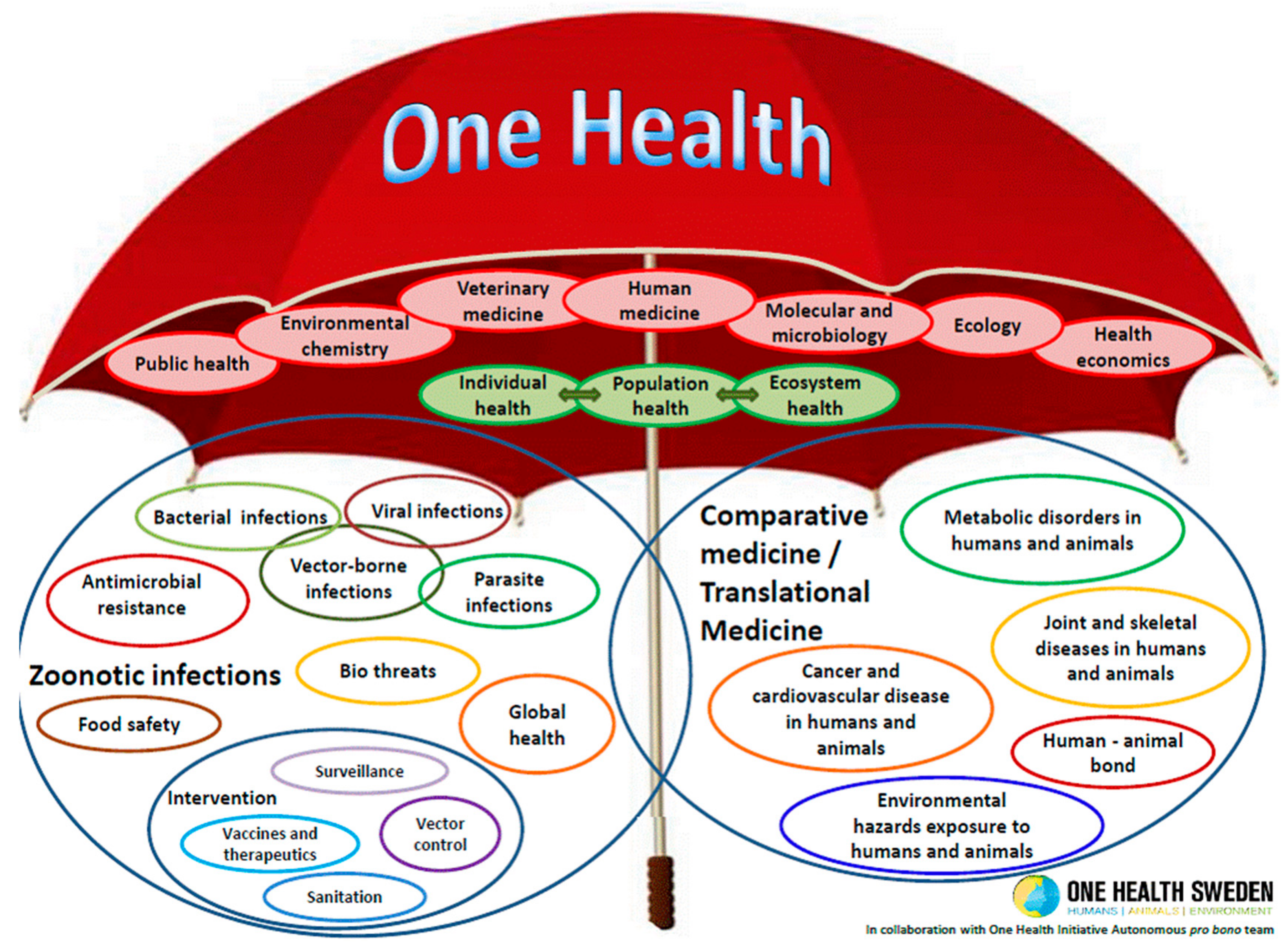
The One Health Approach—Why Is It So Important?
Funding
Conflicts of Interest
References
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; Shope, R.E.; Oaks, S.C. (Eds.) Institute of Medicine. Emerging Infections. Microbial Threats to the United States; National Academy Press: Washington, DC, USA, 1992. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25121245 (accessed on 23 May 2019).
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecologyof zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Wildlife Conservation Society. One World-One Health: Building Interdisciplinary Bridges. 2004. Available online: http://www.oneworldonehealth.org/sept2004/owoh_sept04.html (accessed on 22 May 2019).
- Mackenzie, J.S.; McKinnon, M.; Jeggo, M. One Health: From Concept to Practice. In Confronting Emerging Zoonoses: The One Health Paradigm; Yamada, A., Kahn, L.H., Kaplan, B., Monath, T.P., Woodall, J., Conti, L., Eds.; Springer: Tokyo, Japan, 2014; pp. 163–189. [Google Scholar] [CrossRef]
- IMCAPI. International Ministerial Conference: Animal and Pandemic Influenza: The Way Forward. In Hanoi Declaration; IMCAPI: Hanoi, Vietnam, 2010; Available online: http://www.un-influenza.org/?q=content/hanoi-declaration (accessed on 22 May 2019).
- IMCAPI. Contributing to One World, One Health: A Strategic Framework for Risks of Infectious Diseases at the Animal-Human-Ecosystems Interface. Available online: http://www.fao.org/3/aj137e/aj137e00.pdf (accessed on 23 May 2019).
- Atlas, R.M. One Health: Its origins and future. Curr. Top. Microbiol. Immunol. 2013, 365, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Sharp, J.; Abela-Ridder, B.; Allan, K.J.; Buza, J.; Crump, J.A.; Davis, A.; Del Rio Vilas, V.J.; de Glanville, W.A.; Kazwala, R.R.; et al. One Health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160168. [Google Scholar] [CrossRef] [PubMed]
- Welburn, S.C.; Beange, I.; Ducrotoy, M.J.; Okello, A.L. The neglected zoonoses--the case for integrated control and advocacy. Clin. Microbiol. Infect. 2015, 21, 433–443. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO, FAO, and OIE Unite in the Fight Antimicrobial Resistance. Available online: https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/amr_tripartite_flyer.pdf?ua=1 (accessed on 24 May 2019).
- WHO. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. Available online: https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf;jsessionid=1853DF68D3CE5791633C651325955956?sequence=1 (accessed on 24 May 2019).
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Ceric, O.; Tyson, G.H.; Goodman, L.B.; Mitchell, P.K.; Zhang, Y.; Prarat, M.; Cui, J.; Peak, L.; Scaria, J.; Antony, L.; et al. Enhancing the One Health initiative by using whole genome sequencing to monitor antimicrobial resistance of animal pathogens: Vet-LIRN collaborative project with veterinary diagnostic laboratories in United States and Canada. BMC Vet. Res. 2019, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef] [PubMed]
- Boqvist, S.; Söderqvist, K.; Vågsholm, I. Food safety challenges and One Health. within Europe. Acta Vet. Scand. 2018, 60, 1. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, P.M.; Natterson-Horowitz, B.J.; Kahn, L.H.; Kock, R.; Pappaioanou, M. Incorporating one health into medical education. BMC Med. Educ. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. https://doi.org/10.3390/tropicalmed4020088
Mackenzie JS, Jeggo M. The One Health Approach—Why Is It So Important? Tropical Medicine and Infectious Disease. 2019; 4(2):88. https://doi.org/10.3390/tropicalmed4020088
Chicago/Turabian StyleMackenzie, John S, and Martyn Jeggo. 2019. "The One Health Approach—Why Is It So Important?" Tropical Medicine and Infectious Disease 4, no. 2: 88. https://doi.org/10.3390/tropicalmed4020088
APA StyleMackenzie, J. S., & Jeggo, M. (2019). The One Health Approach—Why Is It So Important? Tropical Medicine and Infectious Disease, 4(2), 88. https://doi.org/10.3390/tropicalmed4020088





