A Serological Survey of Selected Papua New Guinea Blood Donors for Hepatitis B and Related Co-Infections
Abstract
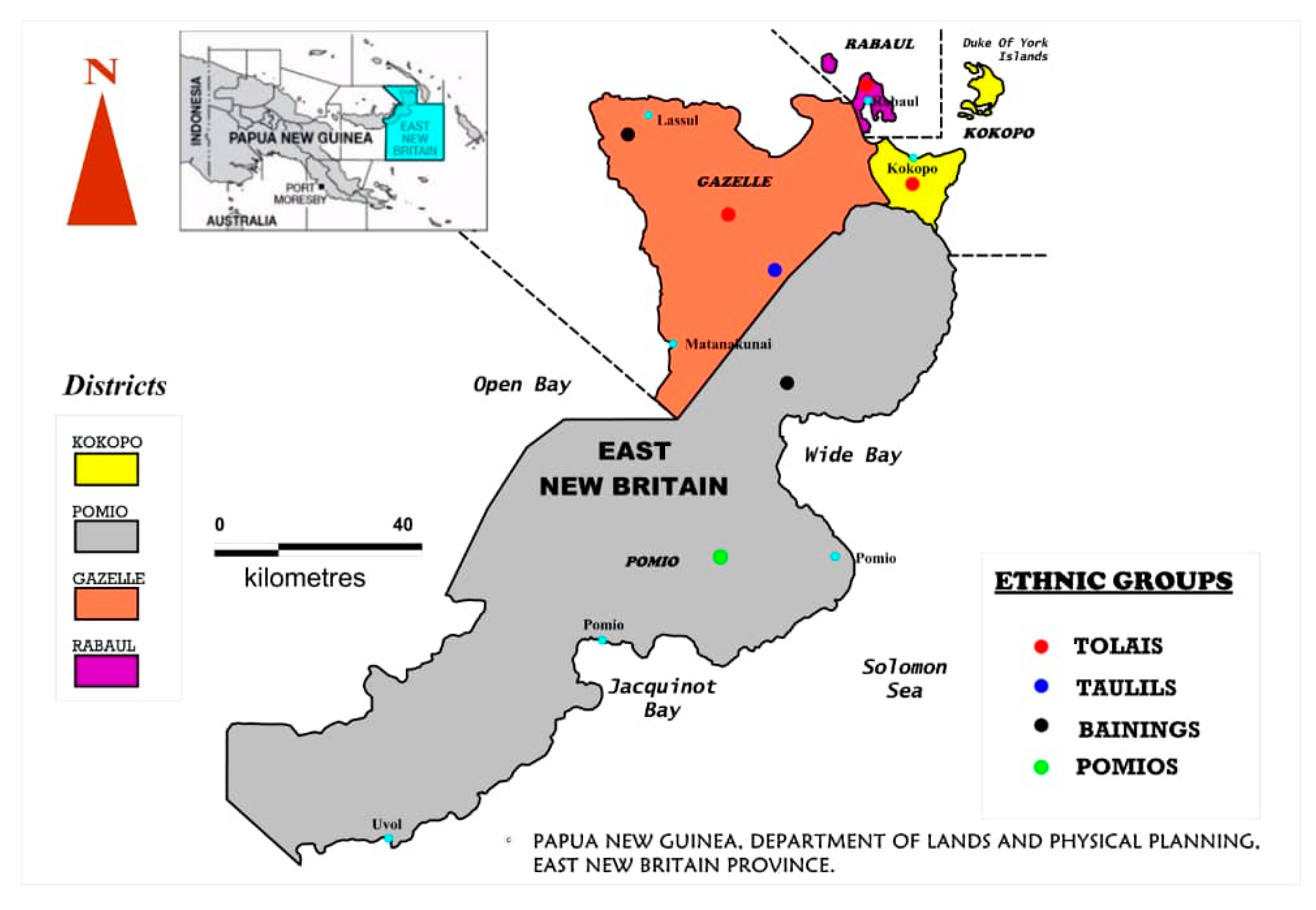
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. HBV Infection among the Districts and Ethnic Groups of This Study
4.2. HBV Infection among Each Gender and Sub-Age Groups
4.3. HBV Co- and Triple Infection with Syphilis, HIV and HCV
5. Conclusions
6. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Year | HBsAg Single Infection | HIV Single Infection | ||
|---|---|---|---|---|
| n = | Prevalence | n = | Prevalence | |
| (95%CI) | (95% CI) | |||
| 2003 | 645/1832 | 35 (34.11–36.30) | 0/1832 | 0 |
| 2004 | 439/1414 | 31 (29.84–32.26) | 2/1414 | 0.1 (0–0.3) |
| 2005 | 200/712 | 28 (26.44–29.74) | 0/712 | 0 |
| 2006 | 378/1462 | 26 (24.73–26.98) | 1/1462 | 0.1 (0–0.2) |
| 2007 | 451/1641 | 27 (27.00–29.18) | 2/1640 | 0.1 (0–0.3) |
| 2008 | 145/952 | 15(14.09–16.38) | 0/952 | 0 |
| 2009 | 68/687 | 10 (9.33–11.63) | 2/685 | 0.3 (0–0.8) |
| 2010 | 144/882 | 16 (17.97–20.58) | 27/867 | 3.1 (2.0–4.3) |
| 2011 | 52/880 | 6 (20.34–23.07) | 154/855 | 18 (15.4–20.6) |
| 2012 | 87/820 | 11 (11.07–13.32) | 41/781 | 5.3 (3.7–6.8) |
| 2013 | 10/515 | 2 (1.68–2.98) | 4/510 | 0.8 (0.02–1.6) |
| 2014 | 2/531 | 0.4 (0.12–0.64) | 4/528 | 0.8 (0–1.5) |
| 2015 | 24/242 | 10 (11.47–15.80) | 3/240 | 1.3 (0–2.7) |
| 2016 | 78/445 | 18 (24.90–29.03) | 5/442 | 1.1 (0.2–2.1) |
| 2017 | 20/129 | 16 (19.60–26.91) | 2/129 | 1.6 (0–3.7) |
| 2018 | 60/1206 | 5 (4.44–5.68) | 25/1888 | 1.3 (0.8–1.8) |
| Total | 2804/14350 | 19.21–19.87 | 272/14237 | 1.9 (1.7–2.1) |
| Year | Syphilis Single | Infection | HCV Single Infection | |
| 2003 | 0/1832 | Not done | 0/1832 | 0 |
| 2004 | 0/1414 | Not done | 0/1414 | 0 |
| 2005 | 0/712 | Not done | 0/712 | 0 |
| 2006 | 0/1462 | Not done | 0/1462 | 0 |
| 2007 | 20/1633 | 1.2 (0.7–1.8) | 0/1641 | 0 |
| 2008 | 5/947 | 0.5 (0.1–1.0) | 0/952 | 0 |
| 2009 | 16/674 | 2.4 (1.2–3.5) | 0/687 | 0 |
| 2010 | 53/849 | 6.2 (4.6–7.9) | 0/882 | 0 |
| 2011 | 217/810 | 26.8 (23.7–29.8) | 0/880 | 0 |
| 2012 | 99/732 | 13.5 (11.1–16.0) | 0/820 | 0 |
| 2013 | 92/425 | 21.7 (17.7–25.6) | 0/515 | 0 |
| 2014 | 120/411 | 29.2 (24.8–33.6) | 0/531 | 0 |
| 2015 | 56/194 | 28.9 (22.5–35.2) | 0/242 | 0 |
| 2016 | 111/375 | 29.6 (25.0–34.2) | 0/445 | 0 |
| 2017 | 31/107 | 29 (20.4–37.6) | 0/129 | 0 |
| 2018 | 133/1037 | 12.8 (10.8–14.9) | 37/1169 | 3.2 (2.58–3.56) |
| Total | 953/13614 | 7.0 (6.6–7.4) | 37/14313 | 0.3 (0.22–0.30) |
| Age Group (Years) | 15–29 | Prevalence | 30–44 | Prevalence | 45–59 | Prevalence | ≥60 | Prevalence | Annual | Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 95% CI | 95% CI | 95% CI | 95% CI | Total | 95% CI | ||||
| 2003 | 521/1460 | 36 (33–38) | 98/320 | 31 (26–36) | 24/50 | 48 (34–62) | 2/2 | 100 (100–100) | 649/1832 | 35 (33–38) |
| 2004 | 284/804 | 35 (32–39) | 125/477 | 26 (22–30) | 28/122 | 23 (16–30) | 2/11 | 18 (−5–41) | 439/1414 | 31 (28–33) |
| 2005 | 137/471 | 29 (25–33) | 57/207 | 28 (22–34) | 6′/33 | 18 (5–31) | 0/1 | 0 (0–0) | 200/712 | 28 (25–31) |
| 2006 | 157/660 | 24 (21–27) | 169/609 | 28 (22–34) | 49/180 | 27 (21–34) | 3/13 | 23 (0.2–46) | 378/1462 | 26 (24–28) |
| 2007 | 247/833 | 30 (27–33) | 159/577 | 28 (24–31) | 41/205 | 20 (15–26) | 12/26 | 46 (27–65) | 459/1641 | 28 (26–30) |
| 2008 | 78/405 | 19 (15–23) | 52/392 | 13 (10–17) | 14/145 | 10 (5–15) | 1/10 | 10 (-9–29) | 145/952 | 15 (13–18) |
| 2009 | 25/292 | 9 (5–12) | 39/266 | 15 (10–19) | 6/126 | 5 (1–9) | 0/3 | 0 (0–0) | 70/687 | 10 (8–13) |
| 2010 | 91/339 | 27 (22–32) | 61/385 | 16 (12–20) | 16/146 | 11 (6–16) | 2/12 | 17 (−4–38) | 170/882 | 19 (17–22) |
| 2011 | 58/300 | 19 (15–24) | 63/305 | 21 (16–25) | 63/251 | 25 (20–31) | 7/24 | 29 (11–47) | 191/880 | 22 (19–24) |
| 2012 | 34/290 | 12 (8–15) | 35/289 | 12 (8–16) | 25/216 | 12 (7–16) | 6/25 | 24 (7–41) | 100/820 | 12 (10–14) |
| 2013 | 3/195 | 2 (-0.2–3) | 7/163 | 4 (1–7) | 2/140 | 1 (-0.5–3) | 0/17 | 0 (0–0) | 12/515 | 2 (1–4) |
| 2014 | 2/271 | 1 (-0.3–2) | 0/179 | 0 (0–0) | 0/81 | 0 (0–0) | 0/0 | 0 (0–0) | 2/531 | 0.4 (−0.1–1) |
| 2015 | 18/107 | 17 (10–24) | 11′/79 | 14 (6–22) | 3/52 | 8 (1–15) | 0/4 | 0 (0–0) | 33/242 | 14 (9–18) |
| 2016 | 56/224 | 25 (19–31) | 51/138 | 37 (29–45) | 12/81 | 15 (7–23) | 1/2 | 50 (−19–119) | 120/445 | 27 (23–31) |
| 2017 | 13/61 | 21 (11–32) | 9′/31 | 29 (13–45) | 8′/34 | 24 (9–38) | 0/3 | 0 (0–0) | 30/129 | 23 (16–31) |
| 2018 | 41/544 | 8 (5–10) | 40/402 | 10 (7–13) | 22/240 | 9 (6–13) | 1′/20 | 5 (−5–15) | 104/1206 | 9 (7–10) |
| Total | 1765/7256 | 24 (23–25) | 976/4819 | 20 (19–21) | 324/2102 | 15 (14–17) | 37/173 | 21 (15–28) | 3102/14350 | 22 (21–22) |
References
- Grove, D.I.; McGregor, A.; Forbes, I.J. Impaired Humoral Immunity in Papua New Guinea Highlanders. P. N. G. Med. J. 1975, 18, 1–7. [Google Scholar] [PubMed]
- Woodfield, D.G. Acute Viral Hepatitis in Papua New Guinea: Results of hepatitis B antigen and antibody studies. Trop. Geogr. Med. 1975, 27, 399–404. [Google Scholar]
- Simons, M.J.; Binns, C.W.; Malcom, L.A.; Yap, E.H. Australia antigen frequencies in two groups of Highland New Guineans. P. N. G. Med. J. 1972, 15, 91–97. [Google Scholar]
- Hawkes, R.A.; Boughton, C.R.; Ferguson, V.; Vale, T.G. The sero-epidemiology of hepatitis in Papua New Guinea II. A long term study of hepatitis B. Am. J. Epidemiol. 1981, 114, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.C.; Purcell, R.H.; Rosen, L. Prevalence of antibody to hepatitis A and hepatitis B viruses in selected populations of the South Pacific. J. Epidemiol. 1979, 110, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.; Naraqi, S. The aetiology of Jaundice in Adult patients at PMGH, Papua New Guinea. P. N. G. Med. J. 1987, 30, 259–264. [Google Scholar] [PubMed]
- Tozer, R.A. Papua New Guinea Red Cross Blood Transfusion Service: Present status and future considerations. P. N. G. Med. J. 1996, 39, 38–42. [Google Scholar] [PubMed]
- Foster, H.M.; Avera, K. Healthy Adults with HBsAg in North Solomons. P. N. G. Med. J. 1987, 30, 247. [Google Scholar]
- Mathai, E.; Krishna, M. Hepatitis B in the Pacific. Pac. Health Dialog. 1998, 5, 142–146. [Google Scholar]
- Powell, K.C.; McGovern. Liver disease in Papua New Guinea. P. N. G. Med. J. 1976, 19, 140–148. [Google Scholar]
- Kitau, R.; Datta, S.S.; Patel Minal, K.; Patel Hennessey, K.; Wannemuehler, K.; Sui, G.; Lagani, W. Hepatitis B Surface Antigen Seroprevalence among Children in Papua New Guinea. Am. J. Trop. Med. Hyg. 2015, 92, 501–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamu, E.; Varpit, F. Comparative study of hepatitis B infection and related co-infections in Voluntary and family replacement donors at Port Moresby General Hospital Blood Transfusion Service: A retrospective study. P. N. G. Med. J. 2016, 16. Available online: https://pdfs.semanticscholar.org/4929/6b5e13823360f79ab3e9f20c4d9aa6ef2341.pdf?_ga=2.168913825.102974277.1593425257-859342083.1582701467 (accessed on 18 May 2020).
- Khageshan, A.P.; Kulkarni, K.R.; Baragundi, M.C. Seroprevalence of Co-Infections Among Blood Donors in A Blood Bank of A Tertiary Health Care Centre. Ann. Pathol. Lab. Med. 2016, 3, A29–A32. [Google Scholar]
- Papua New Guinea National Statistical Office. The Papua New Guinea 2011 National Report, Port Moresby. NSO. 2015. Available online: http://sdd.spc.int/en/resources/document-library?view=preview&format=raw&fileId=218 (accessed on 18 July 2016).
- Hausples.com.pg, PNGs Home of Real Estate. ENB: The fastest growing province in PNG. 17 March 2015. Available online: www.hausples.com.pg/the-fastest-growing-province-in-png/ (accessed on 11 July 2016).
- Papua New Guinea Department of Health. National Blood Policy; Department of Health: Port Moresby, Papua New Guinea, August 2015; pp. 1–35.
- World Health Organization. WHO Prequalification of In Vitro Diagnostics. Acceptance Criteria for HBsAg IVDs; WHO: Geneva, Switzerland, 2020; pp. 1–4. [Google Scholar]
- Katz, M.H. Multivariable Analysis: A Practical Guide for Clinicians; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Hintze, J. NCSS Statistical Software, 9th ed.; NCSS Statistical Software: Kaysville, UT, USA, 2013. [Google Scholar]
- Armitage, P.; Berry, G. Statistical Methods in Medical Research, 3rd ed.; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Mazzur, S.; Jones, N. Distribution and prevalence of hepatitis B surface antigen and antibody in a Melanesian population. Am. J. Epidemiol. 1977, 105, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Coulepis, A.G.; Zimmet, P.; Taylor, R.; Ram, P.; Banuve, S.; Gust, I.D. Sero-epidemiology of infection with hepatitis B virus in Fiji. Am. J. Epidemiol. 1982, 118, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Tibbs, C.J. Delta Hepatitis in Kiribati: A Pacific Focus. J. Med. Virol. 1989, 29, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, R.B.; McMahon, B.J.; Bender, T.R.; Heyward, W.L.; Nakanishi, S.; Wainwright, K.Y.; Foliaki, S.; Erickson, S.L.; Fields, H.A. Prevalence of Hepatitis B virus Infection in Tonga: Identifying High Risk groups for Immunization with hepatitis B Vaccine. Int. J. Epidemiol. 1986, 15, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Foaogali, J.L. The serological status of Solomon Island Blood Donors. Southesat Asian J. Trop. Med. Public Health 1999, 30, 542–545. [Google Scholar]
- Utsumi, T.; Yano, Y.; Truong Bui, X.; Tanaka, Y.; Mizokami, M.; Seo, Y.; Kasuga, M.; Kawabata, M.; Hayashitake, H. Molecular epidemiology study of hepatitis B virus Infection in two different ethnic populations in the Solomon Islands. J. Med. Virol. 2007, 79, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Tsukakoshi, T.; Samuela, J.; Rafai, E.V.; Rabuatoka, U.; Honda, S.; Kamiya, Y.; Buerano, C.C.; Morita, K. Hepatitis B serologic survey and review of immunization records of children, adolescents and adults in Fiji, 2008–2009. Virol. J. 2015, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Papua New Guinea National Health Plan 2011–2020 Volume 2 (Part A). Reference Data and National Health Profile Government of Papua New Guinea June 2010. pp. 1–154. Available online: https://www.health.gov.pg/pdf/PNGNHP%20Vol2%20PartA_2014.pdf (accessed on 18 May 2020).
- Papua New Guinea: WHO and UNICEF Estimates of Immunization Coverage: 2018 Revision. Available online: https://www.who.int/immunization/monitoring_surveillance/data/png.pdf (accessed on 18 May 2020).
- Coursaget, P.; Gharbi, Y.; Khruf, N.; Depril, N.; Boukhris, N.; Fritzell, B.; Kastally, R. Familial clustering of hepatitis B virus infections and prevention of perinatal transmission by immunization with a reduced number of doses in an area of intermediate endemicity (Tunisia). Vaccine 1994, 12, 275–278. [Google Scholar] [CrossRef]
- Boisier, P.; Rabarijaona, L.; Piollet, M.; Roux, J.F.; Zeller, H.G. Hepatitis B virus infection in general population Madagascar: Evidence for different epidemiological patterns in urban and rural areas. Epidemiol. Infect. 1996, 117, 133–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinson, F.A.; Weigle, K.A.; Royce, R.A.; Weber, D.J.; Suchindran, C.M.; Lemon, S.M. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. Am. J. Epidemiol. 1997, 105, 107–112. [Google Scholar]
- Mu, S.C.; Wang, G.M.; Jow, G.M.; Chen, B.F. Impact of Universal Vaccination on Intrafamilial Transmission of Hepatitis B Virus. J. Med. Virol. 2011, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Kuberski, T.; LeGonidec, G.; Gust, I.D.; Dimitrakakis, M.; Cantaloube, D.; Zimmet, P. Hepatitis B infections in Melanesians and Polynesians in New Caledonia. Am. J. Epidemiol. 1981, 114, 355–360. [Google Scholar] [CrossRef]
- Gust, I.D.; Dimitrakakis, M.; Faaiuso, S.; Ainuu, J.; Zimmet, P. The prevalence of hepatitis B infection amongst urban and rural populations in Western Samoa. J. Hygiene Comb. 1981, 86, 87–93. [Google Scholar] [CrossRef]
- Mujeeb, S.; Pearce, M. Temporal trends in hepatitis B and C infection in family blood donors from interior Sindh, Pakistan. BMC Infec. Dis. 2008, 8, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Vahid, T.; Alavian, S.M.; Kabis, A.; Kafaee, J.; Yektaparast, B. Hepatitis B prevalence and risk factors in blood donors in Ghazvin, Iran. Hepat. Mon. 2005, 5, 117–122. [Google Scholar]
- World Bank. Papua New Guinea Public Expenditure & Service Delivery. Education Finance (G). Papua New Guinea. 30 June 2004. Available online: http://siteresources.worldbank.org/INTPUBSERV/Resources/PNG.PESD.Education.Final(G).jun.2004.pdf (accessed on 12 July 2016).
- Luksamijarulkul, P.; Maneesri, P.; Kitigul, L. Hepatitis B sero-prevalence and risk factors among school-age children in a low socioeconomic community, Bangkok. Asia-Pac. J. Public Health 1995, 8, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Zali, M.R.; Mohammad, K.; Farhadi, A.; Masjedi, M.R.; Zargar, A.; Nowroozi, A. Epidemiology of hepatitis B in the Islamic Republic of Iran. East. Mediterr. Health J. 1996, 2, 290–298. [Google Scholar]
- Nkrumak, B.; Owusu, M.; Frempong, H.O.; Averu, P. Hepatitis B and C viral infections among blood donors from rural Ghana. Ghana Med. J. 2011, 45, 97–100. [Google Scholar]
- Sharma, D.C.; Jain, A.; Woike, P.; Rai, S.; Tripathi, L.; Bharat, S.; Gaur, R. Prevalence of HIV Infection Among Blood Donors at a Tertiary Care Centrebin Gwalior, India. Int. Blood Res. Rev. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Gust, I.D.; Lehmann, N.I.; Dimitrakakis, M.; Zimmet, P. Sero-epidemiology of Infection with Hepatitis A and B Viruses in an Isolated Pacific Population. J. Infect. Diseases. 1979, 139, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.M.; Goldstein, S.T.; Kuartei, S. Endemic hepatitis B virus infection and chronic liver disease mortality in the Republic of Palau, 1990–2002. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Walana, W.; Hokey, P.; Ahiaba, S. Seroprevalence of hepatitis B virus infection among blood donors: A retrospective study in the Kintapo Municipal Hospital, Ghana. Open J. Med. Microbiol. 2014, 4, 64–69. [Google Scholar] [CrossRef]
- Mandal, R.; Mondal, K. Transfusion transmissible infections among blood donors from sub-Himalayan rural tertiary care centre in Darjeeling, India. J. Tradit. Complement. Med. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis B, Key Facts. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 20 April 2019).
- Hoyos-Orrego, A.; Massaro-Ceballos, M.; Ospina-Ospina, M.; Gòmez-Builes, C.; Arroyave-Vanegas, N.; Pereira-Tobòn, J.; Hurtado-Jaramillo, J.; Lòpez-Rugeles, M.T. Serological markers and risk factors for hepatitis B and C viruses in patients infected with human immunodeficiency virus. Rev. Inst. Med. Trop. São Paulo 2006, 48, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Stabinski, L.; Reynolds, S.J.; Ocama, N.; Laeyendecker, O.; Serwadda, D.; Gray, R.H.; Wawe, M.T.D.L.; Quinn, T.C.; Gregory, D.K. Hepatitis B Virus and Sexual Behaviour in Rakai, Uganda. J. Med. Virol. 2011, 83, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Pandey, R.P.; Rana, V.A. Clinical Study of prevalence of hepatitis B virus, Hepatitis C virus and Syphilis (T.pallidum) in HIV positive patients. Sch. J. Appl. Med. Sci. 2015, 3, 1490–1496. [Google Scholar]
- Segura, M.; Bautista, C.; Marone, R.; Estani, S.S.; Rey, J.; Montano, S.M.; Griemberg, G.; Weissenbacher, M.; Avila, M.M. HIV/STI co-infections, syphilis incidence, and hepatitis B vaccination: The Buenos Aires cohort of men who have sex with man. AIDS Care. 2010, 22, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Yan, H.; Yang, H.; Huan, X.; Guan, W.; Xu, X.; Hang, M.; Tang, W.; Wang, N.; Gu, J.; et al. The incidence of syphilis, HIV and HCV and associated factors in a cohort of men who have sex with men in Nanjing, China. Sex. Transm. Infect. 2011, 87, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Butsashvili, M.; Tsertsvadze, T.; McNutt, L.A.; Kamkamidze, G.; Gvetadze, R.; Bdridze, N. Prevalence of hepatitis B, hepatitis C, syphilis and HIV in Georgian blood donors. Eur. J. Epidemiol. 2001, 17, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Burek, V.; Horvat, J.; Butorac, K.; Mikulic, R. Viral hepatitis B, C and HIV infection in Croatian prisons. Epidemiol. Infect. 2010, 138, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Shieh, B.; Chang, M.J.; Ko, W.C.; Chen, E.J.; Wu, J.C.; Lee, C.F.; Chang, T.T.; Li, C. Effects of Multiple Virus Co-infections on Disease Progression in HIV-Positive Patients. Intervirology 2003, 46, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jobarteh, M.; Malfroy, M.; Peterson, I.; Jeng, A.; Sarge-Njie, R.; Alabi, A.; Peterson, K.; Cotton, M.; Hall, A.; Rowland-Jones, S.; et al. Sero-prevalence of hepatitis B and C virus in HIV-1 and HIV-2 infected Gambians. Virol. J. 2010, 7, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Beadsworth, M.B.J.; Chaponda, M.; Mhango, B.; Faragher, B.; Njala, J.; Hofland, H.W.C.; Davies, J.; Hart, I.J.; Beeching, N.J.; et al. Favourable one-year ART outcomes in adult Malawians with hepatitis B and C co-infection. J. Infect. 2010, 61, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Spooner, V.; Richeus, J.; Saunders, R. HBsAg, e Antigen and HBV DNA in Healthy Antenal patients attending Goroka Hospital and their relationaship to Tottooing Practices. P. N. G. Med. J. 1990, 33, 11–15. [Google Scholar] [PubMed]
- Papua New Guinea Department of Health. Papua New Guinea National Health Plan. 2001–2010; Department of Health: Port Moresby, Papua New Guinea, August 2000.
- Papua New Guinea National Aids Council Secretariat and Partners. National Health Plan, 2011–2020; National Aids Council Secretariat and Partners: Boroko, NCD, Papua New Guinea, 2008. [Google Scholar]
- Perazzo, P.; Eguibar, N.; González, R.H.; Nusblat, A.D.; Cuestas, M.L. Hepatitis B Virus (Hbv) and S-Escape Mutants: From the Beginning until Now. J. Hum. Virol. Retrovirol. 2015, 2, 46. [Google Scholar] [CrossRef][Green Version]
- Mokaya, J.; McNaughton, A.L.; Hadley, M.J.; Beloukas, A.; Geretti, A.-M.; Goedhals, D.; Matthews, P.C. A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: A call for urgent action. PLoS Negl. Trop. Dis. 2018, 12, e0006629. [Google Scholar] [CrossRef]
- Marks, G.; Crepaz, N.; Janssen, R. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006, 20, 1447–1450. [Google Scholar] [CrossRef] [PubMed]




| Demographic | Proportion Positive | Prevalence % (95% CI) | x² | p-Value |
|---|---|---|---|---|
| Total HBsAg Infection | 3102/14350 | 21 (21.0–22.0) | ||
| Single infection | 2804/14350 | 19 (19.0–19.8) | ||
| Age group | ||||
| 15–29 | 1669/7272 | 23 (22.5–23.4) | ||
| 30–44 | 843/4809 | 17 (17.0–18.1) | 130.41 | ˂0.001 |
| 45–59 | 270/2100 | 13 (12.0–13.6) | ||
| ≥60 | 22/169 | 6 (4.6–8.4) | ||
| Donor frequency | ||||
| Repeat donor | 986/6269 | 16 (14.8–16.6) | 102.89 | ˂0.001 |
| First time donor | 1818/8081 | 23 (21.6–23.4) | ||
| District | ||||
| Kokopo | 622/2826 | 22 (21.2–22.8) | ||
| Gazelle | 1775/7904 | 22 (22.0–22.9) | 27.38 | ˂0.001 |
| Pomio | 11/152 | 7 (5.2–9.3) | ||
| Rabaul | 694/3468 | 20 (19.3–20.7) | ||
| Ethnicity | ||||
| Bainings | 99/360 | 21 (19.7–23.5) | ||
| Pomios | 13/142 | 8 (6.2–10.6) | 19.51 | ˂0.001 |
| Taulils | 30/73 | 29 (24.7–33.5) | ||
| Tolais | 2960/10673 | 21 (21.0–22.1) | ||
| Co & Triple infection | ||||
| HBsAg/SYPHILIS | 120 | 0.8 (0.6–1.0) | ||
| HBsAg/HIV | 101 | 0.7 (0.5–0.9) | 38.21 | ˂0.001 |
| HBsAg/HCV | 65 | 0.5 (0.3–0.6) | ||
| Triple Infection | 12 | 0.1 (0.0–0.2) | ||
| Total | 298/14350 | 2 (2.0–2.2) |
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Demographic | Proportion | Prevalence % | Proportion | Prevalence % | X² | p-Value |
| Positive | 95 CI% | Positive | 95% CI | |||
| Gender | 2236/9686 | 23 (22.6–23.5) | 866/4664 | 18 (18.0–19.1) | 39.91 | ˂0.001 |
| Single HBsAg Infection | 2023/9686 | 21 (20.5–21.3) | 781/4664 | 16 (16.0–17.3) | ||
| Donor Frequency | ||||||
| Repeat Donors | 517/4487 | 12 (10.6–12.5) | 469/1782 | 26 (24.3–28.4) | 714.58 | ˂0.001 |
| First Time Donors | 1506/5199 | 29 (27.7–30.2) | 312/2882 | 11 (9.7–12.0) | ||
| District | ||||||
| Kokopo | 428/1924 | 22 (21.3–23.2) | 194/902 | 21 (20.1–22.9) | ||
| Gazelle | 1270/5255 | 24 (23.6–24.8) | 505/2649 | 19 (18.3–19.8) | ||
| Pomio | 8/101 | 8 (5.3–10.6) | 3/51 | 5 (2.7–9.1) | 45.99 | ˂0.001 |
| Rabaul | 530/2406 | 22 (21.2–22.9) | 164/1062 | 15 (14.4–16.5) | ||
| Ethnicity | ||||||
| Bainings | 70/294 | 23 (21.4–26.3) | 29/165 | 17 (14.7–20.5) | ||
| Pomios | 9/103 | 8 (6.0–11.5) | 4/52 | 7 (4.1–11.3) | 26.11 | 0.002 |
| Taulils | 26/73 | 35 (30.1–41.1) | 4/30 | 13 (7.2–19.4) | ||
| Tolais | 2131/9216 | 23 (22.7–23.6) | 829/4417 | 18 (18.0–19.4) | ||
| Age Group | ||||||
| 15–29 | 1306/5120 | 25 (24.9–26.1) | 483/2152 | 22 (21.6–23.3) | ||
| 30–44 | 663/3054 | 21 (21–22.4) | 281/1755 | 16 (15.2–16.9) | 183.98 | ˂0.001 |
| 45–59 | 236/1366 | 17(16.3–18.3) | 99/734 | 13 (12.3–14.7) | ||
| ≥60 | 31/146 | 21 (17.9–24.6) | 23-Mar | 13 (6.1–19.9) | ||
| Double and Triple HBsAg Infections | ||||||
| HBsAg/Syphilis | 100 | 1.0 (0.8–1.2) | 20 | 0.4 (0.2–0.6)) | ||
| HBsAg/HIV | 67 | 0.7 (0.5–0.9) | 34 | 1.0 (0.5–1.0) | ||
| HBsAg/HCV | 36 | 0.4 (0.3–0.5) | 29 | 0.6 (0.4–0.9)) | 149.1 | ˂0.001 |
| Triple Infection | 10 | 0.1 (0.0–0.2) | 2 | 0.0 (0.0–0.1) | ||
| Total | 213/9686 | 2 (2.0–2.4) | 85/4664 | 2 (1.6–2.0) | ||
| Independent Variable | Regression Coefficient | SE | Wald Z-Value | Wald p-Value | Odds Ratio | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|
| B0: Intercept | 0.00 | 0.04 | −0.02 | 0.99 | 0.99 | 0.92 | 1.08 |
| DISTRICT = “KOKOPO” | −0.03 | 0.05 | −0.50 | 0.62 | 0.97 | 0.88 | 1.08 |
| DISTRICT = “POMIO” | −1.30 | 0.32 | −4.10 | <0.01 | 0.27 | 0.15 | 0.51 |
| DISTRICT = “RABAUL” | −0.25 | 0.05 | −4.736 | <0.01 | 0.78 | 0.70 | 0.86 |
| HIV = 1 | 1.55 | 0.13 | 12.353 | <0.01 | 4.71 | 3.68 | 6.02 |
| Age = 15–29 years vs. 30–34 years | −0.23 | 0.05 | −4.873 | <0.01 | 0.80 | 0.73 | 0.87 |
| Age = 15–29 years vs. 25–59 years | −0.59 | 0.07 | −8.770 | <0.01 | 0.55 | 0.48 | 0.63 |
| Age = 15–29 years vs. ≥60 years | −0.33 | 0.20 | −1.683 | 0.09 | 0.72 | 0.49 | 1.06 |
| Gender = ”M” | 0.27 | 0.05 | 5.866 | <0.01 | 1.31 | 1.19 | 1.43 |
| Independent Variable | Regression Coefficient | Standard Error | Wald Z-Value | Wald Prob Level | Odds Ratio | Lower 95% C | Upper 95% C |
|---|---|---|---|---|---|---|---|
| B0: Intercept | −0.02 | 0.12 | −0.12 | 0.90 | 0.99 | 0.78 | 1.25 |
| Gender = “M” | 0.29 | 0.05 | 6.27 | <0.01 | 1.33 | 1.22 | 1.45 |
| HIV = 1 | 1.58 | 0.13 | 12.57 | <0.01 | 4.84 | 3.79 | 6.19 |
| OLD_NEW = “OLD” | −0.18 | 0.04 | −4.16 | <0.01 | 0.84 | 0.77 | 0.91 |
| Age = 15–29 years vs. 30–34 years | −0.21 | 0.05 | −4.59 | <0.01 | 0.81 | 0.74 | 0.89 |
| Age = 15–29 years vs. 25–59 years | −0.57 | 0.07 | −8.33 | <0.01 | 0.57 | 0.50 | 0.65 |
| Age = 15–29 years vs. ≥60 years | −0.28 | 0.20 | −1.41 | 0.16 | 0.76 | 0.51 | 1.11 |
| ETHNICITY = “POMIO” | −0.56 | 0.27 | −2.03 | 0.04 | 0.57 | 0.33 | 0.98 |
| ETHNICITY = “TAULIL” | 0.21 | 0.25 | 0.86 | 0.39 | 1.24 | 0.76 | 2.01 |
| ETHNICITY = “TOLAI” | 0.00 | 0.12 | 0.03 | 0.98 | 1.00 | 0.80 | 1.26 |
| HCV = 1 | −0.94 | 0.48 | −1.97 | 0.05 | 0.39 | 0.15 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varpit, F.; Gummow, B. A Serological Survey of Selected Papua New Guinea Blood Donors for Hepatitis B and Related Co-Infections. Trop. Med. Infect. Dis. 2020, 5, 108. https://doi.org/10.3390/tropicalmed5030108
Varpit F, Gummow B. A Serological Survey of Selected Papua New Guinea Blood Donors for Hepatitis B and Related Co-Infections. Tropical Medicine and Infectious Disease. 2020; 5(3):108. https://doi.org/10.3390/tropicalmed5030108
Chicago/Turabian StyleVarpit, Francisca, and Bruce Gummow. 2020. "A Serological Survey of Selected Papua New Guinea Blood Donors for Hepatitis B and Related Co-Infections" Tropical Medicine and Infectious Disease 5, no. 3: 108. https://doi.org/10.3390/tropicalmed5030108
APA StyleVarpit, F., & Gummow, B. (2020). A Serological Survey of Selected Papua New Guinea Blood Donors for Hepatitis B and Related Co-Infections. Tropical Medicine and Infectious Disease, 5(3), 108. https://doi.org/10.3390/tropicalmed5030108





