Evaluating the Gaps in the Diagnosis and Treatment in Extra-Pulmonary Tuberculosis Patients Under National Tuberculosis Elimination Programme (NTEP) Guidelines: A Multicentric Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Operational Definitions
2.4.1. EPTB Patients
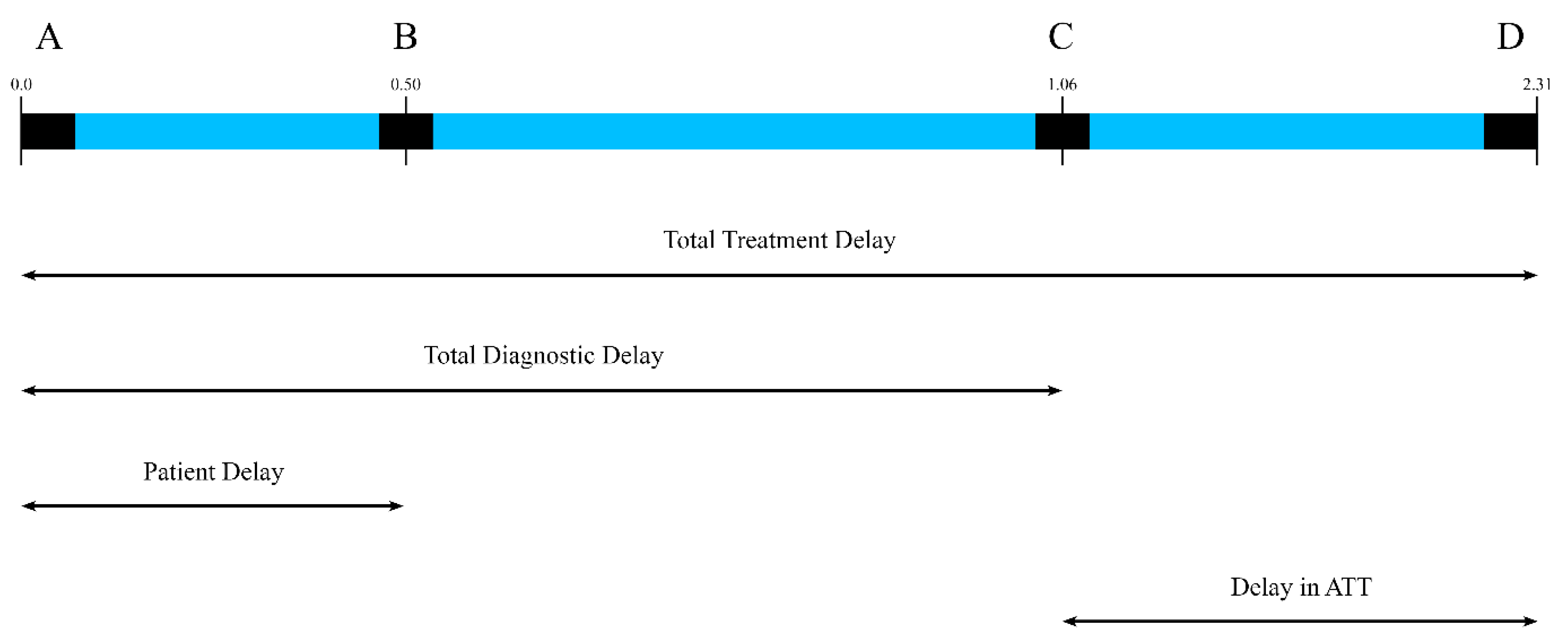
2.4.2. Delays
2.4.3. Survival Outcome
2.4.4. Protocol for Follow-Up
2.4.5. Sample Size Calculation
2.4.6. Statistical Analysis
2.4.7. Ethical Consideration
3. Results
3.1. Patient Characteristics
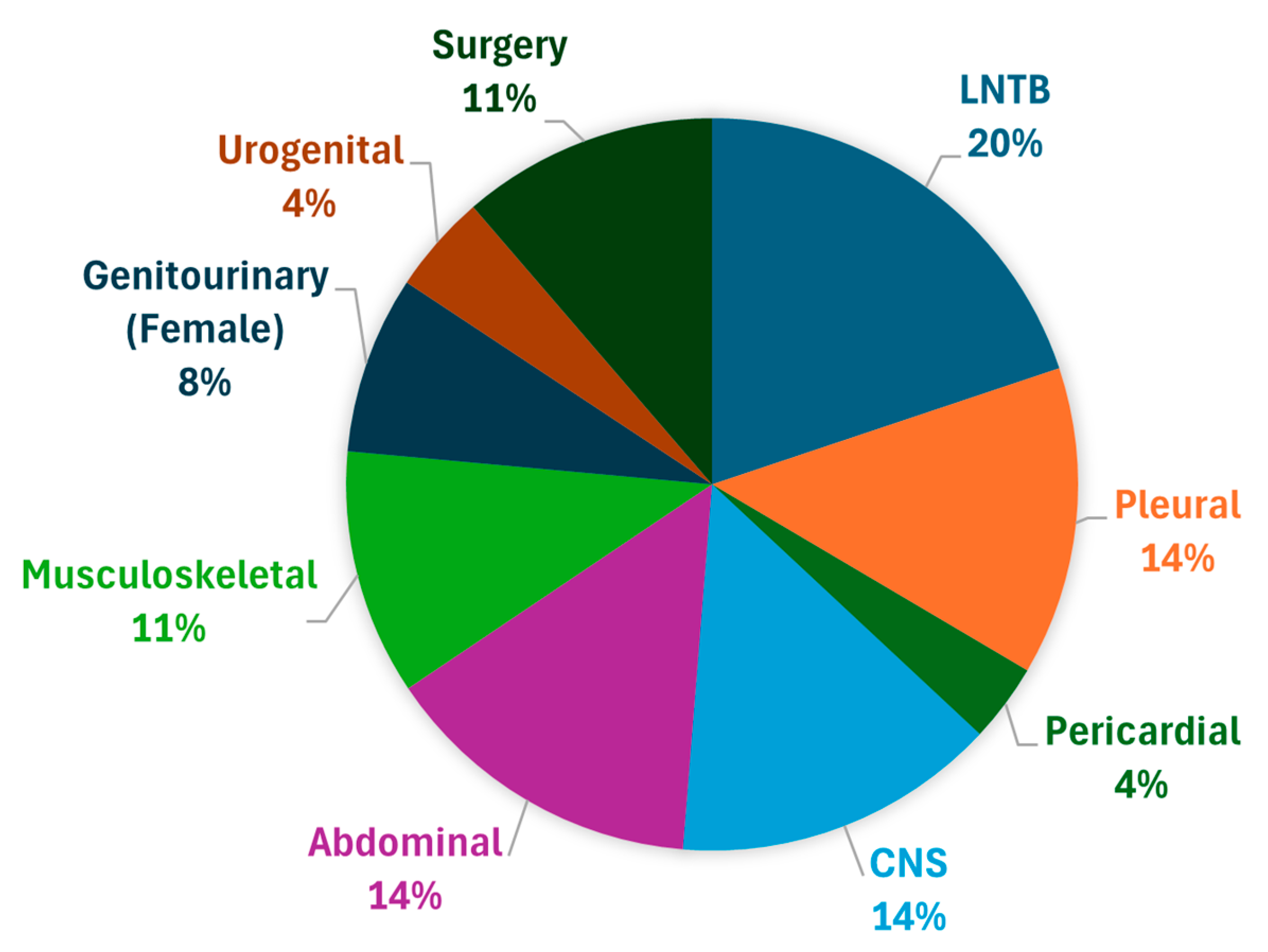
3.2. Primary Outcome
3.2.1. CNS Tuberculosis
3.2.2. Abdominal Tuberculosis
3.2.3. Lymph Node TB
3.2.4. Musculoskeletal TB
3.2.5. Pericardial TB
3.2.6. Pleural TB
3.2.7. Surgical TB
3.2.8. Genitourinary TB (Female)
3.2.9. Urogenital TB
3.3. Survival Outcomes
4. Discussion
4.1. Gaps in Diagnosis of EPTB Case
4.2. Gaps in Treatment of EPTB Patients
4.3. Determinants of Survival Outcome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- India TB Report 2023–Central Tuberculosis Division. (n.d.). Tbcindia.mohfw.gov.in. Available online: https://tbcindia.mohfw.gov.in/2023/06/06/india-tb-report-2023/ (accessed on 1 March 2025).
- Ministry of Health and Family Welfare Government of India Coming Together to End TB Altogether. (n.d.). Available online: https://tbcindia.mohfw.gov.in/wp-content/uploads/2023/05/TBAnnaulReport2022.pdf (accessed on 1 March 2025).
- Atif, M.; Fatima, R.; Ahmad, N.; Babar, Z.U. Treatment outcomes of extrapulmonary tuberculosis in Bahawalpur, Pakistan; A record review. J. Pharm. Policy Pract. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, M.M.; Tasew, T.W.; Richard, M.J. The quality of tuberculosis diagnosis in districts of Tigray region of Northern Ethiopia. Ethiop. J. Health Dev. 2005, 19, 13–20. [Google Scholar] [CrossRef]
- Steen, T.W.; Mazonde, G.N. Ngaka ya setswana, ngaka ya sekgoa or both? Health seeking behaviour in Batswana with pulmonary tuberculosis. Soc. Sci. Med. 1999, 48, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Sharma, M.; Rosales-Klintz, S.; Lundborg, C.S. ‘Multiple-test’ approach to the laboratory diagnosis of tuberculosis -perception of medical doctors from Ujjain, India. BMC Infect. Dis. 2015, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Virenfeldt, J.; Rudolf, F.; Camara, C.; Furtado, A.; Gomes, V.; Aaby, P.; Petersen, E.; Wejse, C. Treatment delay affects clinical severity of tuberculosis: A longitudinal cohort study. BMJ Open 2014, 4, e004818. [Google Scholar] [CrossRef] [PubMed]
- Chandru, B.A.; Varma, R.P. Factors affecting ability of TB patients to follow treatment guidelines - applying a capability approach. Int. J. Equity Health 2023, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Bamgboye, E.A.; Abuljadayel, N.; Smadi, H.; Abouzeid, M.S.; Al Hakeem, R.F. Incidence of and risk factors associated with pulmonary and extra-pulmonary tuberculosis in Saudi Arabia (2010–2011). PLoS ONE 2014, 9, e95654. [Google Scholar] [CrossRef] [PubMed]
- Sreeramareddy, C.T.; Panduru, K.V.; Verma, S.C.; Joshi, H.S.; Bates, M.N. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect. Dis. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, O.Y.; Adams, G.; Teeter, L.D.; Bui, T.T.; Musser, J.M.; Graviss, E.A. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int. J. Tuberc. Lung Dis. 2003, 7, 1178–1185. [Google Scholar] [PubMed]
- Subbaraman, R.; Nathavitharana, R.R.; Satyanarayana, S.; Pai, M.; Thomas, B.E.; Chadha, V.K.; Rade, K.; Swaminathan, S.; Mayer, K.H. The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis. PLoS Med. 2016, 13, e1002149. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Purohit, R.; Mustafa, T. Patient Health Seeking and Diagnostic Delay in Extrapulmonary Tuberculosis: A Hospital Based Study from Central India. Tuberc. Res. Treat. 2019, 2019, 4840561. [Google Scholar] [CrossRef] [PubMed]
- Tahseen, S.; Ambreen, A.; Masood, F.; Qadir, M.; Hussain, A.; Jamil, M.; Safdar, N.; Sviland, L.; Mustafa, T. Primary drug resistance in extra-pulmonary tuberculosis: A hospital-based prospective study from Pakistan. Int. J. Tuberc. Lung Dis. 2019, 23, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Sen, M.K.; Tyagi, J.S. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J. Clin. Microbiol. 2005, 43, 4357–4362. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A. Care seeking and treatment related delay among childhood tuberculosis patients in Delhi, India. Int. J. Tuberc. Lung Dis. 2017, 21, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.; Hinderaker, S.G.; Enarson, D.; Malla, P.; Mørkve, O. Delay in the diagnosis of tuberculosis in Nepal. BMC Public Health 2009, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Gele, A.A.; Bjune, G.; Abebe, F. Pastoralism and delay in diagnosis of TB in Ethiopia. BMC Public Health 2009, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Meintjes, G.; Schoeman, H.; Morroni, C.; Wilson, D.; Maartens, G. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: A cross-sectional study. BMC Infect. Dis. 2008, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Ryan, H.; Khaparde, S.; Sachdeva, K.S.; Singh, A.D.; Mohan, A.; Sarin, R.; Paramasivan, C.N.; Kumar, P.; Nischal, N.; et al. Index-TB guidelines: Guidelines on extrapulmonary tuberculosis for India. Indian J. Med. Res. 2017, 145, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Schiller, I.; Dendukuri, N.; Dheda, K.; Denkinger, C.M.; Schumacher, S.G.; Steingart, K.R. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst. Rev. 2018, 8, CD012768. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Gopi, P.G.; Santha, T.; Jaggarajamma, K.; Charles, N.; Prabhakaran, E.; Narayanan, P.R. Course of action taken by smear negative chest symptomatics: A report from a rural area in South India. Indian J. Tuberc. 2006, 53, 4–6. [Google Scholar]
- Qin, Z.Z.; Pai, M.; Van Gemert, W.; Sahu, S.; Ghiasi, M.; Creswell, J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur. Respir. J. 2015, 45, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Salje, H.; Andrews, J.R.; Deo, S.; Satyanarayana, S.; Sun, A.Y.; Pai, M.; Dowdy, D.W. The importance of implementation strategy in scaling up Xpert MTB/RIF for diagnosis of tuberculosis in the Indian health-care system: A transmission model. PLoS Med. 2014, 11, e1001674. [Google Scholar] [CrossRef] [PubMed]
- Sant’ Anna, F.M.; Araújo-Pereira, M.; Schmaltz, C.A.; Arriaga, M.B.; Andrade, B.B.; Rolla, V.C. Impact of adverse drug reactions on the outcomes of tuberculosis treatment. PLoS ONE 2023, 18, e0269765. [Google Scholar] [CrossRef] [PubMed]
- Katrak, S.M. Central nervous system tuberculosis. J. Neurol. Sci. 2021, 421, 117278. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Meintjes, G.; Wilkinson, R.J. Treatment of Tuberculous Meningitis and Its Complications in Adults. Curr. Treat. Options Neurol. 2018, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Goyal, V. Spinal Tuberculosis Treatment: An Enduring Bone of Contention. Ann. Indian Acad. Neurol. 2020, 23, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, J.; Sarkar, S.; Chinnakali, P.; Lakshminarayanan, S.; Sahu, S.K.; Reshma, A.; Knudsen, S.; Das, M.; Thekkur, P.; Venugopal, V.; et al. Risk factors for death during treatment in pulmonary tuberculosis patients in South India: A cohort study. Indian J. Tuberc. 2021, 68, 32–39. [Google Scholar] [CrossRef] [PubMed]
- VidyaRaj, C.K.; Vadakunnel, M.J.; Mani, B.R.; Anbazhagi, M.; Pradhabane, G.; Venkateswari, R.; Palavesam, S.; Venkatesh, K.; Usharani, B.; Sriramkumar, S.R.; et al. Prevalence of extrapulmonary tuberculosis and factors influencing successful treatment outcomes among notified cases in South India. Sci. Rep. 2025, 15, 8290. [Google Scholar] [CrossRef] [PubMed]
- Berihe Hiluf, S.; Abera, A.; Bahiru, M.; Kassie, B. Determinants of unsuccessful tuberculosis treatment outcome in Southwest Ethiopia regional state public hospitals, 2022: A multi-center case control study. Front. Public Health 2024, 12, 1406211. [Google Scholar] [CrossRef] [PubMed]
- Waitt, C.J.; Squire, S.B. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2011, 15, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.J.; Foote, M.; Ray, S.M.; Gandhi, N.R.; Kempker, R.R. Diabetes mellitus and extrapulmonary tuberculosis: Site distribution and risk of mortality. Epidemiol. Infect. 2016, 144, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Ginisha, G.; Preeti, G.; Dwivedi, S.; Swamai, P. The association between diabetes and tuberculosis may be the next challenge for global tuberculosis control worldwide. Indian J. Endocrinol. Metab. 2016, 20, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Sulaiman, S.A.S.; Laghari, M.; Hassali, M.A.; Muttalif, A.R.; Bhatti, Z.; Ming, L.C.; Talpur, B.A. Treatment outcomes and risk factors of extra-pulmonary tuberculosis in patients with co-morbidities. BMC Infect. Dis. 2019, 19, 691. [Google Scholar] [CrossRef] [PubMed]
| Term | Definition |
|---|---|
| Microbiologically confirmed TB | A presumptive TB patient with a biological specimen positive for AFB, or positive for MTB on culture, or positive for TB through Quality Assured Rapid Diagnostic molecular test. |
| Clinically diagnosed TB case | A presumptive TB patient who is not microbiologically confirmed but diagnosed with active TB by a clinician based on X-ray, histopathology, or clinical signs, with a decision to treat with a full course of anti-TB treatment. |
| Presumptive EPTB case | A patient with symptoms and signs of EPTB who needs to be investigated. |
| Bacteriologically confirmed case | A patient with a microbiological diagnosis of EPTB, based on positive microscopy, culture, or a validated PCR-based test. Note: A presumptive case started on ATT empirically, without microbiological testing, is considered a clinically diagnosed case. If subsequently found bacteriologically positive, the case is reclassified as bacteriologically confirmed. |
| Non-EPTB case | A patient was investigated for EPTB and diagnosed with another condition, with no microbiological evidence of EPTB. |
| Surgical EPTB | A patient with TB infections affecting the ear, nose, throat, and related head and neck structures, or having ulcers/discharging sinuses over lymph nodes, bones and joints, or ocular structures. |
| Term | Definition |
|---|---|
| Patient delay | Duration from symptom onset to first visit with a health care provider (HCP), measured in weeks. |
| Healthcare system diagnostic delay | Time from first visit with a healthcare professional to confirmatory diagnosis, measured in weeks. |
| Total treatment delay | Time from onset of first symptom to initiation of ATT, measured in weeks. |
| Demographic Parameters | (n = 433) | n (%) | |
|---|---|---|---|
| Age | (Mean ± SD) Years | 34.1 ± 14.24 | |
| Gender | Male | 205 | 47 |
| Female | 228 | 53 | |
| Residence | Urban | 319 | 74 |
| Rural | 114 | 26 | |
| History of TB | No | 333 | 85 |
| Yes | 61 | 15 | |
| History of current chronic lung disease | No | 426 | 98 |
| Yes | 7 | 2 | |
| History of Diabetes Mellitus | No | 391 | 90 |
| Yes | 42 | 10 | |
| EPTB Type (n = 433) | Patient Delay Median (min, max) (Weeks) | Health Care system Diagnostic Delay Median (min, max) (Weeks) | Total Treatment Delay Mean ± SD (Weeks) | No Microbiological Diagnosis, n (%) | No Radiological Investigations n (%) | No HIV Testing n (%) | Payment Was Needed n (%) |
|---|---|---|---|---|---|---|---|
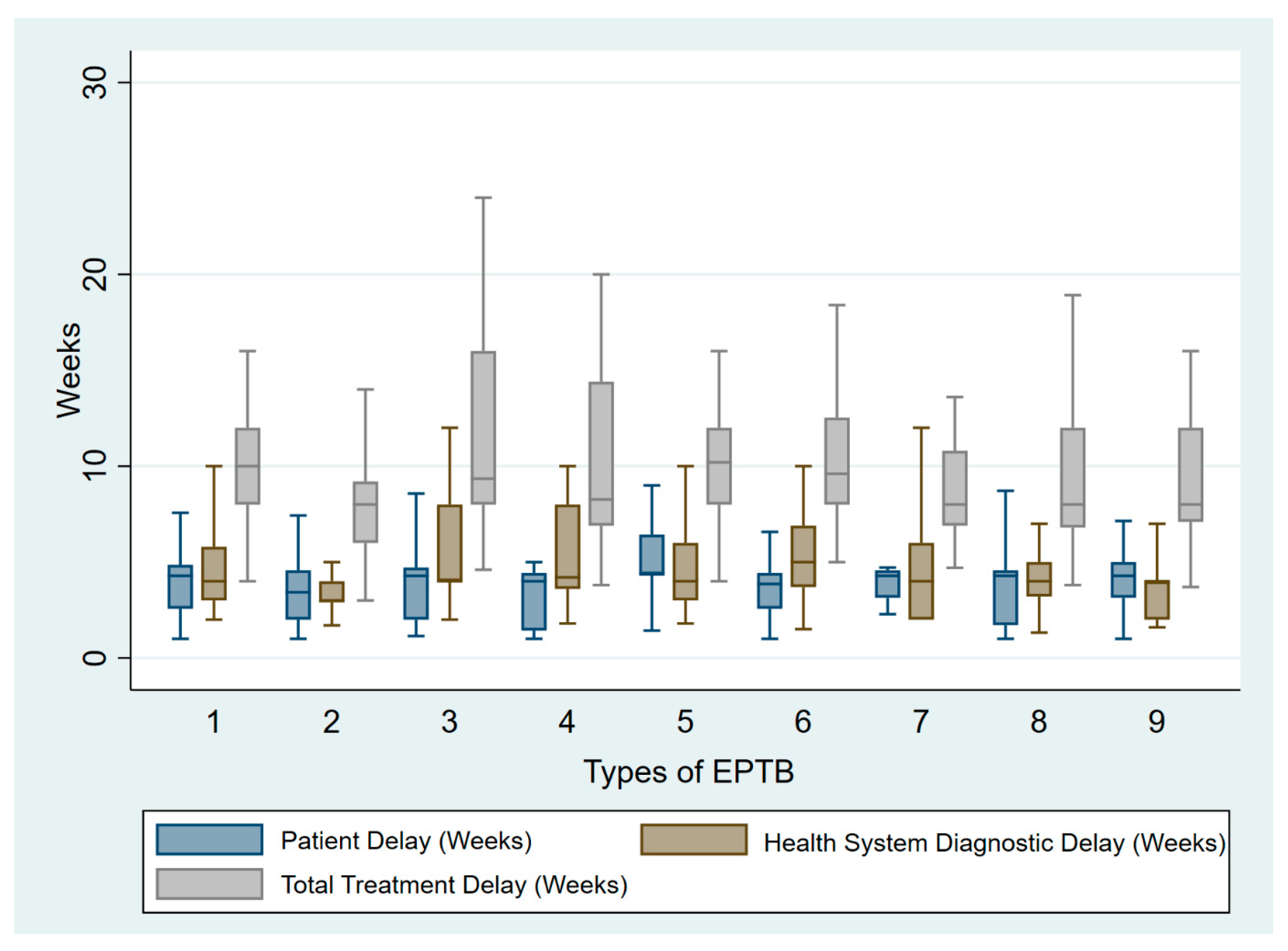
| Lymph node TB (n = 86) | 4.4 (1.4–21.5) | 4 (1.8–10) | 11.1 ± 4.4 | 7 (8) | 9 (10) | 8 (9) | 9 (10) |
| CNS TB (n = 62) | 3.4 (1–17.4) | 3 (1.2–10.3) | 8.2 ± 3.4 | 27 (4) | 0 | 3 (5) | 7 (11) |
| Abdominal TB (n = 62) | 4.2 (1–21.8) | 4 (2–14) | 10 (4–20) | 15 (24) | 0 | 5 (8) | 3 (5) |
| Pleural TB (n = 59) | 4.2 (1–21) | 4 (1.3–9.8) | 8 (3.8–26.5) | 9 (15) | 2 (3) | 3 (5) | 7 (12) |
| Surgical TB (n = 49) | 4.2 (1–17.4) | 4 (1.6–12) | 8 (3.7–20) | 14 (28) | 0 | 7 (14) | 7 (14) |
| Musculoskeletal TB (n = 47) | 3.8 (1–16.5) | 5 (1.5–10) | 9.6 (5–28) | 28 (60) | 1 (0.02) | 5 (11) | 5 (11) |
| Genitourinary TB Female (n = 34) | 4.2 (1.1—8.5) | 4 (2–12) | 9.35 (4.6–24) | 7 (20) | 13 (38) | 4 (12) | 1 (3) |
| Urogenital TB (n = 19) | 4 (1–14.4) | 4.2 (1.8–10) | 8.2 (3.8–20) | 5 (26.3) | 2 (10) | 2 (10) | 1 (5) |
| Pericardial TB (n = 15) | 4.2 (1–21) | 4 (1.3–9.8) | 8 (3.8–26.5) | 3 (20) | 0 | 0 | 4 (27) |
| p-value | 0.0014 | 0.013 | 0.0005 | <0.001 | <0.001 |
| EPTB Type (n = 433) | 1st Line ATT Not Initiated n (%) | Mean Duration of Treatment Mean ± SD (Months) | No Pyridoxine Supplementation n (%) | Modified ATT n (%) | Adverse Effects n (%) |
|---|---|---|---|---|---|
| CNS TB (n = 62) | 18 (29) | 11.4 ± 7.9 | 0 | 23 (37) | 10 (16) |
| Abdominal TB (n = 62) | 21 (33) | 9 ± 4.50 | 0 | 7 (11) | 9 (15) |
| Lymph node TB (n = 86) | 3 (3) | 7.9 ± 3.07 | 9 (10) | 6 (7) | 9 (10) |
| Musculoskeletal TB (n = 47) | 12 (25) | 13.4 ± 5.40 | 1 (2) | 5 (11) | 11 (23) |
| Pericardial TB (n = 15) | 0 | 9.5 ± 5.30 | 0 | 5 (33) | 1 (0.06) |
| Pleural TB (n = 59) | 31 (52) | 7.1 ± 2.26 | 14 (23) | 5 (8) | 21 (35) |
| Surgical TB (n = 49) | 11 (22) | 9.6 ± (4.3) | 0 | 5 (10) | 4 (8) |
| Genitourinary TB Female (n = 34) | 0 | 7.11 ± 1.64 | 1 (3) | 3 (9) | 3 (9) |
| Urogenital TB (n = 19) | 1 (15) | 7.78 ± 3.5 | 0 | 2 (10) | 2 (10) |
| Characteristics | Values | Died (N = 18) | Survived (N = 415) n (n%) | Unadjusted R (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (Years) | <34 | 8 (44) | 250 (60) | 1 | |||
| >34 | 10 (56) | 165 (40) | 1.89 (0.73, 4.90) | 0.188 | |||
| Gender | Male | 10 (56) | 186 (45) | ||||
| Female | 8 (44) | 229 (55) | 0.65 (0.25, 1.68) | 0.374 | |||
| History of DM | Yes | 7 (39) | 38 (9) | <0.001 | 7.57 (2.64–21.72) | <0.001 | |
| No | 11 (61) | 377 (91) | 6.31 (2.31, 17.24) | ||||
| History of TB disease | Yes | 7 (39) | 58 (14) | 0.007 | 4.82 (1.69–13.7) | <0.003 | |
| No | 11 (61) | 357 (86) | 3.91 (1.46, 10.51) | ||||
| Patient delay (weeks) | <4.43 | 12 (67) | 288 (69) | 1 | |||
| ≥4.43 | 6 (33) | 127 (31) | 1.13 (0.42, 3.09) | 0.806 | |||
| Health system delay (weeks) | <4.53 | 10 (56) | 275 (66) | ||||
| ≥4.53 | 8 (44) | 140 (34) | 1.57 (0.60, 4.07) | 0.352 | |||
| Total treatment delay (weeks) | <10.1 | 10 (56) | 254(61) | 1 | |||
| ≥10.1 | 8 (44) | 161 (39) | 1.26 (0.48, 3.26) | 0.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, S.; Titiyal, R.; Mohapatra, P.R.; Palvai, R.K.; Kar, I.; Mishra, B.; Ajayababu, A.; Sinha, A.; Bhuniya, S.; Pandey, S. Evaluating the Gaps in the Diagnosis and Treatment in Extra-Pulmonary Tuberculosis Patients Under National Tuberculosis Elimination Programme (NTEP) Guidelines: A Multicentric Cohort Study. Trop. Med. Infect. Dis. 2025, 10, 206. https://doi.org/10.3390/tropicalmed10080206
Sinha S, Titiyal R, Mohapatra PR, Palvai RK, Kar I, Mishra B, Ajayababu A, Sinha A, Bhuniya S, Pandey S. Evaluating the Gaps in the Diagnosis and Treatment in Extra-Pulmonary Tuberculosis Patients Under National Tuberculosis Elimination Programme (NTEP) Guidelines: A Multicentric Cohort Study. Tropical Medicine and Infectious Disease. 2025; 10(8):206. https://doi.org/10.3390/tropicalmed10080206
Chicago/Turabian StyleSinha, Sanjeev, Renuka Titiyal, Prasanta R. Mohapatra, Rajesh K. Palvai, Itishree Kar, Baijayantimala Mishra, Anuj Ajayababu, Akanksha Sinha, Sourin Bhuniya, and Shivam Pandey. 2025. "Evaluating the Gaps in the Diagnosis and Treatment in Extra-Pulmonary Tuberculosis Patients Under National Tuberculosis Elimination Programme (NTEP) Guidelines: A Multicentric Cohort Study" Tropical Medicine and Infectious Disease 10, no. 8: 206. https://doi.org/10.3390/tropicalmed10080206
APA StyleSinha, S., Titiyal, R., Mohapatra, P. R., Palvai, R. K., Kar, I., Mishra, B., Ajayababu, A., Sinha, A., Bhuniya, S., & Pandey, S. (2025). Evaluating the Gaps in the Diagnosis and Treatment in Extra-Pulmonary Tuberculosis Patients Under National Tuberculosis Elimination Programme (NTEP) Guidelines: A Multicentric Cohort Study. Tropical Medicine and Infectious Disease, 10(8), 206. https://doi.org/10.3390/tropicalmed10080206






