Does Drug-Resistant Extrapulmonary Tuberculosis Hinder TB Elimination Plans? A Case from Delhi, India
Abstract
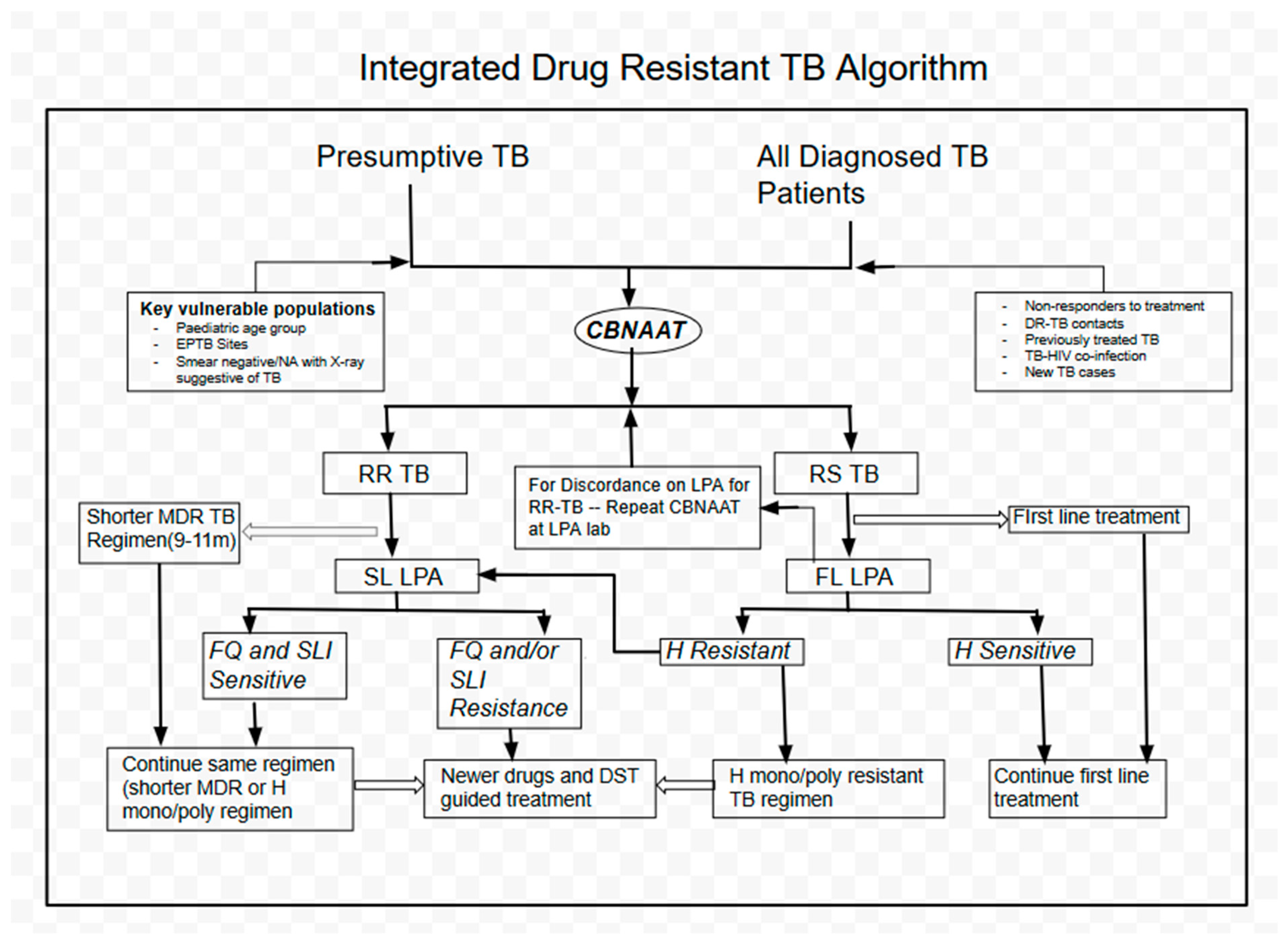
1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
General Setting
- Cure: Treatment completed as recommended by the national policy without evidence of failure, and three or more consecutive cultures taken at least 30 days apart during CP are negative, including culture at the end of treatment.
- Treatment completed: Treatment completed as recommended by the national policy without evidence of failure, but no record that three or more consecutive cultures taken at least 30 days apart are negative after the intensive phase.
- Treatment success: This is a combination of cure plus treatment completed.
- Treatment failure: Treatment terminated or a need for permanent regimen change of at least two or more anti-TB drugs in CP because of the lack of microbiological conversion by the end of the extended intensive phase or microbiological reversion in the continuation phase after conversion to negative or evidence of additional acquired resistance to FQ or SLI drugs or adverse drug reactions (ADR).
- Death: A patient who dies for any reason during the course of treatment.
- Treatment lost-to-follow-up: A patient whose treatment was interrupted for one month or more for any reasons prior to being declared as failed.
- Not evaluated: A patient for whom no treatment outcome is assigned.
- Regimen changed: A TB patient’s need for permanent regimen change of at least one or more anti-TB drugs prior to being declared as failed.
- Treatment stopped due to adverse drug reactions: A patient who develops adverse drug reactions and cannot continue the M/XDR-TB treatment in spite of the management of adverse drug reactions as per the defined protocols and a decision has been taken by the DR-TB Centre committee to stop treatment.
2.3. Study Site
2.4. Study Population
2.4.1. Data Variables, Sources of Data and Data Collection
2.4.2. Data Analysis and Statistics
3. Ethics Approval
4. Results
4.1. Patient Characteristics
4.2. Adverse Drug Reactions (ADRs)
4.3. Delay in Treatment Initiation
4.4. Treatment Outcomes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2019 [Internet]. [Cited 8 March 2020]. Available online: https://www.who.int/tb/global-report-2019 (accessed on 28 May 2020).
- Bastos, M.L.; Hussain, H.; Weyer, K.; Garcia-Garcia, L.; Leimane, V.; Leung, C.C.; Narita, M.; Penã, J.M.; Ponce-de-Leon, A.; Seung, J.K.; et al. Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: An individual patient data meta-analysis. Clin. Infect. Dis. 2014, 59, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.D.; Ashkin, D.; Avendano, M.; Banerjee, R.; Bauer, M.; Bayona, J.N.; Becerra, M.C.; Benedetti, A.; Burgos, M.; Centis, R.; et al. Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients. PLoS Med. 2012, 9, e1001300. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahuja, S.D.; Akkerman, O.W.; Alffenaar, J.-W.C.; Anderson, L.F.; Baghaei, P.; Bang, D.; Barry, P.M.; Bastos, M.L.; Behera, D.; et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet 2018, 392, 821–834. [Google Scholar] [CrossRef]
- Parmar, M.; Sachdeva, K.S.; Dewan, P.K.; Rade, K.; Nair, S.A.; Pant, R.; Khaparde, S.D. Unacceptable treatment outcomes and associated factors among India’s initial cohorts of multidrug-resistant tuberculosis (MDR-TB) patients under the revised national TB control programme (2007–2011): Evidence leading to policy enhancement. PLoS ONE 2018, 13, e0193903. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multi-Drug Resistant Tuberculosis (MDR-TB) 2018 Update [Internet]. World Health Organization (WHO), 2020. [Cited 20 May 2020]. Available online: https://www.who.int/tb/areas-of-work/drug-resistant-tb/MDR-RR_TB_factsheet_2018_Apr2019.pdf?ua=1 (accessed on 28 May 2020).
- Central TB Division. National Strategic Plan for Tuberculosis Elimination 2017–2025; Ministry of Health with Family Welfare: New Delhi, India, 2017. [Google Scholar]
- Central TB Division Directorate General of Health Services. India TB Report 2018 Revised National TB Control Programme; Ministry of Health with Family Welfare: New Delhi, India, 2018. [Google Scholar]
- Nair, D.; Velayutham, B.; Kannan, T.; Tripathy, J.P.; Harries, A.D.; Natrajan, M.; Swaminathan, S. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. Public Heal. Action 2017, 7, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.L.; Shewade, H.D.; Nagaraja, S.B.; Nair, S.A.; Parmar, M. Unfavourable outcomes among patients with MDR-TB on the standard 24-month regimen in Maharashtra, India. Public Health Action 2017, 7, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Jain, A. Epidemiological perspective of drug resistant extrapulmonary tuberculosis. World J. Clin. Infect. Dis. 2015, 5, 77. [Google Scholar] [CrossRef]
- Kant, S.; Maurya, A.K.; Nag, V.L.; Bajpai, J. Rising trend of drug resistance among extra pulmonary TB in Northern India. Tuberculosis 2018, 52, PA3681. [Google Scholar] [CrossRef]
- Vadwai, V.; Shetty, A.; Supply, P.J.; Rodrigues, C. Evaluation of 24-locus MIRU-VNTR in extrapulmonary specimens: Study from a tertiary centre in Mumbai. Tuberculosis 2012, 92, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Dusthackeer, A.; Sekar, G.; Chidambaram, S.; Kumar, V.; Mehta, P. Drug resistance among extrapulmonary TB patients: Six years experience from a supranational reference laboratory. Indian J. Med Res. 2015, 142, 568–574. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Density of Population Census 2011 [Internet]. [Cited 20 May 2020]. Available online: https://censusindia.gov.in/2011-prov-results/data_files/india/Final_PPT_2011chapter7.pdf (accessed on 28 May 2020).
- Central TB Division Ministry of Health and Family Welfare. India TB Report 2019 Revised National TB Control Programme Annual Report; Ministry of Health and Family Welfare: New Delhi, India, 2019. [Google Scholar]
- Central TB Division Ministry of Health and Family Welfare. Guidelines for Programmatic Management of Drug Resistant Tuberculosis in India 2017 [Internet]. 2017. Available online: https://tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=4780&lid=3306 (accessed on 28 May 2020).
- Lee, J.Y. Diagnosis and Treatment of Extrapulmonary Tuberculosis. Tuberc. Respir. Dis. 2015, 78, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Central TB Division Ministry of Health and Family Welfare. TB India 2017 Revised National Tuberculosis Control Programme Annual Status Report [Internet]. Available online: https://tbcindia.gov.in/index1.php?lang=1&level=1&sublinkid=4160&lid=2807 (accessed on 28 May 2020).
- Pang, Y.; An, J.; Shu, W.; Huo, F.; Chu, N.; Gao, M.; Qin, S.; Huang, H.; Chen, X.; Xu, S. Epidemiology of Extrapulmonary Tuberculosis among Inpatients, China, 2008–2017. Emerg. Infect. Dis. 2019, 25, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.K.; Rajnish, G. Trends of extra-pulmonary tuberculosis under Revised National Tuberculosis Control Programme: A study from South Delhi. Indian J. Tuberc. 2006, 53, 77–83. [Google Scholar]
- Joshi, J.M.; Desai, U. Extrapulmonary drug-resistant tuberculosis at a drug-resistant tuberculosis center, Mumbai: Our experience – Hope in the midst of despair! Lung India 2019, 36, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Tuli, S.; Jvn, J.; Gupta, N.; Raju, S.; Indrani, T.; Padhiary, K.; Madala, B. Tuberculosis of the Skeletal System; Jaypee Brothers Medical Publishing: New Delhi, India, 2004. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, A.; Maug, A.K.J.; Salim, A.H.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, Highly Effective, and Inexpensive Standardized Treatment of Multidrug-resistant Tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Updates Its Treatment Guidelines for Multidrug- and Rifampicin-Resistant Tuberculosis; WHO: Geneva, Switzerland, 2019. [Google Scholar]
| Characteristics | Number | (%) | |
|---|---|---|---|
| Sex | Male | 92 | (45.3) |
| Female | 111 | (54.7) | |
| Age (years) | < 15 | 50 | (24.6) |
| 15–44 | 147 | (72.4) | |
| 45 and above | 06 | (3.0) | |
| Weight (in kilograms) | < 30 | 41 | (20.2) |
| 31–50 | 93 | (45.8) | |
| Above 50 | 69 | (34.0) | |
| DR-TB Centre | Centre 1 | 56 | (27.6) |
| Centre 2 | 59 | (29.1) | |
| Centre 3 | 88 | (43.3) | |
| Site of disease | Peripheral lymph node | 108 | (53.2) |
| Deep lymph node | 10 | (4.9) | |
| Brain | 09 | (4.4) | |
| Bone | 69 | (34.0) | |
| Pleural effusion | 19 | (9.4) | |
| Abdomen | 10 | (4.9) | |
| Heart | 0 | (0) | |
| Genital | 02 | (1.0) | |
| Basis of diagnosis | CBNAAT | 173 | (85.2) |
| Culture | 2 | (1.0) | |
| Line Probe Assay | 20 | (9.9) | |
| Clinical | 8 | (3.9) | |
| Drug Resistance | HR | 19 | (9.4) |
| Rifampicin | 195 | (96.1) | |
| Fluoroquinolone | 05 | (2.5) | |
| Other injectables | 02 | (1.0) | |
| History of TB | Yes | 178 | 87.7 |
| No | 24 | 11.8 | |
| Not recorded | 01 | (0.5) | |
| HIV status | Negative | 200 | (98.5) |
| Positive | 03 | (1.5) | |
| Diabetes | Yes | 05 | (2.5) |
| No | 198 | (97.5) | |
| Severe adverse reaction | Yes | 58 | (28.6) |
| No | 145 | (71.4) |
| Adverse Drug Reactions | Number | (%) |
|---|---|---|
| Severe vomiting | 26 | 12.8 |
| Behavior disorder | 15 | 7.4 |
| Hearing loss | 7 | 3.4 |
| Severe joint pain | 21 | 10.3 |
| Renal disturbance | 03 | 1.5 |
| Allergic reaction | 04 | 2.0 |
| Recurrent hepatitis | 01 | 0.5 |
| Gynaecomastia | 02 | 1.0 |
| Thyroid disturbance | 02 | 1.0 |
| Peripheral neuropathy | 01 | 0.5 |
| Vision loss | 01 | 0.5 |
| Others | 02 | 1.0 |
| Adverse Drug Reactions | Number | (%) |
|---|---|---|
| Treatment success | 134 | 66.0 |
| Unfavourable treatment outcome | 69 | 34.0 |
| Death | 14 | 6.9 |
| Treatment failure | 1 | 0.5 |
| Lost to follow up | 40 | 19.7 |
| Regimen changed | 3 | 1.5 |
| Treatment stopped d/t ADR | 0 | 0.0 |
| Not evaluated | 11 | 5.4 |
| Variables | Treatment Outcome | RR (95% CI) | p-Value | aRR (95% CI) | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Total | Unfavourable | |||||||
| N | n | % | ||||||
| Sex | Male | 92 | 35 | (38.0) | 1.2 (0.8–1.8) | 0.2 | 1.3 (0.9–1.9) | 0.13 |
| Female | 111 | 34 | (30.6) | 1.0 | 1.0 | |||
| Age (years) | ≥ 15 years | 153 | 57 | (37.3) | 1.6 (0.9–2.7) | 0.08 | 1.6 (0.8–3.0) | 0.14 |
| < 15 years | 50 | 12 | (24.0) | 1.0 | 1.0 | |||
| Weight (in Kilograms) | < 30 | 41 | 8 | (19.5) | 1.0 | 1.0 | ||
| 31–50 | 93 | 37 | (39.8) | 2.0 (1.1–4.0) | 0.02 | 1.8 (1.2–3.4) | 0.02 | |
| Above 50 | 69 | 24 | (34.8) | 1.8 (0.9–3.6) | 0.09 | 1.6(0.8–3.0) | 0.1 | |
| DR-TB Centre | Centre 1 | 59 | 16 | (27.1) | 1.0 | 1.0 | ||
| Centre 2 | 88 | 37 | (42.0) | 1.6 (1.0–2.5) | 0.06 | 1.5 (1.0–2.5) | 0.05 | |
| Centre 3 | 56 | 16 | (28.6) | 1.05 (0.6–1.9) | 0.8 | 1.0 (0.7–2.0) | 0.8 | |
| Site of disease | Others | 104 | 38 | (36.5) | 1.2 (0.8–1.7) | 0.4 | ||
| Lymph node | 99 | 31 | (31.3) | 1.0 | ||||
| Basis of diagnosis | Others | 30 | 10 | (33.3) | 1.0 (0.6–1.7) | 0.9 | ||
| CBNAAT | 173 | 59 | (34.1) | 1.0 | ||||
| History of previous TB | Yes | 178 | 65 | (36.5) | 2.3 (1.0–5.7) | 0.04 | 2.1 (1.1–4.8) | 0.03 |
| No | 25 | 4 | 16.0) | 1.0 | 1.0 | |||
| HIV status | Negative | 200 | 67 | (33.5) | 1.0 | 0.3 | ||
| Positive | 3 | 2 | (66.7) | 2.0 (0.9–4.5) | ||||
| Diabetes | Yes | 5 | 3 | (60.0) | 1.8 (0.9–3.8) | 0.2 | 1.9 (0.9–3.4) | 0.18 |
| No | 198 | 66 | (33.3) | 1.0 | 1.0 | |||
| Severe adverse reaction | Yes | 58 | 16 | 27.6 | 1.0 | 0.2 | 1.0 | |
| No | 145 | 53 | 36.6 | 1.3 (0.8–2.1) | 1.4 (0.9–2.2) | 0.15 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohiya, S.; Tripathy, J.P.; Sagili, K.; Khanna, V.; Kumar, R.; Ojha, A.; Bhatnagar, A.; Khanna, A. Does Drug-Resistant Extrapulmonary Tuberculosis Hinder TB Elimination Plans? A Case from Delhi, India. Trop. Med. Infect. Dis. 2020, 5, 109. https://doi.org/10.3390/tropicalmed5030109
Lohiya S, Tripathy JP, Sagili K, Khanna V, Kumar R, Ojha A, Bhatnagar A, Khanna A. Does Drug-Resistant Extrapulmonary Tuberculosis Hinder TB Elimination Plans? A Case from Delhi, India. Tropical Medicine and Infectious Disease. 2020; 5(3):109. https://doi.org/10.3390/tropicalmed5030109
Chicago/Turabian StyleLohiya, Sheelu, Jaya Prasad Tripathy, Karuna Sagili, Vishal Khanna, Ravinder Kumar, Arun Ojha, Anuj Bhatnagar, and Ashwani Khanna. 2020. "Does Drug-Resistant Extrapulmonary Tuberculosis Hinder TB Elimination Plans? A Case from Delhi, India" Tropical Medicine and Infectious Disease 5, no. 3: 109. https://doi.org/10.3390/tropicalmed5030109
APA StyleLohiya, S., Tripathy, J. P., Sagili, K., Khanna, V., Kumar, R., Ojha, A., Bhatnagar, A., & Khanna, A. (2020). Does Drug-Resistant Extrapulmonary Tuberculosis Hinder TB Elimination Plans? A Case from Delhi, India. Tropical Medicine and Infectious Disease, 5(3), 109. https://doi.org/10.3390/tropicalmed5030109





