Abstract
The tuberculosis (TB) health burden in Fiji has been declining in recent years, although challenges remain in improving control of the diabetes co-epidemic and achieving adequate case detection across the widely dispersed archipelago. We applied a mathematical model of TB transmission to the TB epidemic in Fiji that captured the historical reality over several decades, including age stratification, diabetes, varying disease manifestations, and incorrect diagnoses. Next, we simulated six intervention scenarios that are under consideration by the Fiji National Tuberculosis Program. Our findings show that the interventions were able to achieve only modest improvements in disease burden, with awareness raising being the most effective intervention to reduce TB incidence, and treatment support yielding the highest impact on mortality. These improvements would fall far short of the ambitious targets that have been set by the country, and could easily be derailed by moderate increases in the diabetes burden. Furthermore, the effectiveness of the interventions was limited by the extensive pool of latent TB infection, because the programs were directed at only active cases, and thus were unlikely to achieve the desired reductions in burden. Therefore, it is essential to address the co-epidemic of diabetes and treat people with latent TB infection.
1. Introduction
Tuberculosis (TB) remains endemic in the Republic of Fiji (hereafter referred to as Fiji) with an estimated incidence of 59 new cases per 100,000 population in 2016 (95% confidence interval [CI] 45–75) [1]. The fight against TB in the South Pacific Ocean region in general and in Fiji in particular is complicated by local challenges, including a substantial part of the population living in isolated rural areas and high rates of type 2 diabetes mellitus (T2DM) [2]. Fiji comprises more than 100 inhabited islands, most of which are remote from the main two islands (Viti Levu and Vanua Levu) where the TB clinics are located, constituting a challenge for case detection and patient management. The TB epidemic in Fiji is significantly driven by T2DM, which is on the rise and affects around 16% of the Fijian population aged 25 years and above [3]. T2DM is estimated to increase the risk of developing TB disease around threefold [4], making this comorbidity an important concern for the national TB control program (NTP). By contrast, HIV is not a major driver of the TB epidemic in Fiji, due to its very low prevalence in the country [5].
Directly observed therapy short course (DOTS) has been the standard of TB care in Fiji since 1997, with most patients being hospitalised at the start of the treatment, but later receiving community-based treatment [6]. This has contributed to sustained high rates of treatment success over the last two decades, and to minimal multidrug-resistant TB (MDR-TB) in the country. Although the first ever case of MDR-TB was identified in 2016, there is no evidence of transmission within the country.
Since 2010, the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM) has been the major donor to the Fiji NTP, contributing approximately half the country’s total funding for TB. This funding has enabled strengthened active case-finding (ACF) activities and the implementation of GeneXpert for rapid diagnosis and the identification of rifampicin resistance. However, following the updated classification of Fiji as an upper–middle income (previously lower–middle income) country by the World Bank in 2012, Fiji has become ineligible for future GFATM funding, and the final grant from GFATM ended in 2017. With this return to almost fully domestically funded TB services, it is critical to strengthen and improve the efficiency of TB programs in Fiji. This is particularly important if the country is to achieve the NTP’s targets of 80% reduction in TB incidence and 90% reduction in TB mortality by 2030, which are in line with the Sustainable Development Goals (SDGs) and the End TB Strategy.
Transmission modelling is increasingly used to make predictions about the future trajectory of the TB epidemic, as well as effectiveness and costs associated with planned control interventions. It has become a critical tool for policy makers in the cyclical process of evaluating current programs, setting future priorities, and developing funding requests to donors. We previously developed and described the AuTuMN software platform to guide country-level TB decision making and strategic planning [7]. Here, we describe the application of this tool to TB control in Fiji.
2. Materials and Methods
2.1. General Approach
The approach to development of the AuTuMN software platform is described in detail in Trauer et al. [7]. Briefly, the software assists countries with TB decision making and strategic planning by providing (i) predictions of the future TB epidemic trajectory based on current epidemiology and programmatic responses, (ii) the likely epidemiological and economic impact resulting from future changes to the programmatic response, and (iii) the cost-effectiveness of TB programs. The full details of the model are described in the Supplementary Materials and summarised as follows. Given the slow moving and complex nature of the TB epidemic, we aimed for consistency with historical TB dynamics in Fiji for many decades into the past, and allowed a high degree of model complexity. To achieve this, we first loaded the publicly available data for birth, death, Bacillus Calmette–Guérin (BCG) vaccination and TB-related programmatic measures and fit functions mapping parameter values to time for these quantities using polynomial spline functions (Supplement, pages 3–8).
2.2. Background Demographics
Patients are first born into the model with the time-variant birth rate fit to World Bank data (Figure S1) and proportions of births vaccinated and unvaccinated are split according to the vaccination coverage data from the World Health Organization (WHO) or the United Nations Children’s Fund (UNICEF) (Figure S2). Population-wide natural mortality was also parameterised to data from the World Bank (Figure S3) and was applied to all the compartments. All the compartments were stratified by age, with age groups being: under 5 years, 5 to 15 years, 15 to 25 years, and 25 years and above. The proportion of the population with T2DM was considered as a time-variant parameter and applied to the oldest age group only (Figure S9).
2.3. Model of TB Transmission and Progression
The force of infection was calculated as the number of infectious persons, including undiagnosed persons, missed false-negative presentations who had yet to seek additional care, and patients in very early stage treatment. All the paediatric (<15 years old) and smear-negative TB patients were considered to have markedly reduced infectiousness compared to adults. The force of infection was further modified according to the susceptibility/immunity status of the susceptible group, with BCG-vaccinated children retaining immunity from vaccination until 15 years of age, and latently infected persons also having a markedly reduced risk of infection (Supplement Table S1 and page 16). The relative immunity attributable to previously treated disease episodes is highly uncertain, and so was varied as a calibration/uncertainty parameter.
Following infection, the latency period to active TB disease was simulated as two sequential compartments, with infected persons initially entering a high-risk early latent state before transitioning to a lower risk late latent compartment [8,9]. The proportion of infected persons progressing to active TB disease from early latency is greater for children than adults, and each new episode of active TB disease is distinguished according to smear status into smear-positive, smear-negative pulmonary, and extrapulmonary using country-specific notification data. Persons with T2DM have an approximately threefold increased risk of progression to active TB disease following infection (Supplement pages 24–25) [4].
An untreated episode of active TB was assumed to last for around three years (although uncertainty was included around this parameter), with a greater case fatality rate for smear-positive active TB than for smear-negative and extrapulmonary disease (Supplement page 17) [10].
2.4. Simulation of the Health Care System
The rate of detection of active TB patients by the health care system was set such that the proportion of cases that were detected—rather than reaching an outcome as a consequence of the natural history of their disease episode (i.e., spontaneous recovery or death)—was equal to the reported time-variant case detection rate (Supplement pages 18–19). Smear-positive and extrapulmonary patients commenced treatment after a shorter delay than smear-negative patients, since their diagnosis relies on clinical judgement and sputum smear rather than culture. Treatment outcomes were divided into success, death, and/or unfavourable outcomes other than death, with treatment rendering the patient non-infectious after 10 days and lasting for six months in total. The probability of reaching each of these three outcomes varied with time and differed according to treatment history (Supplement pages 20–21).
2.5. Automatic Calibration and Uncertainty
We used a Metropolis algorithm to calibrate the model to align with the TB incidence estimated by the WHO for the years 2010 to 2016, by adjusting five important but uncertain parameters: the effective contact rate, the TB epidemic starting time, the relative risk of reinfection in previously treated individuals, the case fatality ratio for untreated TB cases, and the duration of untreated active disease. A Metropolis algorithm is a Markov chain Monte Carlo method that enables sampling from the posterior distribution of the model parameters [11]. That is, it identifies the model parameter sets that are best able to replicate the trends observed in the data. Details about the Metropolis algorithm used in this study are available in the Supplement (pages 12–14). To match reported mortality rates, it was necessary to incorporate a parameter reflecting the proportion of deaths among TB patients who never reached the health system that contributed to the reported mortality. Comparison to prevalence and trends in reported mortality over time were assessed to validate calibration, but were not part of the calibration algorithm. The model runs accepted by the Metropolis algorithm (which were associated with the posterior parameter distributions) were used to assess uncertainty around the reported epidemiological outputs, and the parameter set with the greatest likelihood from 10,000 iterations was used to project the future epidemiology under scenario conditions.
2.6. Interventions
In addition to the baseline conditions in which all the time-variant parameters were maintained at their 2016 values, we developed a set of six programmatic interventions in collaboration with the Fiji NTP (Table 1). These interventions were intended to reflect the real-world programmatic changes that are currently under consideration by the country stakeholders. The interventions were modelled by changing the relevant model parameters from their baseline values, using the continuous scale-up function to increase the values progressively from 2017 to 2020. These changes were informed by reviews of the literature undertaken by all the authors, after which two authors (J.T., T.D.) who were blinded to each other’s assessments assessed the standard of evidence for the interventions implemented. Then, agreement between the two blinded assessments was completed. We also categorised the type of evidence that was used to estimate the effectiveness of interventions, and distinguish between the directly applicable programmatic evidence and the evidence from clinical studies that was extrapolated to the assumed programmatic effect.

Table 1.
Intervention implementation.
2.7. Economic Analysis
To estimate the costs of interventions, we used a logistic function to describe the association between cost and coverage of an intervention, as previously described [7]. Estimations of economic inputs were conducted in collaboration with the Fiji NTP and are described in the Supplement (pages 44–49), with the economic parameters presented in Table S2 and the cost-coverage curves presented in Figure S27. The economic analysis was performed from a health care provider perspective, with all costs in 2017 US dollars.
3. Results
3.1. Calibration and Validation
Figure 1 presents the results of the automatic calibration. The model predictions for TB prevalence remarkably matched the official statistics, although these were not included as calibration targets. Similarly, the reported notifications were also very closely matched by calibration to incidence only, with both the absolute numbers of notified cases as well as the variation in numbers with time accurately reflected by the model. Although the pattern of mortality over time is similar to official statistics, it must be stressed that this model output primarily reflects deaths occurring in the health system, as discussed in the Methods and Discussion sections.
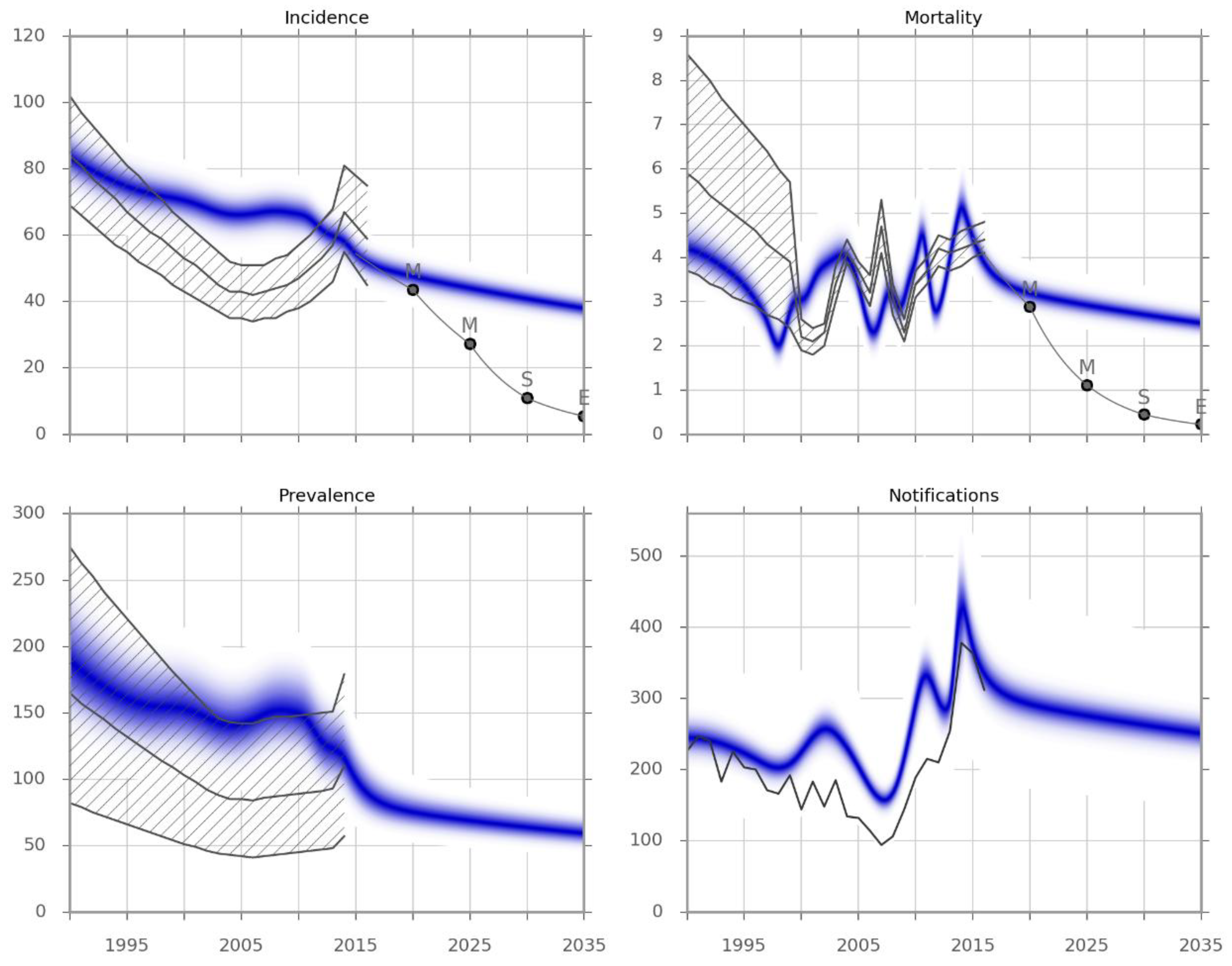
Figure 1.
Model calibration results. Incidence and observed mortality (per 100,000 per year), prevalence (per 100,000) and the number of notifications by calendar year. The blue-shaded areas represent the calibrated model predictions obtained from the Metropolis simulation. The grey lines represent point estimates, and the hatched areas represent the confidence limits for each indicator from the Global TB Report 2017. The light grey line indicates the epidemic trajectory that would be required to achieve the different targets, and is a piecewise exponential function. M, Milestone; S, Sustainable Development Goal; E, End TB Strategy Target.
The baseline conditions that were carried forward—which included maintenance of the recent improvements in the case detection rate (above 60%) and the treatment success rates (above 85%)—are expected to result in a slow but steady decline in disease burden. However, this decline is far from that required to meet the End TB Strategy milestones or targets.
3.2. Intervention Effectiveness
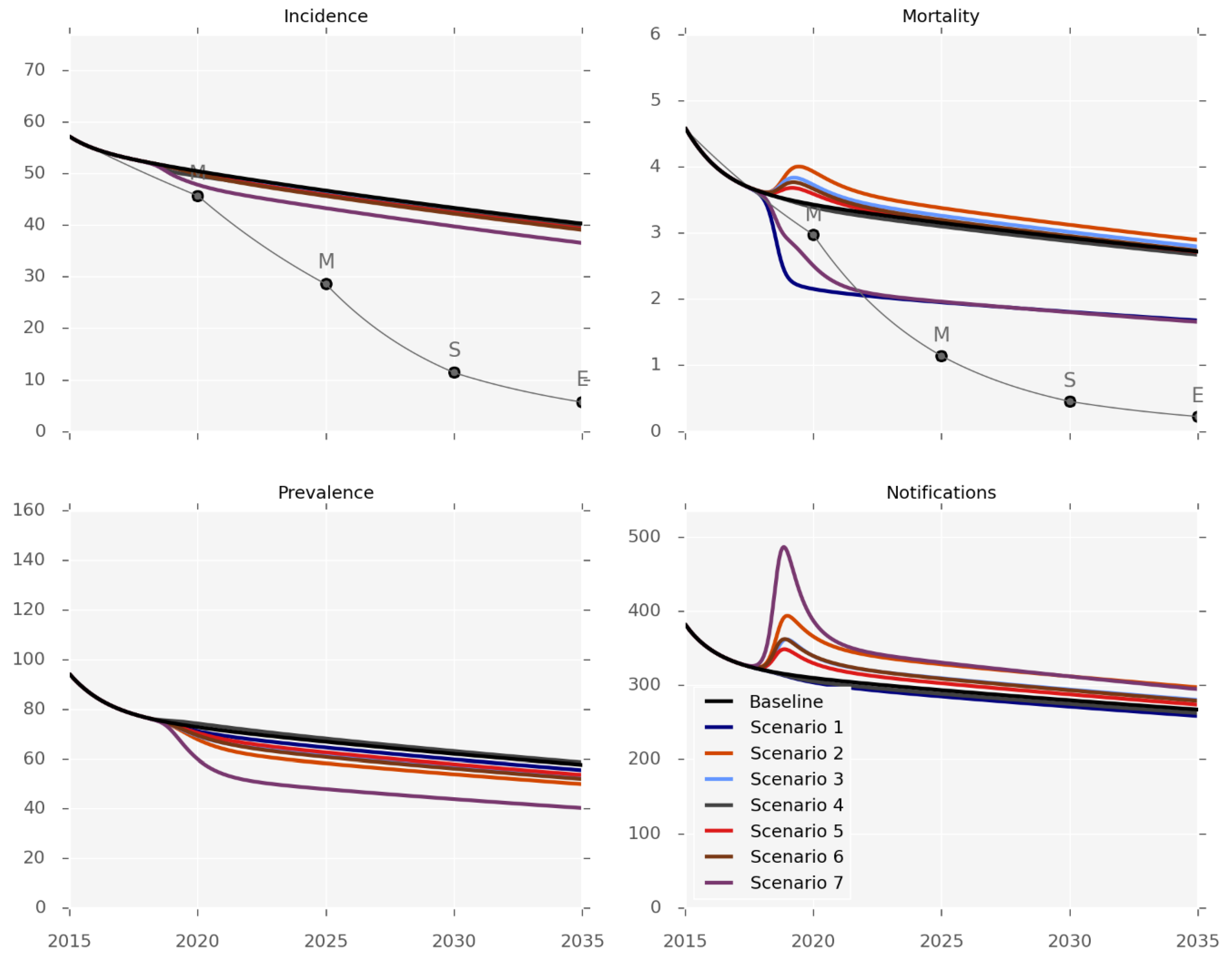
Figure 2, Table 2 and the Supplement (pages 30–43) present the anticipated effectiveness of each of the interventions. The outputs should be interpreted in the context of the differing levels and applicability of evidence for each one, and the caveat that changes in mortality predominantly reflect health system-related deaths. None of the six interventions was sufficient to achieve any but the first set of the End TB Strategy milestones (for 2020). The impact on disease incidence would be particularly limited, as none of the individual interventions could achieve more than an incidence reduction of 3% by 2035 (Table 2), and only a 10% reduction would be achieved by the very optimistic and arguably unrealistic mix of implementing all the programs simultaneously (Scenario 7). In contrast, the effect on disease prevalence was predicted to be more pronounced, as a 17% reduction by 2035 could be achieved by using decentralisation or GeneXpert replacing smear-microscopy (scenarios 2 and 3). Identifying additional cases would increase the number of notifications under all of the scenarios except for treatment support (Scenario 1).
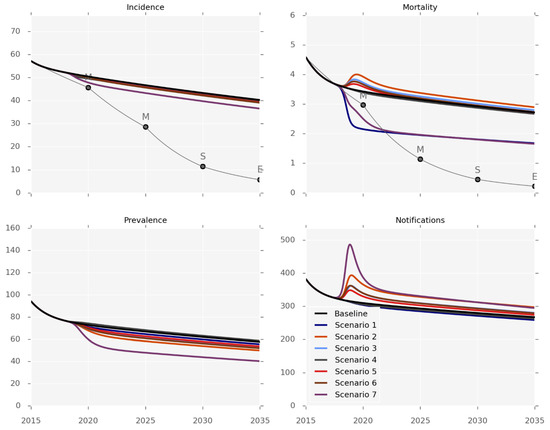
Figure 2.
Intervention effectiveness. Incidence and observed mortality (per 100,000 per year), prevalence (per 100,000) and number of notifications by calendar year.

Table 2.
Predicted intervention effectiveness. Values in brackets represent 95% simulation intervals.
The mortality predictions that are presented in Figure 2 pertain to the mortality rates captured by surveillance systems, such that most of the control interventions increased the observed TB-related mortality by increasing the proportion of active cases known to the health system. The intervention impact on the true mortality (including undetected TB deaths) is presented in Figure S26, with treatment support being the most effective intervention for reducing TB-related deaths. We estimate that the true mortality could be reduced by 23% by 2035 with this program, while none of the other interventions reduced mortality by more than 12%.
The costs of each program are markedly different, ranging from isoniazid preventive therapy (IPT), which is predicted to be very cheap, to GeneXpert-based ACF, which would cost approximately USD 31 million a year.
3.3. Impact of T2DM Burden on TB Epidemic
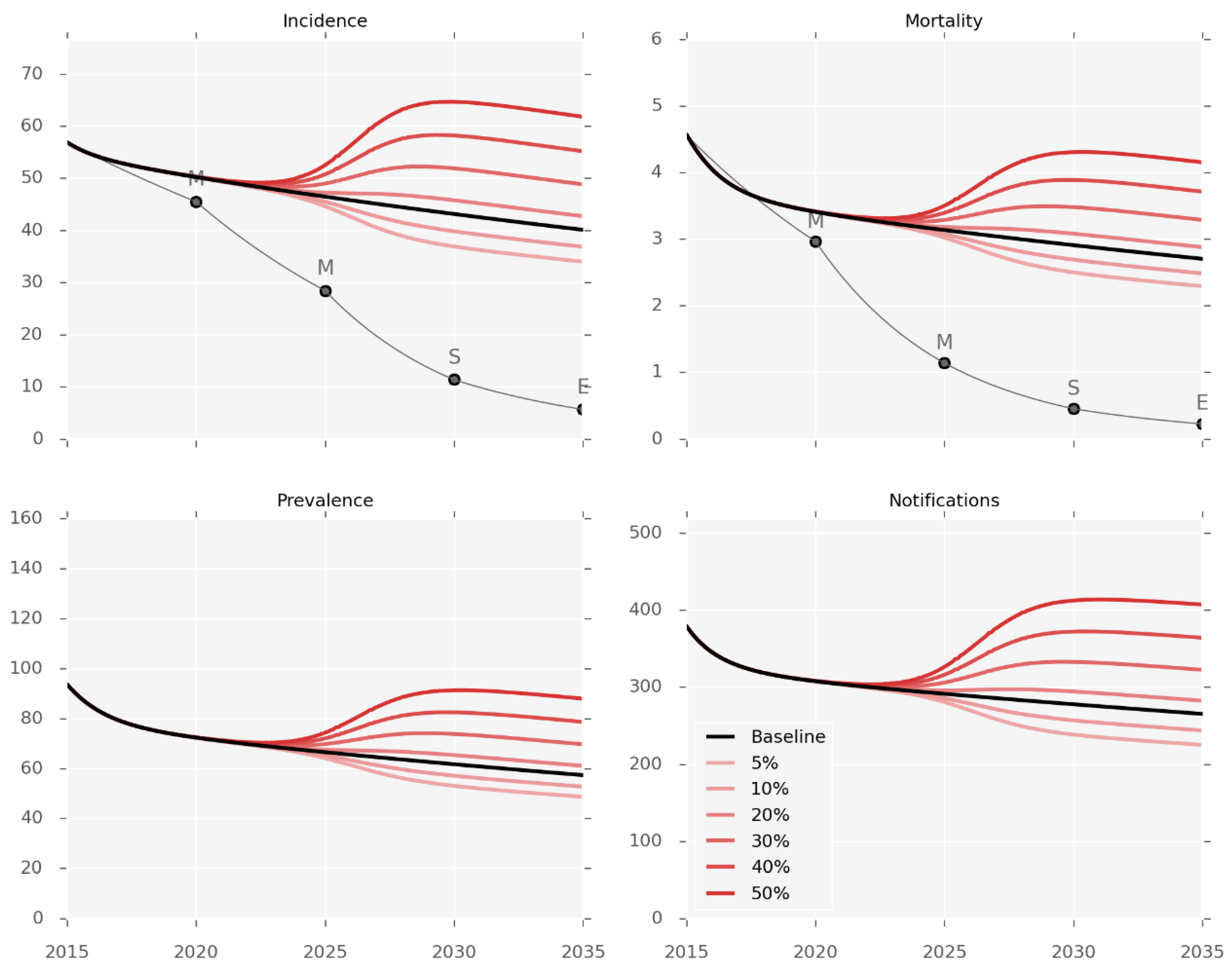
Simulations of changes in the future burden of T2DM predict a marked impact on the burden of TB (Figure 3). The population prevalence of T2DM was changed from the baseline value of 15.6% in 2017 to reach each counterfactual prevalence by 2035, using a sigmoidal function. These changes are associated with considerable changes in disease burden as compared to the baseline prediction for 2035, ranging from an incidence decrease of 16% with T2DM prevalence of 5%, to an incidence increase of 58% when T2DM prevalence reaches 50%.
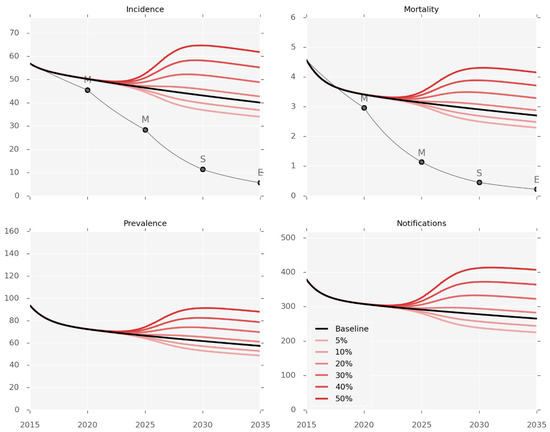
Figure 3.
Diabetes prevalence counterfactuals. Incidence and observed mortality (per 100,000 per year), prevalence (per 100,000), and number of notifications by calendar year. Red lines represent the different levels of T2DM prevalence varying from 5% to 50%. The baseline scenario (15.6% T2DM prevalence) is shown in black.
4. Discussion
We predict that TB rates are likely to fall in the coming years in Fiji, but to an extent far from that required to achieve the ambitious goals set by the Fiji NTP. Support for patients under treatment (Scenario 1) is likely to have the greatest impact on observed mortality rates, while raising awareness (Scenario 6) should have the greatest impact on incidence. However, realistic changes in diabetes prevalence are predicted to result in changes to disease burden that are comparable to or greater than these intervention-related effects.
Calibration of our model reveals important insights into TB transmission dynamics in Fiji. Calibration to incidence did not closely match the marked swings in incidence reported in WHO estimates over recent years. However, although prevalence, notifications, and mortality were not calibration targets, our results closely matched each of these important outputs, validating the model results and suggesting that the reported wide swings in incidence may be artefactual. Changes in a range of other programmatic and epidemiological processes over recent years result in a steady decline in each of these markers of TB burden. The recent peak in notifications reflects the improvement in case detection, which—if maintained—would be expected to be followed by a decline in notifications through decreased TB transmission. Moreover, it should be noted that our approach to determining incidence and notifications through the fitting of a mechanistic model would typically result in directly opposite results to the WHO approach of inferring incidence partly from notifications.
Baseline projections of continuing the current programmatic response in Fiji predicts a gradual decline in disease burden that would fall far short of the country’s targets for incidence and mortality for 2020 or the End TB Targets for 2035. Even under the scenario in which all the interventions are simultaneously implemented with high efficacy and coverage (Scenario 7), the End TB Targets still cannot be reached. This is because the pool of latent TB infection (LTBI) acquired before 2016 will continue to drive incidence rates long after this time, especially in the adult population. We estimate that incidence will remain at least 20 per 100,000 in 2035 even if transmission were immediately and completely curtailed. Therefore, any “game changer” intervention must address the issue of reactivation of LTBI, whether by treatment of LTBI or novel vaccination to prevent reactivation.
Increasing the use of IPT to include older children aged between 5–15 years and increasing the coverage in younger children aged between 0–5 years has a substantial impact on reducing incidence, mortality, and prevalence. This is in part attributable to children under 15 years accounting for a relatively large proportion (30%) of the total population [18]. In our economic analysis, this intervention is also cheap, as it requires nothing more than providing isoniazid to patients who are already receiving DOTS visits.
Achieving targets for mortality is problematic, as it is believed that a large proportion of TB-related deaths are unknown to the NTP. Depending on the registry, missing deaths can occur. Using the vital registry, a death can be missed if the NTP does not make the necessary communications with the vital registry and a cause of death other than TB is given. Similarly, missing deaths can occur when a case of TB is not notified to the NTP, only diagnosed post-mortem, and not communicated with the NTP. The proportion of cases that are potentially missed can be estimated using a capture–recapture method, which has been performed in Fiji, suggesting that the mortality rates may be more than three times higher than those estimated by NTP. Indeed, our estimates for total mortality are considerably higher than the estimates for known mortality.
Fiji comprises more than 100 islands, many of which have poor infrastructure and access to care. If hard-to-reach population groups—such as people living on remote islands—are targeted, the strategy would be able to reach larger numbers of missed cases and have a greater impact on TB epidemiology. The targeting of hard-to-reach individuals was considered in two of the scenarios. Scenarios 2 and 5 examined the impact of decentralisation and ACF using mobile health clinics (vans or ferries) equipped with GeneXpert, respectively. The aim of these simulated interventions is to have treatment and diagnostic clinics closer to remote communities. We found that these programs are impactful in improving the detection of cases that would have been otherwise missed, and reducing TB incidence and prevalence. However, these interventions are resource-intensive, and decentralisation is supported by evidence that is of poorer quality or has less direct applicability than many of the other forms.
Replacing sputum smear microscopy with GeneXpert as the primary diagnostic test for passive case detection across the health service (Scenario 3) was found to have a modest effect. This is because there is very little rifampicin resistance, and because cultures are currently being widely used in addition to sputum smear microscopy as a standard diagnostic. Therefore, GeneXpert does not offer greater sensitivity than the current algorithm, and its only effect is to achieve slightly quicker diagnosis, which does not result in a significant epidemiological impact.
Changes in the burden of diabetes could have a marked effect on the burden of TB in Fiji, with a change in prevalence of 10% predicted to lead to a change in TB burden that is equivalent to the most effective intervention simulated. Therefore, targeting diabetes and TB as interwoven co-epidemics in Fiji is critical [19].
Limitations of this work include the lack of evidence for the epidemiological and economic parameterisation of some interventions being actively considered by the Fiji NTP. However, the uncertainty analyses performed around the effect of the different interventions did not reveal any changes to the main conclusions. Other limitations are linked to the structural aspects of the model. For example, an explicit spatial structure would be desirable to fully capture the effects of outreach interventions, but this would require additional data that are currently unavailable. Another example is the assumption of homogeneous mixing, which may not be realistic, as there has been evidence of age-assortative mixing in other settings [20]. Again, the lack of local data on social mixing at the time of the model elaboration was the main barrier to heterogeneous mixing implementation. Lastly, as transmission rates continue to fall and case numbers per year decline to double digits, stochastic simulations will be important to capture the full range of possible outcomes. Some of the findings presented here may be applicable to other parts of the Pacific, due to the high rates of diabetes and the geographical challenges for case detection that are common to many such countries [21]. However, there are also dramatic differences between Pacific countries, including huge differences in disease rates between the highest (e.g., Kiribati, the Marshall islands) and lowest (e.g., Samoa) burden countries [22].
5. Conclusions
If current programmatic responses are continued, we predict further gradual declines in the TB burden in Fiji, although these will be well short of the SDGs and End TB targets. Finding additional TB cases over and above the current (mostly passive) case detection is an important component of reducing TB burden. However, addressing the underlying burden of LTBI will be essential if the country is to achieve its disease-related goals. The TB-diabetes co-epidemic is also of particular importance in this setting, and could derail attempts to achieve the ambitious targets of the Fiji NTP.
Supplementary Materials
The following are available online at https://www.mdpi.com/2414-6366/4/2/71/s1, Figures S1 to S27, Tables S1 and S2.
Author Contributions
Conceptualisation, all authors; methodology, R.R., T.D., J.T. and E.M.; software, R.R., J.T.; validation, F.U. and E.R.; formal analysis, R.R., T.D., J.T. and E.M.; investigation, all authors; resources, F.U. and E.R; data curation, F.U. and E.R; writing—original draft preparation, R.R., T.D., J.T. and E.M.; writing—review and editing, all authors; visualisation, R.R. and J.T.; supervision, J.T. and E.M.; project administration, F.U., E.R and E.M.; funding acquisition, T.D., J.T. and E.M.
Funding
This work was primarily funded by the Global Fund to Fight AIDS, Tuberculosis and Malaria, and was partly funded by the Australian Government through the Department of Foreign Affairs and Trade. The views expressed in this publication are the authors’ alone, and are not necessarily the views of the Australian Government.
Acknowledgments
We thank the members of the Fiji National Tuberculosis Program who are not listed as authors for facilitating this study, especially by providing some of the data required to inform the model.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the study.
References
- World Health Organization. WHO TB Country Profile of Fiji; WHO: Geneva, Switzerland; Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=FJ&LAN=EN&outtype=html (accessed on 19 June 2017).
- Fiji Ministry of Health and Medical Services. TB National Strategic Plan 2015–2019; Fiji Ministry of Health and Medical Services: Suva, Fiji, 2014.
- Lin, S.; Tukana, I.; Linhart, C.; Morrell, S.; Taylor, R.; Vatucawaqa, P.; Magliano, D.J.; Zimmet, P. Diabetes and obesity trends in Fiji over 30 years. J. Diabetes 2016, 8, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, 1091–1101. [Google Scholar]
- World Health Organization. Country Profiles on HIV. Available online: http://www.wpro.who.int/hiv/data/countries/fji/en/ (accessed on 19 June 2017).
- Alo, A.; Gounder, S.; Graham, S.M. Clinical characteristics and treatment outcomes of tuberculosis cases hospitalised in the intensive phase in Fiji. Public Heal. Action 2014, 4, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Trauer, J.M.; Ragonnet, R.; Doan, T.N.; McBryde, E.S. Modular programming for tuberculosis control, the ‘AuTuMN’ platform. BMC Infect. Dis. 2017, 17, 546. [Google Scholar] [CrossRef] [PubMed]
- Ragonnet, R.; Trauer, J.M.; Scott, N.; Meehan, M.T.; Denholm, J.T.; McBryde, E.S. Optimally capturing latency dynamics in models of tuberculosis transmission. Epidemics 2017, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Trauer, J.M.; Moyo, N.; Tay, E.-L.; Dale, K.; Ragonnet, R.; McBryde, E.S.; Denholm, J.T. Risk of Active Tuberculosis in the Five Years Following Infection... 15%? Chest 2016, 149, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Tiemersma, E.W.; van der Werf, M.J.; Borgdorff, M.W.; Williams, B.G.; Nagelkerke, N.J. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS ONE 2011, 6, e17601. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.K. Monte Carlo Sampling Methods Using Markov Chains and Their Applications. Biometrika 1970, 57, 97–109. [Google Scholar] [CrossRef]
- Thiam, S.; LeFevre, A.M.; Hane, F.; Ndiaye, A.; Ba, F.; Fielding, K.L.; Ndir, M.; Lienhardt, C. Effectiveness of a Strategy to Improve Adherence to Tuberculosis Treatment in a Resource-Poor Setting. JAMA 2007, 297, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef]
- Sollai, S.; Galli, L.; de Martino, M.; Chiappini, E. Systematic review and meta-analysis on the utility of Interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: A 2013 update. BMC Infect. Dis. 2014, 14, S6. [Google Scholar] [CrossRef] [PubMed]
- Smieja, M.J.; Marchetti, C.; Cook, D.J.; Smaill, F.M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Bandason, T.; Duong, T.; Dauya, E.; Makamure, B.; Churchyard, G.J.; Williams, B.G.; Munyati, S.S.; Butterworth, A.E.; Mason, P.R.; et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): A cluster-randomised trial. Lancet 2010, 376, 1244–1253. [Google Scholar] [CrossRef]
- Jaramillo, E. The impact of media-based health education on tuberculosis diagnosis in Cali, Colombia. Health Policy Plan. 2001, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Bank Data: Population Ages 0–14. Washington, DC, USA, 2017. Available online: https://data.worldbank.org/indicator/SP.POP.0014.TO.ZS?locations=FJ (accessed on 19 June 2017).
- Lönnroth, K.; Roglic, G.; Harries, A.D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014, 2, 730–739. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. PLoS MED 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Viney, K.; Brostrom, R.; Nasa, J.; Defang, R.; Kienene, T. Diabetes and tuberculosis in the Pacific Islands region. Lancet Diabetes Endocrinol 2014, 2, 932. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2016. WHO: Geneva, Switzerland, 2017. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 19 June 2017).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).