Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Farm Selection
2.3. Data Collection
2.4. Sample Collection
2.5. Serological Analysis
2.6. Data Analysis
3. Results
3.1. Brucella Seroprevalence
3.2. Risk Factor Analyses for Herds with No Previous Vaccination
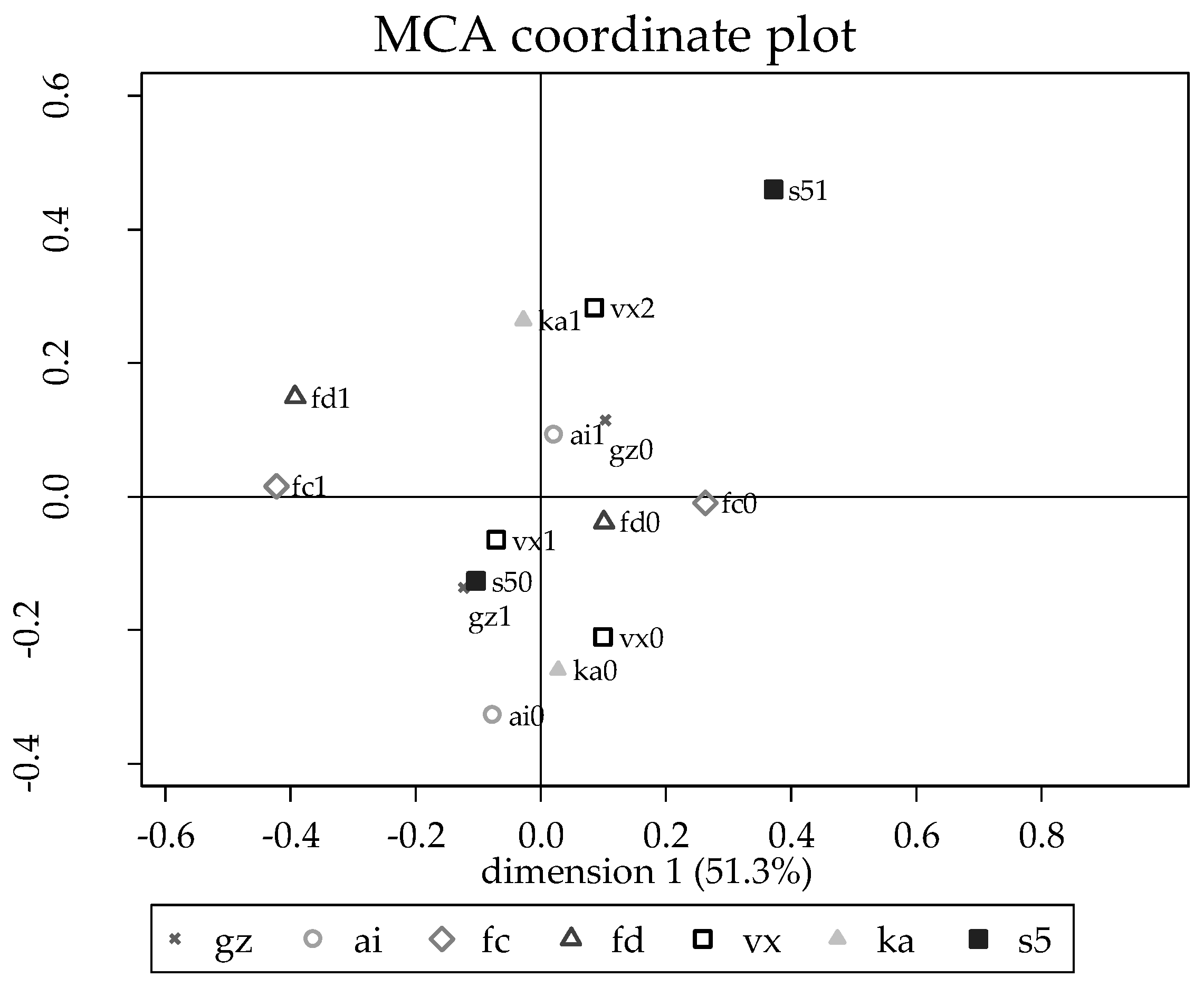
3.3. Risk Factors for Brucella Seropositivity in Guwahati
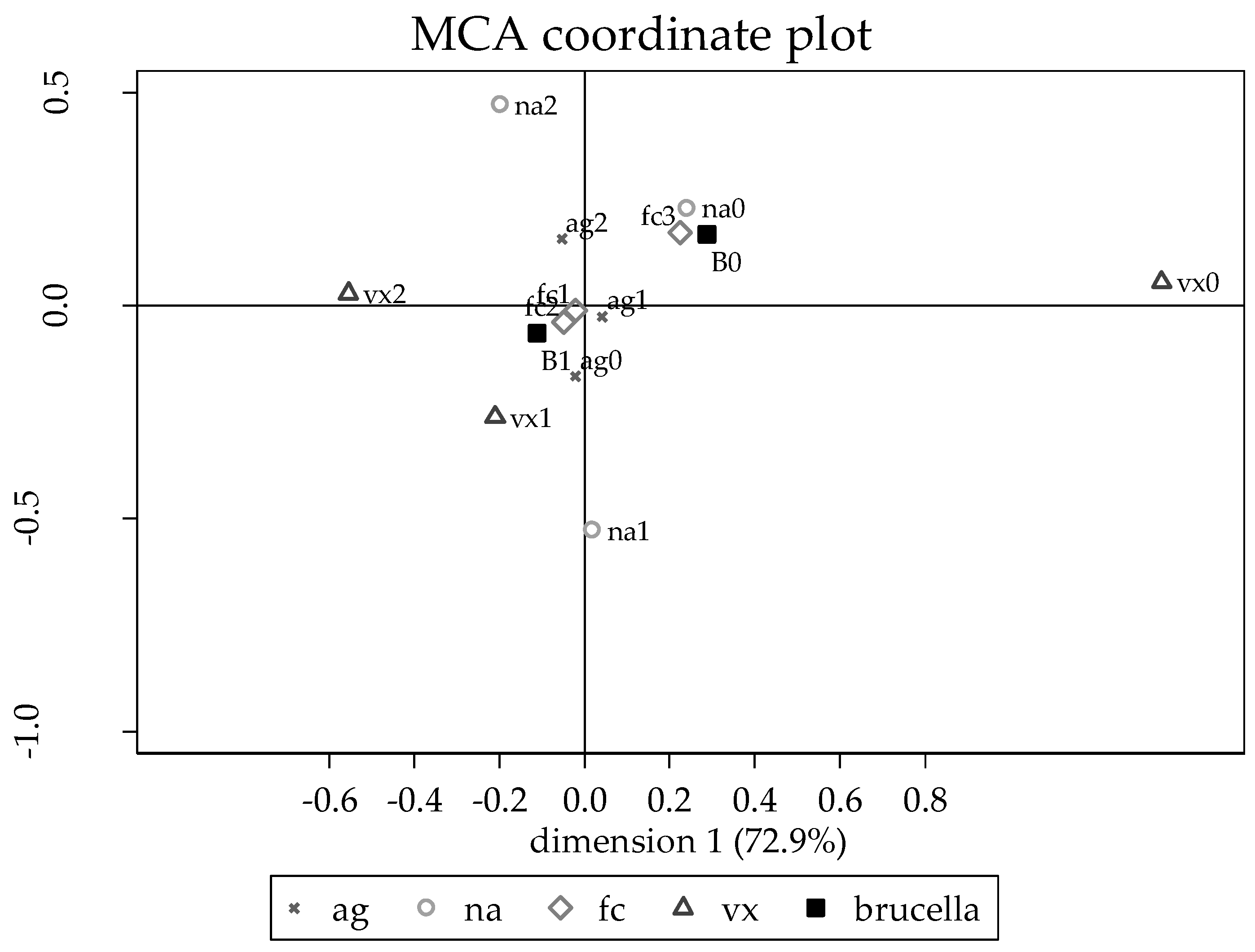
3.4. Risk Factors for Brucella Seropositivity in Bangalore, Bhubaneswar, Ludhiana, and Udaipur
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO FAO OIE; Corbel, M.J. Brucellosis in Humans and Animals; WHO: Rome, Italy, 2006. [Google Scholar]
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet. Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- OIE. Bovine Brucellosis: OIE Terrestrial Manual 2009; Office International de Epizootie: Paris, France, 2009. [Google Scholar]
- McDermott, J.J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Sci. Tech. Rev. Off. Int. Des Epizoot. 2013, 32, 249–261. [Google Scholar] [CrossRef]
- Godfroid, J.; Nielsen, K.; Saegerman, C. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 2010, 51, 296–305. [Google Scholar] [CrossRef] [PubMed]
- OIE. Infection with Brucella abortus, Brucella melitensis and Brucella suis. In OIE Terrestrial Manual 2016; OIE (World Organisation for Animal Health): Rome, Italy, 2016. [Google Scholar]
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.; Stack, J. Brucellosis—What every practitioner should know. Practice 2012, 34, 532–539. [Google Scholar] [CrossRef]
- Delgado, C.L. Rising Consumption of Meat and Milk in Developing Countries Has Created a New Food Revolution. J. Nutr. 2003, 133, 3907S–3910S. [Google Scholar] [CrossRef]
- FAOSTAT. Milk Total Production in India. 2015. Available online: http://faostat3.fao.org/browse/Q/QL/E (accessed on 12 April 2015).
- Douphrate, D.I.; Hagevoort, G.R.; Nonnenmann, M.W.; Lunner Kolstrup, C.; Reynolds, S.J.; Jakob, M.; Kinsel, M. The dairy industry: A brief description of production practices, trends, and farm characteristics around the world. J. Agromed. 2013, 18, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Thumbi, S.M.; Njenga, M.K.; Marsh, T.L.; Noh, S.; Otiang, E.; Munyua, P.; Ochieng, L.; Ogola, E.; Yoder, J.; Audi, A.; et al. Linking Human Health and Livestock Health: A “One-Health” Platform for Integrated Analysis of Human Health, Livestock Health, and Economic Welfare in Livestock Dependent Communities. PLoS ONE 2015, 10, e0120761. [Google Scholar] [CrossRef]
- Grace, D.; Wanyoike, F.; Lindahl, J.; Bett, B.; Randolph, T.; Rich, K.M. Poor livestock keepers: Ecosystem-poverty-health interactions. Philos. Trans. R. Soc. B-Econ. 2017, 372, 20160166. [Google Scholar] [CrossRef]
- Omemo, P.; Ogola, E.; Omondi, G.; Wasonga, J.; Knobel, D. Knowledge, attitude and practice towards zoonoses among public health workers in Nyanza province, Kenya. J. Public Health Afr. 2012, 3, 22. [Google Scholar] [CrossRef]
- de Glanville, W.A.; Conde-Álvarez, R.; Moriyón, I.; Njeru, J.; Díaz, R.; Cook, E.A.J.; Morin, M.; de, C.; Bronsvoort, B.M.; Thomas, L.F.; Kariuki, S.; et al. Poor performance of the rapid test for human brucellosis in health facilities in Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005508. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Vrentas, C.E.; Ram, P.; Deka, R.A.; Hazarika, H.; Rahman, R.G.; Bambal, J.S.; Bedi, C.; Pallab Chaduhuri, B.; Fairoze, N.M.; et al. Brucellosis in India: Results of a collaborative workshop to define One Health priorities. Trop. Anim. Health Prod. 2019. (submitted). [Google Scholar]
- Lindahl, J.F.; Chauhan, A.; Gill, J.P.S.; Hazarika, R.A.; Fairoze, N.M.; Grace, D.; Kakkar, M. The extent and structure of peri-urban smallholder dairy farming in five cities in India. Trop. Anim. Health Prod. 2019. (submitted). [Google Scholar]
- Emmerzaal, A.; de Wit, J.J.; Dijkstra, T.; Bakker, D.; van Zijderveld, F.G. The Dutch Brucella abortus monitoring programme for cattle: The impact of false-positive serological reactions and comparison of serological tests. Vet. Q. 2002, 24, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gall, D.; Nielsen, K. Serological Diagnosis of Bovine Brucellosis: A Review of Test Performance and Cost Comparison. Rev. sci. tech. Off. int. Epiz. 2004, 23, 3. [Google Scholar] [CrossRef]
- Deka, R.P.; Magnusson, U.; Grace, D.; Lindahl, J. Bovine brucellosis: Prevalence, risk factors, economic cost and control options with particular reference to India—A review. Infect. Ecol. Epidemiol. 2018, 8, 1556548. [Google Scholar] [CrossRef]
- Makita, K.; Fèvre, E.M.; Waiswa, C.; Eisler, M.C.; Thrusfield, M.; Welburn, S.C. Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Vet. Res. 2011, 7, 60. [Google Scholar] [CrossRef]
- Mugizi, D.R.; Boqvist, S.; Nasinyama, G.W.; Waiswa, C.; Ikwap, K.; Rock, K.; Lindahl, E.; Magnusson, U.; Erume, J. Prevalence of and factors associated with Brucella sero-positivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J. Vet. Med. Sci. 2015, 77, 557–564. [Google Scholar] [CrossRef]
- Gill, J.; Kaur, S.; Joshi, D.; Sharma, J. Epidemiological studies on brucellosis in farm animals in Punjab state of India and its public health significance. In Proceedings of the 9th International Symposium on Veterinary Epidemiology and Economics, Breckenridge, CO, USA, 6–11 August 2000. [Google Scholar]
- Patel, M.; Patel, P.; Prajapati, M.; Kanani, A.N.; Tyagi, K.K.; Fulsoundar, A.B. Prevalence and risk factor’s analysis of bovine brucellosis in peri-urban areas under intensive system of production in Gujarat, India. Vet. World 2014, 7, 509–516. [Google Scholar] [CrossRef]
- Aulakh, H.K.; Patil, P.K.; Sharma, S.; Kumar, H.; Mahajan, V.; Sandhu, K.S. A study on the epidemiology of bovine brucellosis in Punjab (India) using milk-ELISA. Acta Vet. Brno. 2008, 77, 393–399. [Google Scholar] [CrossRef]
- Ul-Islam, M.R.; Gupta, M.P.; Filia, G.; Sidhu, P.K.; Shafi, T.A.; Bhat, S.A.; Hussain, S.A.; Mustafa, R.; Verma, A.K.; Sinha, D.K. Sero-epidemiology of brucellosis in organized cattle and buffaloes in Punjab (India). Adv. Anim. Vet. Sci. 2013, 1, 5–8. [Google Scholar]
- Dhand, N.K.; Gumber, S.; Singh, B.B.; Aradhana; Bali, M.S.; Kumar, H.; Sharma, D.R.; Singh, J.; Sandhu, K.S. A study on the epidemiology of brucellosis in Punjab (India) using Survey Toolbox. Rev. Sci. Tech. 2005, 24, 879–885. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Deka, R.P.; Melin, D.; Berg, A.; Lundén, H.; Lapar, M.L.; Asse, R.; Grace, D. An inclusive and participatory approach to changing policies and practices for improved milk safety in Assam, northeast India. Glob. Food Sec. 2018, 17, 9–13. [Google Scholar] [CrossRef]
- Chakraborty, M.; Patgiri, G.P.; Barman, N.N. Application of delayed-type hypersensitivity test (DTH) for the diagnosis of bovine brucellosis. Indian Vet. J. 2000, 77, 849–851. [Google Scholar]
- Gogoi, S.B.; Hussain, P.; Sarma, P.C.; Barua, A.G.; Mahato, G.; Bora, D.P.; Konch, P.; Gogoi, P. Prevalence of bovine brucellosis in Assam, India. J. Entomol. Zool. Stud. 2017, 5, 179–185. [Google Scholar]
- Renukaradhya, G.; Isloor, S.; Rajasekhar, M. Epidemiology, zoonotic aspects, vaccination and control/eradication of brucellosis in India. Vet. Microbiol. 2002, 90, 183–195. [Google Scholar] [CrossRef]
- Hussain, S.A.; Rahman, H.; Pal, D.; Ahmed, K. Sero-prevalence of bovine and human brucellosis in Assam. Indianj. Comp. Microbiol. Immunol. Infect. Dis. 2000, 21, 165–166. [Google Scholar]
- Yohannes Gemechu, M.; Paul Singh Gill, J. Seroepidemiological survey of human brucellosis in and around Ludhiana, India. Emerg. Health Threat. J. 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Heffernan, C.; Thomson, K.; Nielsen, L. Caste, livelihoods and livestock: An exploration of the uptake of livestock vaccination adoption among poor farmers in India. J. Int. Dev. 2011, 23, 103–118. [Google Scholar] [CrossRef]
- Basunathe, V.K.; Sawarkar, S.W.; Sasidhar, P.V.K. Adoption of Dairy Production Technologies and Implications for Dairy Development in India. Outlook Agric. 2010, 39, 134–140. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Young, J.; Wyatt, A.; Young, M.; Alders, R.; Bagnol, B.; Kibaya, A.; Grace, D. Do vaccination interventions have effects? A study on how poultry vaccination interventions change smallholder farmer knowledge, attitudes, and practice in villages in Kenya and Tanzania. Trop. Anim. Health Prod. 2018, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
| All Sites | Guwahati | Bangalore | Bhubaneswar | Ludhiana | Udaipur | p-Value * | |
|---|---|---|---|---|---|---|---|
| Age of farmer | 46.2 (12.3) | 43.9 (12.9) | 44.8 (13.2) | 51.1 (10.8) | 46.6 (12.7) | 44.6 (10.2) | 0.035 |
| Female respondents | 21.8% (18.3–25.6) | 15.7% (9.2–24.2) | 41.2% (31.5–51.4) | 11.8% (6.2–19.6) | 14.7% (8.5–23.1) | 25.5% (17.4–35.1) | 0.096 |
| Illiterate respondents | 35.4% (31.0–39.9) | 51.1% (40.2–61.9) | 41.1% (30.8–52.0) | 23.5% (15.7–33.0) | 15.7% (8.6–25.3) | 44.9% (34.8–55.3) | 0.001 |
| Number of dairy animals | 7.7 (4.0) | 10.3 (4.1) | 5.4 (2.7) | 8.1 (3.6) | 7.2 (3.7) | 7.5 (4.0) | <0.001 |
| Zero-grazing | 53.3% (48.9–57.7) | 95.1% (88.9–98.3) | 9.8% (4.8–17.2) | 4.9% (1.6–11.1) | 93.1% (86.4–97.2) | 63.7% (53.6–73.0) | <0.001 |
| Using AI | 76.0% (72.1–79.7) | 92.2% (85.1–96.6) | 98.0% (93.1–99.8%) | 47.5% (37.5–57.7) | 69.6% (59.7–78.3) | 72.5% (62.8–80.9) | <0.001 |
| Purchasing cows from neighboring farms | 57.6% (52.8–62.3) | 98.9% (94.0–100) | 71.7% (57.7–83.2) | 21.1% (13.4–30.6) | 34.4% (24.9–45.0) | 69.0% (59.0–77.9) | <0.001 |
| Dirty floors in cow sheds | 11.1% (8.5–14.2) | 24.8% (16.7–34.3) | 11.0% (5.6–18.8) | 10.8% (5.5–18.5) | 6.1% (2.3–12.7) | 3.0% (0.6–8.4) | <0.001 |
| Well-drained floors | 19.8% (16.4–23.6) | 8.9% (4.2–16.2) | 22.8% (15.2–32.5) | 23.5% (15.7–33.0) | 35.4% (26.0–45.6) | 8.9% (4.2–16.2) | 0.007 |
| Never vaccinate animals | 15.5% (12.4–18.9) | 25.5% (17.4–35.1) | 0% (0–3.6) | 2.0% (0.2–6.9) | 7.8% (3.4–14.9) | 42.42% (32.4–52.3) | <0.001 |
| Vaccinate young animals routinely | 26.7% (22.9–30.7) | 61.8% (51.6–71.2) | 2.9% (0.6–8.4) | 44.1% (34.3–54.3) | 0% (0–3.6) | 24.5% (16.5–34.0) | <0.001 |
| Records of sick animals | 11.6% (8.0–14.7) | 0% (0–3.6) | 8.8% (4.1–16.1) | 24.5% (16.5–34.0) | 1.0% (0–5.3) | 23.5% (15.7–33.0) | <0.001 |
| Alpha score for cleaning routines | 2.19 (0.28) | 2.14 (0.23) | 2.10 (0.28) | 2.16 (0.08) | 2.06 (0.13) | 2.50 (0.34) | 0.034 |
| Alpha score for observed hygiene | 0.32 (0.28) | 0.10 (0.11) | 0.31 (0.21) | 0.60 (0.31) | 0.25 (0.21) | 0.35 (0.23) | <0.001 |
| Regular health checks | 23.9% (20.3–27.9) | 3.9% (1.1–9.7) | 14.7% (8.5–23.1) | 33.3% (24.3–43.4) | 46.1% (36.2–56.2) | 21.6% (14.0–30.8) | <0.001 |
| Let veterinarian check animals before purchase, or test the animal | 25.9% (22.1–29.9) | 2.9% (0.6–8.4) | 35.3% (26.1–45.4) | 58.8% (48.6–68.5) | 9.8% (4.8–17.3) | 22.6% (14.9–31.9) | <0.001 |
| Quarantine new animals | 24.6% (20.8–28.4) | 17.7% (10.8–26.4) | 19.6% (12.4–28.6) | 41.4% (31.6–51.8) | 19.6% (12.4–28.6) | 24.5% (16.5–34.0) | 0.074 |
| Brucella Farm Positivity | Brucella Farm Positivity Excluding Farms with Vaccination | |
|---|---|---|
| Bangalore | 2.9% (0.6–8.4) | 3.0% (0.6–8.6) |
| Bhubaneswar | 2.0% (0.2–6.9) | 1.4% (0–7.4) |
| Guwahati | 73.5% (63.9–81.8) | 72.5% (62.5–81.0) |
| Ludhiana | 4.9% (1.6–11.1) | 4.3% (1.2–10.6) |
| Udaipur | 5.9% (2.2–12.4) | 5.2% (1.7–11.6) |
| Overall | 17.8% (14.6–21.34) | 18.3% (14.8–22.1) |
| Risk Factor | Odds Ratio | 95% Confidence Interval | Standard Error | p-Value |
|---|---|---|---|---|
| Farmer age (year) | 0.96 | 0.90–1.02 | 0.03 | 0.20 |
| Floor cleanliness | ||||
| Clean | Reference | |||
| Average | 11.6 | 1.29–105.18 | 13.1 | 0.03 |
| Dirty | 42.8 | 1.87–978.57 | 68.3 | 0.02 |
| Vaccination | ||||
| No vaccination | Reference | |||
| Vaccinate irregularly | 44.1 | 0.73–2669.57 | 92.3 | 0.07 |
| Vaccinate routinely | 12.8 | 1.40–116.80 | 14.4 | 0.02 |
| Number of animals | 1.0 | 0.85–1.30 | 0.4 | 0.7 |
| Quadratic term of number of animals | 0.95 | 0.90–0.999 | 0.02 | 0.05 |
| Constant | 2.2 | 0.05–91.5 | 4.1 | |
| Village level variance | 4.2 | 0.65–26.84 | 4.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindahl, J.F.; Gill, J.P.S.; Hazarika, R.A.; Fairoze, N.M.; Bedi, J.S.; Dohoo, I.; Chauhan, A.S.; Grace, D.; Kakkar, M. Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities. Trop. Med. Infect. Dis. 2019, 4, 70. https://doi.org/10.3390/tropicalmed4020070
Lindahl JF, Gill JPS, Hazarika RA, Fairoze NM, Bedi JS, Dohoo I, Chauhan AS, Grace D, Kakkar M. Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities. Tropical Medicine and Infectious Disease. 2019; 4(2):70. https://doi.org/10.3390/tropicalmed4020070
Chicago/Turabian StyleLindahl, Johanna F., Jatinder Paul Singh Gill, Razibuddin Ahmed Hazarika, Nadeem Mohamed Fairoze, Jasbir S. Bedi, Ian Dohoo, Abhimanyu Singh Chauhan, Delia Grace, and Manish Kakkar. 2019. "Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities" Tropical Medicine and Infectious Disease 4, no. 2: 70. https://doi.org/10.3390/tropicalmed4020070
APA StyleLindahl, J. F., Gill, J. P. S., Hazarika, R. A., Fairoze, N. M., Bedi, J. S., Dohoo, I., Chauhan, A. S., Grace, D., & Kakkar, M. (2019). Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities. Tropical Medicine and Infectious Disease, 4(2), 70. https://doi.org/10.3390/tropicalmed4020070







