Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren
Abstract
1. Introduction
2. Materials and Methods
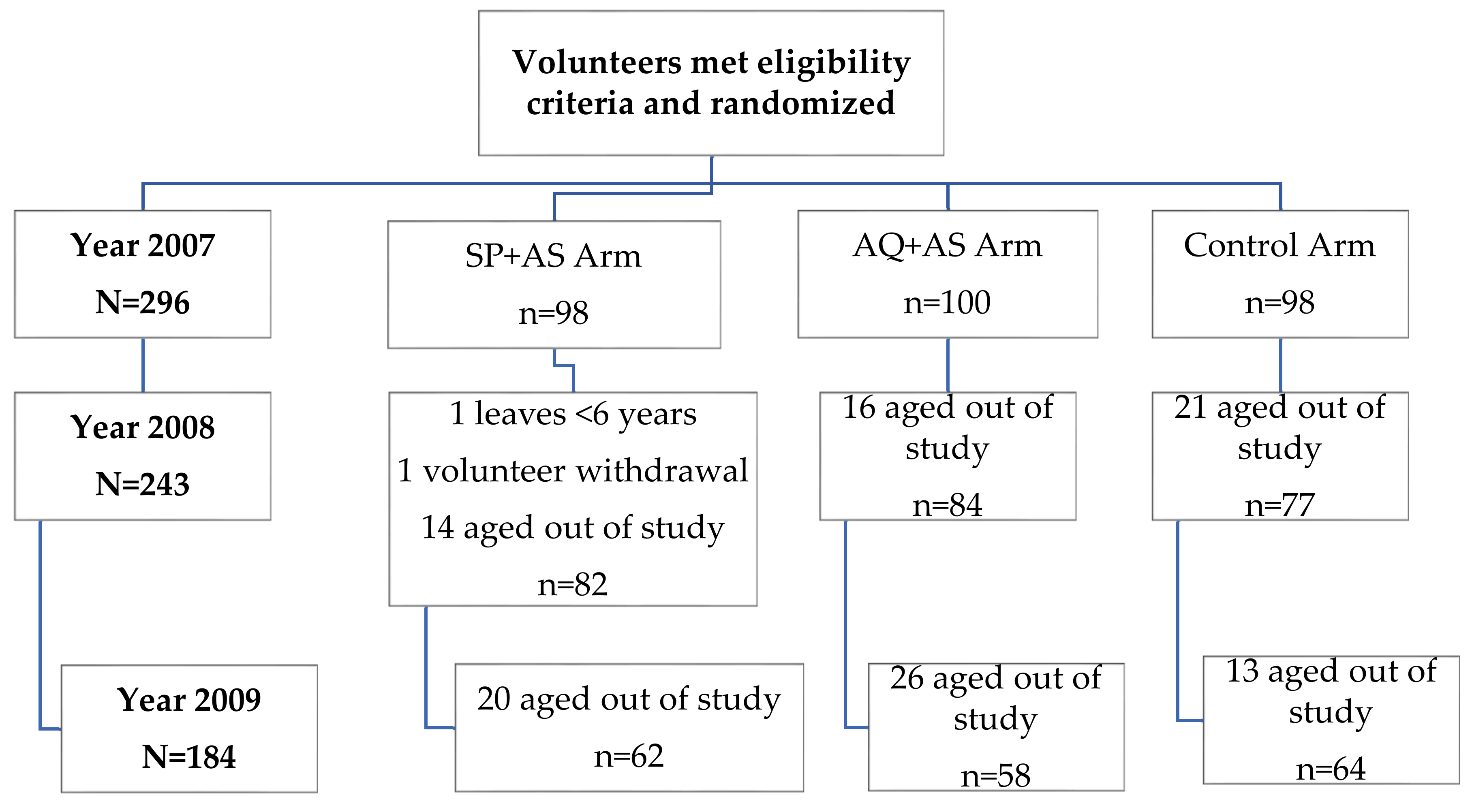
2.1. Participant Flow
2.2. Baseline Data
2.3. Clinical Illness
2.4. Parasitemia
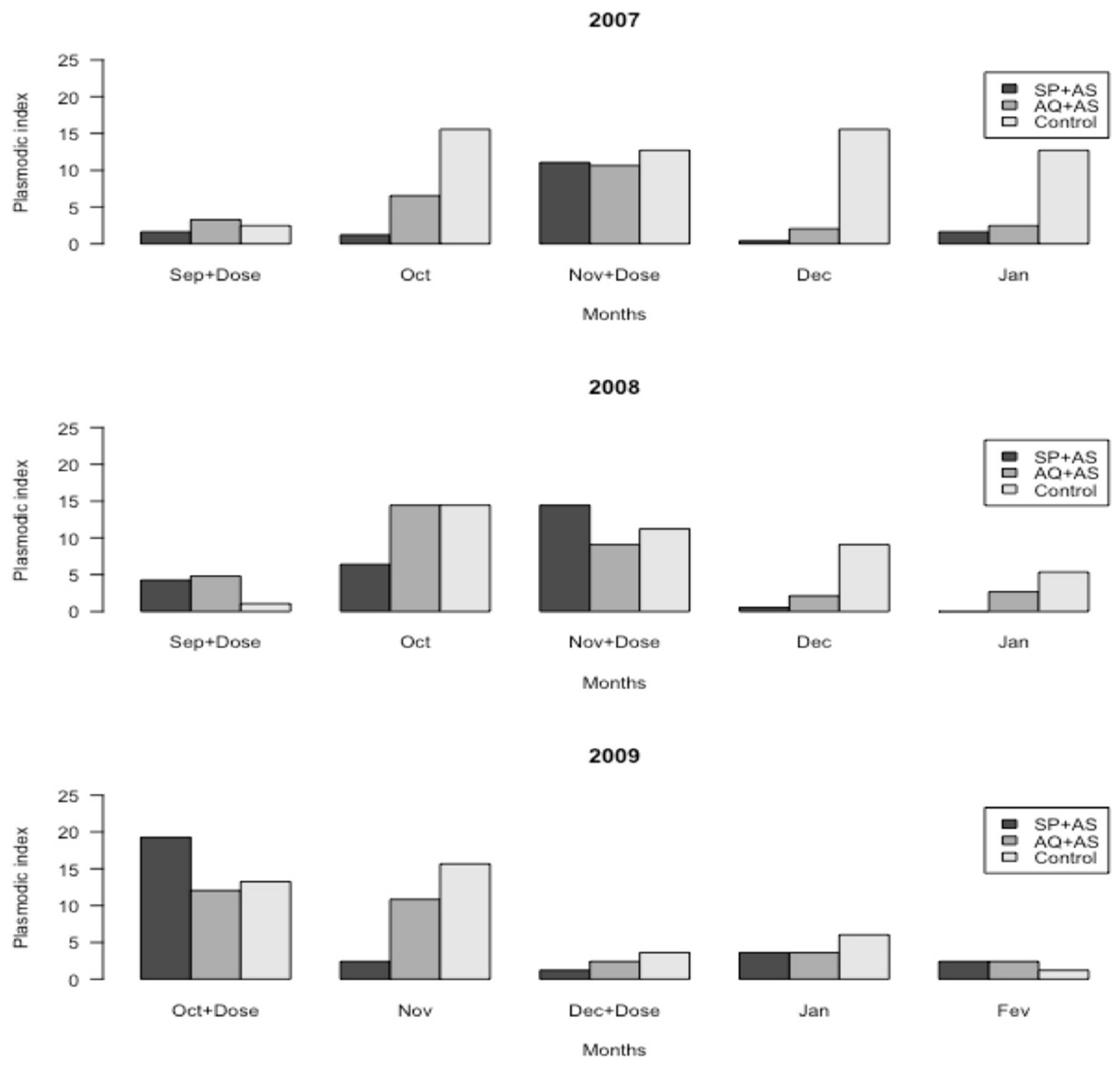
2.5. Plasmodic Index
2.6. Anemia
2.7. Hemoglobin Concentration
2.8. School Performance
2.9. Adverse Events
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meremikwu, M.M.; Donegan, S.; Sinclair, D.; Esu, E.; Oringanje, C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst. Rev. 2012, CD003756. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Taskforce, I.P. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS ONE 2011, 6, e16976. [Google Scholar] [CrossRef] [PubMed]
- WHO. Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium Falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-Region in Africa; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Barger, B.; Maiga, H.; Traore, O.B.; Tekete, M.; Tembine, I.; Dara, A.; Traore, Z.I.; Gantt, S.; Doumbo, O.K.; Djimde, A.A. Intermittent preventive treatment using artemisinin-based combination therapy reduces malaria morbidity among school-aged children in Mali. Trop. Med. Int. Health 2009, 14, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Gosling, R.D.; Cairns, M.E.; Chico, R.M. Chandramohan D: Intermittent preventive treatment against malaria: An update. Expert Rev. Anti Infect Ther. 2010, 8, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.; Bojang, K.; Tagbor, H.; Pagnoni, F. Combining community case management and intermittent preventive treatment for malaria. Trends Parasitol. 2011, 27, 477–480. [Google Scholar] [CrossRef]
- Dicko, A.; Barry, A.; Dicko, M.; Diallo, A.I.; Tembine, I.; Dicko, Y.; Dara, N.; Sidibe, Y.; Santara, G.; Conare, T.; et al. Malaria morbidity in children in the year after they had received intermittent preventive treatment of malaria in Mali: A randomized control trial. PLoS ONE 2011, 6, e23390. [Google Scholar] [CrossRef]
- Konate, A.T.; Yaro, J.B.; Ouedraogo, A.Z.; Diarra, A.; Gansane, A.; Soulama, I.; Kangoye, D.T.; Kabore, Y.; Ouedraogo, E.; Ouedraogo, A.; et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: A randomised, double-blind, placebo-controlled trial. PLoS Med. 2011, 8, e1000408. [Google Scholar] [CrossRef]
- Zongo, I.; Milligan, P.; Compaore, Y.D.; Some, A.F.; Greenwood, B.; Tarning, J.; Rosenthal, P.J.; Sutherland, C.; Nosten, F.; Ouedraogo, J.B. Randomized Noninferiority Trial of Dihydroartemisinin-Piperaquine Compared with Sulfadoxine-Pyrimethamine plus Amodiaquine for Seasonal Malaria Chemoprevention in Burkina Faso. Antimicrob. Agents Chemother. 2015, 59, 4387–4396. [Google Scholar] [CrossRef]
- Athuman, M.; Kabanywanyi, A.M.; Rohwer, A.C. Intermittent preventive antimalarial treatment for children with anaemia. Cochrane Database Syst. Rev. 2015, 1, CD010767. [Google Scholar] [CrossRef]
- Plowe, C.V.; Djimde, A.; Wellems, T.E.; Diop, S.; Kouriba, B.; Doumbo, O.K. Community pyrimethamine-sulfadoxine use and prevalence of resistant Plasmodium falciparum genotypes in Mali: A model for deterring resistance. Am. J. Trop. Med. Hyg. 1996, 55, 467–471. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Greenwood, B. Intermittent preventive antimalarial treatment in infants. Clin. Infect Dis. 2007, 45, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Dicko, A.; Sagara, I.; Sissoko, M.S.; Guindo, O.; Diallo, A.I.; Kone, M.; Toure, O.B.; Sacko, M.; Doumbo, O.K. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children in Mali. Malar. J. 2008, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.E.; Jukes, M.C.; Njagi, J.K.; Khasakhala, L.; Cundill, B.; Otido, J.; Crudder, C.; Estambale, B.B.; Brooker, S. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: A cluster-randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 127–138. [Google Scholar] [CrossRef]
- Nankabirwa, J.I.; Wandera, B.; Amuge, P.; Kiwanuka, N.; Dorsey, G.; Rosenthal, P.J.; Brooker, S.J.; Staedke, S.G.; Kamya, M.R. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: A randomized, placebo-controlled trial. Clin. Infect Dis. 2014, 58, 1404–1412. [Google Scholar] [CrossRef]
- Cairns, M.; Carneiro, I.; Milligan, P.; Owusu-Agyei, S.; Awine, T.; Gosling, R.; Greenwood, B.; Chandramohan, D. Duration of protection against malaria and anaemia provided by intermittent preventive treatment in infants in Navrongo, Ghana. PLoS ONE 2008, 3, e2227. [Google Scholar] [CrossRef]
- Sanjeev, K.; White, N.J. Pharmacokinetics of Quinine, Chloroquine and Amodiaquine. Clin. Pharm. 1996, 30, 263–299. [Google Scholar]
- De Radigues, X.; Diallo, K.I.; Diallo, M.; Ngwakum, P.A.; Maiga, H.; Djimde, A.; Sacko, M.; Doumbo, O.; Guthmann, J.P. Efficacy of chloroquine and sulfadoxine/pyrimethamine for the treatment of uncomplicated falciparum malaria in Koumantou, Mali. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1013–1018. [Google Scholar] [CrossRef][Green Version]
- Tekete, M.; Djimde, A.A.; Beavogui, A.H.; Maiga, H.; Sagara, I.; Fofana, B.; Ouologuem, D.; Dama, S.; Kone, A.; Dembele, D.; et al. Efficacy of chloroquine, amodiaquine and sulphadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria: Revisiting molecular markers in an area of emerging AQ and SP resistance in Mali. Malar. J. 2009, 8, 34. [Google Scholar] [CrossRef]
- Maiga, H.; Djimde, A.A.; Beavogui, A.H.; Toure, O.; Tekete, M.; Sangare, C.P.; Dara, A.; Traore, Z.I.; Traore, O.B.; Dama, S.; et al. Efficacy of sulphadoxine-pyrimethamine + artesunate, sulphadoxine-pyrimethamine + amodiaquine, and sulphadoxine-pyrimethamine alone in uncomplicated falciparum malaria in Mali. Malar. J. 2015, 14, 64. [Google Scholar] [CrossRef]
- Laufer, M.K.; Djimde, A.A.; Plowe, C.V. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am. J. Trop. Med. Hyg. 2007, 77, 160–169. [Google Scholar] [CrossRef]
- Talisuna, A.O.; Okello, P.E.; Erhart, A.; Coosemans, M.; D’Alessandro, U. Intensity of malaria transmission and the spread of Plasmodium falciparum resistant malaria: A review of epidemiologic field evidence. Am. J. Trop. Med. Hyg. 2007, 77, 170–180. [Google Scholar] [PubMed]
- Sokhna, C.; Cisse, B.; Ba el, H.; Milligan, P.; Hallett, R.; Sutherland, C.; Gaye, O.; Boulanger, D.; Simondon, K.; Simondon, F.; et al. A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children. PLoS ONE 2008, 3, e1471. [Google Scholar] [CrossRef] [PubMed]
- Maiga, H.; Lasry, E.; Diarra, M.; Sagara, I.; Bamadio, A.; Traore, A.; Coumare, S.; Bahonan, S.; Sangare, B.; Dicko, Y.; et al. Seasonal Malaria Chemoprevention with Sulphadoxine-Pyrimethamine and Amodiaquine Selects Pfdhfr-dhps Quintuple Mutant Genotype in Mali. PLoS ONE 2016, 11, e0162718. [Google Scholar] [CrossRef]
- Wongsrichanalai, C.; Meshnick, S.R. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg. Infect Dis. 2008, 14, 716–719. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Takala-Harrison, S.; Jacob, C.G.; Arze, C.; Cummings, M.P.; Silva, J.C.; Dondorp, A.M.; Fukuda, M.M.; Hien, T.T.; Mayxay, M.; Noedl, H.; et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J. Infect Dis. 2015, 211, 670–679. [Google Scholar] [CrossRef]
- Fernando, S.D.; Gunawardena, D.M.; Bandara, M.R.; De Silva, D.; Carter, R.; Mendis, K.N.; Wickremasinghe, A.R. The impact of repeated malaria attacks on the school performance of children. Am. J. Trop. Med. Hyg. 2003, 69, 582–588. [Google Scholar] [CrossRef]
- Ter Kuile, F.O.; Terlouw, D.J.; Phillips-Howard, P.A.; Hawley, W.A.; Friedman, J.F.; Kolczak, M.S.; Kariuki, S.K.; Shi, Y.P.; Kwena, A.M.; Vulule, J.M.; et al. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: Cross-sectional survey. Am. J. Trop. Med. Hyg. 2003, 68, 100–107. [Google Scholar] [CrossRef]
- Phillips-Howard, P.A.; Nahlen, B.L.; Kolczak, M.S.; Hightower, A.W.; ter Kuile, F.O.; Alaii, J.A.; Gimnig, J.E.; Arudo, J.; Vulule, J.M.; Odhacha, A.; et al. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 23–29. [Google Scholar] [CrossRef]
- Van Dyk, J.C.; Bouwman, H.; Barnhoorn, I.E.; Bornman, M.S. DDT contamination from indoor residual spraying for malaria control. Sci. Total Environ. 2010, 408, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Treatment Arms | ||
|---|---|---|---|
| SP+AS (N = 98) | AQ+AS (N = 100) | Control (N = 98) | |
| Mean age, years (range) | 10 (6 – 13) | 10 (6 – 13) | 10 (7 – 13) |
| Percent female % | 36.3 | 46.6 | 42.9 |
| Mean weight, kg (range) | 27 (12–55) | 26 (15–55) | 28 (16–48) |
| Mean hemoglobin, g/dL (SD) | 11.4 (+/−1.4) | 11.3 (+/−1.5) | 11.2 (+/−1.4) |
| Geometric mean parasites/µL (SD) | 2107 (+/−5334) | 2627(+/−8123) | 2407(+/−2073) |
| Axillary temperature (°C) | 36.5 | 36.6 | 36.5 |
| Treatment Arms | ||||||
|---|---|---|---|---|---|---|
| SP+AS n (%) | p-Value | AQ+AS n (%) | p-Value | Control n (%) | ||
| 2007 | All-cause clinic visits | 29 (20.1) | <0.001 | 47 (32.6) | 0.04 | 68 (47.2) |
| Malaria cases | 18 (12.5) | <0.001 | 29 (20.1) | 0.009 | 52 (36.1) | |
| 2008 | All-cause clinic visits | 20 (21.7) | 0.005 | 31 (33.7) | 0.14 | 41 (44.6) |
| Malaria cases | 10 (10.9) | 0.98 | 15 (16.3) | 0.63 | 11 (12.0) | |
| 2009 | All-cause clinic visits | 14 (21.2) | 0.02 | 22 (33.7) | 0.56 | 30 (44.6) |
| Malaria cases | 4 (6.1) | 1 | 7 (10.6) | 0.36 | 4 (6.1) | |
| Treatment Arms | ||||||
|---|---|---|---|---|---|---|
| SP+AS | p-Value | AQ+AS | p-Value | Control | ||
| 2007 | n (%) | 116 (29.9) | <0.001 | 122 (30.6) | <0.001 | 162 (42.1) |
| 2008 | n (%) | 80 (29.1) | <0.001 | 100 (35.5) | 0.02 | 122 (44.6) |
| 2009 | n (%) | 29 (13.4) | 0.001 | 36 (18.8) | 0.12 | 55 (25.3) |
| Treatment Arms | Markers | 2007−2008 | 2008−2009 | 2009−2010 |
|---|---|---|---|---|
| SP + AS | Grade average | 5.48 | 4.84 | 5.00 |
| 95% CI | (4.93–6.03) | (45–5.24) | (4.71–5.27) | |
| n | 23 | 21 | 19 | |
| p-value Success (%) | 0.49 91 | 0.41 95 | 0.38 79 | |
| AQ + AS | Grade average | 5.49 | 4.66 | 4.93 |
| 95% CI | (5.07–5.91) | (4.26–5.05) | (4.53–5.33) | |
| n | 22 | 22 | 18 | |
| p-value Success (%) | 0.41 91 | 0.64 86 | 0.57 78 | |
| Control | Grade average | 5.00 | 4.40 | 4.65 |
| 95% CI | (4.50–5.49) | (4.00–4.81) | (4.30–5.00) | |
| n Success (%) | 15 87 | 13 85 | 14 71 |
| Treatment Arms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Events | SP + AS | AQ + AS | Control | |||||
| n | (%) | p-Value | n | (%) | p-Value | n | (%) | |
| Fever | 25 | 1.2 | 0.001 | 53 | 2.5 | 0.77 | 54 | 2.7 |
| Headache | 54 | 2.6 | 0.04 | 79 | 3.8 | 0.97 | 77 | 3.8 |
| Vomiting | 24 | 1.2 | 0.15 | 35 | 1.7 | 0.89 | 35 | 1.7 |
| Abdominal pain | 5 | 1.3 | 0.08 | 14 | 1.7 | 0.45 | 12 | 2.0 |
| Coughing | 28 | 1.4 | <0.001 | 5 | 0.2 | 0.42 | 9 | 0.5 |
| Diarrhea | 5 | 0.3 | 0.12 | 14 | 0.7 | 0.72 | 12 | 0.6 |
| Pruritis | 2 | 0.1 | 1 | 2 | 0.1 | 1 | 1 | 0.05 |
| Lethargic | 0 | 0 | - | 1 | 0.04 | - | 0 | 0 |
| Convulsion | 0 | 0 | - | 0 | 0 | - | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiga, H.; Barger, B.; Sagara, I.; Guindo, A.; Traore, O.B.; Tekete, M.; Dara, A.; Traore, Z.I.; Diarra, M.; Coumare, S.; et al. Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren. Trop. Med. Infect. Dis. 2020, 5, 148. https://doi.org/10.3390/tropicalmed5030148
Maiga H, Barger B, Sagara I, Guindo A, Traore OB, Tekete M, Dara A, Traore ZI, Diarra M, Coumare S, et al. Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren. Tropical Medicine and Infectious Disease. 2020; 5(3):148. https://doi.org/10.3390/tropicalmed5030148
Chicago/Turabian StyleMaiga, Hamma, Breanna Barger, Issaka Sagara, Abdoulaye Guindo, Oumar B. Traore, Mamadou Tekete, Antoine Dara, Zoumana I. Traore, Modibo Diarra, Samba Coumare, and et al. 2020. "Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren" Tropical Medicine and Infectious Disease 5, no. 3: 148. https://doi.org/10.3390/tropicalmed5030148
APA StyleMaiga, H., Barger, B., Sagara, I., Guindo, A., Traore, O. B., Tekete, M., Dara, A., Traore, Z. I., Diarra, M., Coumare, S., Kodio, A., Toure, O. B., Doumbo, O. K., & Djimde, A. A. (2020). Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren. Tropical Medicine and Infectious Disease, 5(3), 148. https://doi.org/10.3390/tropicalmed5030148









