1: Comparison of case distribution of carbapenem susceptible Acinetobacter baumannii (CSAB) and carbapenem non-susceptible Acinetobacter baumannii (CNSAB) based on site, island, and year of sampling.
Author Contributions
Conceptualization, D.A., D.S. and K.K.; methodology, D.A., D.S., U.H. and K.K.; software, N.J. and F.M.S.; validation, D.A., D.S., P.D.E., U.H. and K.K.; formal analysis, D.A., U.H. and K.K.; investigation, D.A., D.S. and P.D.E.; resources, D.A., U.H. and K.K.; data curation, D.A., D.S., P.D.E., N.J., F.M.S. and U.H.; writing—original draft preparation, D.A. and N.J.; writing—review and editing, D.A., D.S., P.D.E., N.J., F.M.S., U.H. and K.K.; supervision, U.H. and K.K.; project administration, D.A. and N.J.; funding acquisition, D.A., U.H. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Doctoral Dissertation Grant from the Ministry of Education and Culture of the Republic of Indonesia (contract no.: 010/E5/PG.02.00.PT/2022, sub-contract no.: 716/UN3.15/PT/2022).
Institutional Review Board Statement
The study protocol was reviewed and approved by the Ethics Unit of the Faculty of Medicine, Universitas Riau, No. B/133/UN19.5.1.1.8/UEPKK/2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
The underlying data of this study are available from the corresponding author on request.
Acknowledgments
We thank the members of the Indonesian Clinical Epidemiology Evidence-Based Medicine (ICE_EBM) network who collaborated on this study. We also thank the directors of the Arifin Achmad Hospital, Soetomo Hospital, and Saiful Anwar Hospital, who facilitated our work in these teaching hospitals. In addition, we thanks to Nadia, Dian Handayani, Rahmi Sagita, Budi Haryadi, Chyntia Isra, Deya Seisora, Inna Fairuuza Firdaus, Ega Rischella, Pristiawan Navy Endraputra, Diani Dwi Indrasari, Dewi Yana, and Nathanael Ibot David who have been involved in the data and isolate collection. Finally, the statistical analysis assistance of Hari Basuki is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nowak, P.; Paluchowska, P. Acinetobacter baumannii: Biology and drug resistance—Role of carbapenemases. Folia Histochem. Cytobiol. 2016, 54, 61–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.B.; Werlang, M.H.B.; Vandresen, D.F.; Fortes, P.C.N.; Pascotto, C.R.; Lúcio, L.C.; Ferreto, L.E.D. Genetic, antimicrobial resistance profile and mortality rates of Acinetobacter baumannii infection in Brazil: A systematic review. Narra J. 2022, 2, e68. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Tal-Jasper, R.; Katz, D.E.; Amrami, N.; Ravid, D.; Avivi, D.; Zaidenstein, R.; Lazarovitch, T.; Dadon, M.; Kaye, K.S.; Marchaim, D. Clinical and epidemiological significance of carbapenem resistance in Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2016, 60, 3127–3131. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhao, C.; Chen, H.; Li, H.; Wang, H.; Cares Network, O. Prospective multi-center evaluation on risk factors, clinical characteristics and outcomes due to carbapenem resistance in Acinetobacter baumannii complex bacteraemia: Experience from the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) Network. J. Med. Microbiol. 2020, 69, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.H.; Liao, C.H.; Lauderdale, T.L.; Ko, W.C.; Chen, Y.S.; Liu, J.W.; Lau, Y.J.; Wang, L.H.; Liu, K.S.; Tsai, T.Y.; et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int. J. Infect. Dis. 2010, 14, e764-9. [Google Scholar] [CrossRef] [PubMed]
- Metan, G.; Sariguzel, F.; Sumerkan, B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur. J. Intern. Med. 2009, 20, 540–544. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.I.; Hong, K.W.; Kim, Y.R.; Park, Y.J.; Kang, M.W. Risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: Impact of appropriate antimicrobial therapy. J. Korean Med. Sci. 2012, 27, 471–475. [Google Scholar] [CrossRef]
- Guo, N.; Xue, W.; Tang, D.; Ding, J.; Zhao, B. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am. J. Infect. Control 2016, 44, e37–e39. [Google Scholar] [CrossRef]
- Lemos, E.V.; de la Hoz, F.P.; Einarson, T.R.; McGhan, W.F.; Quevedo, E.; Castaneda, C.; Kawai, K. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2014, 20, 416–423. [Google Scholar] [CrossRef]
- Piperaki, E.T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Anggraini, D.; Santosaningsih, D.; Dwi Endraswari, P.; Moehario, L.; Riezke, C.V.; Enty, E.; Marindra, F.; Verbrugh, H.A. Epidemiology study of Acinetobacter spp. isolated from blood culture in Indonesia. Int. J. Infect. Dis. 2020, 101, 62–63. [Google Scholar] [CrossRef]
- Anggraini, D.; Kuntaman, K.; Karuniawati, A.; Santosaningsih, D.; Saptawati, L.; Cahyarini; Puspandari, N.; Haryadi, B. Surveilans Resistansi Antibiotik Rumah Sakit Kelas Dan B Indonesia Tahun 2020; Puspandari, N., Ed.; Deepublish: Yogyakarta, Indoneisa, 2020. [Google Scholar]
- Ng, T.M.; Teng, C.B.; Lye, D.C.; Apisarnthanarak, A. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect. Control Hosp. Epidemiol. 2014, 35, 49–55. [Google Scholar] [CrossRef]
- Smolyakov, R.; Borer, A.; Riesenberg, K.; Schlaeffer, F.; Alkan, M.; Porath, A.; Rimar, D.; Almog, Y.; Gilad, J. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: Risk factors and outcome with ampicillin-sulbactam treatment. J. Hosp. Infect. 2003, 54, 32–38. [Google Scholar] [CrossRef]
- Du, X.; Xu, X.; Yao, J.; Deng, K.; Chen, S.; Shen, Z.; Yang, L.; Feng, G. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Chopra, T.; Marchaim, D.; Awali, R.A.; Krishna, A.; Johnson, P.; Tansek, R.; Chaudary, K.; Lephart, P.; Slim, J.; Hothi, J.; et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob. Agents Chemother. 2013, 57, 6270–6275. [Google Scholar] [CrossRef]
- Routsi, C.; Pratikaki, M.; Platsouka, E.; Sotiropoulou, C.; Nanas, S.; Markaki, V.; Vrettou, C.; Paniara, O.; Giamarellou, H.; Roussos, C. Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: Risk factors, clinical features and outcomes. Infection 2010, 38, 173–180. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Du, X.; Li, W.; Zhong, T.; Tang, Y.; Feng, Y.; Tao, C.; Xie, Y. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter Baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS ONE 2015, 10, e0130701. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Chiu, S.K.; Hsueh, P.R.; Wang, N.C.; Wang, C.C.; Fang, C.T. Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: A case-control study. PLoS ONE 2014, 9, e85973. [Google Scholar] [CrossRef]
- Kumar, A.; Randhawa, V.S.; Nirupam, N.; Rai, Y.; Saili, A. Risk factors for carbapenem-resistant Acinetobacter baumanii blood stream infections in a neonatal intensive care unit, Delhi, India. J. Infect. Dev. Ctries. 2014, 8, 1049–1054. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jung, J.Y.; Kang, Y.A.; Lim, J.E.; Kim, E.Y.; Lee, S.K.; Park, S.C.; Chung, K.S.; Park, B.H.; Kim, Y.S.; et al. Risk factors for occurrence and 30-day mortality for carbapenem-resistant Acinetobacter baumannii bacteremia in an intensive care unit. J. Korean Med. Sci. 2012, 27, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Xiao, T.; Guo, L.; Yu, W.; Chen, Y.; Zheng, B.; Huang, C.; Yu, X.; Xiao, Y. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: Cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect. Drug Resist. 2018, 11, 2021–2030. [Google Scholar] [CrossRef]
- Anggraini, D.; Santosaningsih, D.; Saharman, Y.R.; Endraswari, P.D.; Cahyarini, C.; Saptawati, L.; Hayati, Z.; Farida, H.; Siregar, C.; Pasaribu, M. Distribution of carbapenemase genes among carbapenem-non-susceptible Acinetobacter baumanii blood isolates in Indonesia: A Multicenter Study. Antibiotics 2022, 11, 366. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS): Guide to Enrolment for Antimicrobial Resistance National Focal Points. Available online: https://apps.who.int/iris/handle/10665/251556 (accessed on 27 May 2022).
- Paterson, D.L.; Ko, W.C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 2004, 140, 26–32. [Google Scholar] [CrossRef]
- Henderson, H.; Luterbach, C.L.; Cober, E.; Richter, S.S.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; Kaye, K.S.; Evans, S. The Pitt bacteremia score predicts mortality in nonbacteremic infections. Clin. Infect. Dis. 2020, 70, 1826–1833. [Google Scholar] [CrossRef]
- Oltean, S.; Ţăţulescu, D.; Bondor, C.; Slavcovici, A.; Cismaru, C.; Lupşe, M.; Muntean, M.; Jianu, C.; Marcu, C.; Oltean, M. Charlson’s weighted index of comorbidities is useful in assessing the risk of death in septic patients. J. Crit. Care 2012, 27, 370–375. [Google Scholar] [CrossRef]
- Johnson, S.W.; Anderson, D.J.; May, D.B.; Drew, R.H. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum β-lactamase-producing enterobacteriaceae on hospital admission. Infect. Control Hosp. Epidemiol. 2013, 34, 385–392. [Google Scholar] [CrossRef]
- Klompas, M.; Branson, R.; Eichenwald, E.C.; Greene, L.R.; Howell, M.D.; Lee, G.; Magill, S.S.; Maragakis, L.L.; Priebe, G.P.; Speck, K.; et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect. Control. Hosp. Epidemiol. 2014, 35 (Suppl. 2), S133–S154. [Google Scholar] [CrossRef][Green Version]
- Marschall, J.; Mermel, L.A.; Fakih, M.; Hadaway, L.; Kallen, A.; O’Grady, N.P.; Pettis, A.M.; Rupp, M.E.; Sandora, T.; Maragakis, L.L.; et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35 (Suppl. 2), S89–S107. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Hampshire, P.A.; Guha, A.; Strong, A.; Parsons, D.; Rowan, P. An evaluation of the Charlson co-morbidity score for predicting sepsis after elective major surgery. Indian J. Crit. Care Med. 2011, 15, 30–36. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).