Unmasking the Determinants of Loss to Follow-Up in Pulmonary Tuberculosis: A Study in Selangor, Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Data Collection
2.3. Statistical Methods
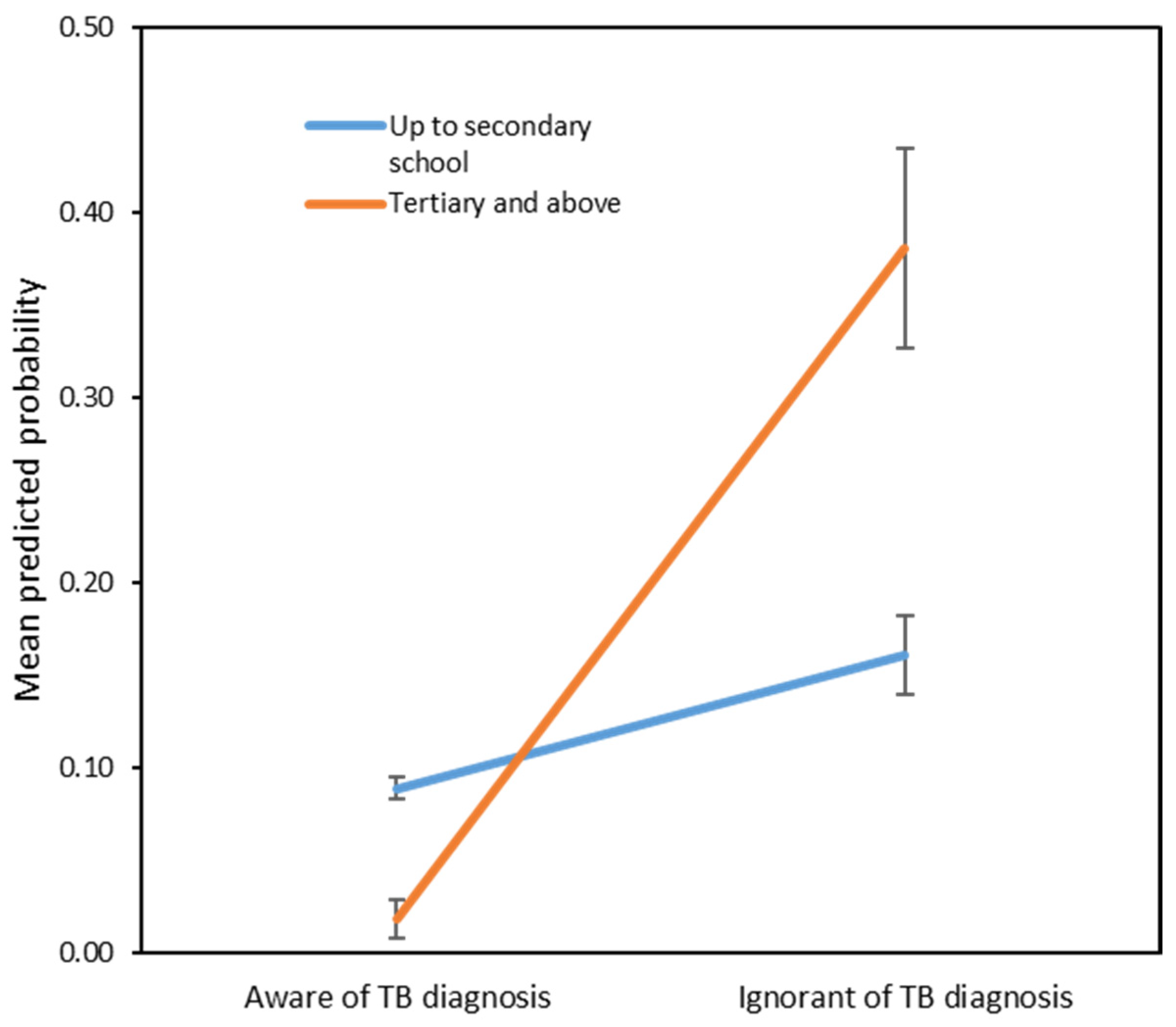
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTFU | Loss to Follow-Up |
| pTB | Pulmonary tuberculosis |
| SES | Socioeconomic status |
| TB | Tuberculosis |
References
- World Health Organization Tuberculosis in Malaysia. Available online: https://worldhealthorg.shinyapps.io/TBrief/?_inputs_&entity_type=%22country%22&iso2=%22MY%22&sidebarCollapsed=true&sidebarItemExpanded=null (accessed on 6 December 2022).
- Floyd, K.; Glaziou, P.; Houben, R.M.G.J.; Sumner, T.; White, R.G.; Raviglione, M. Global tuberculosis targets and milestones set for 2016–2035: Definition and rationale. Int. J. Tuberc. Lung Dis. 2018, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Teferi, M.Y.; El-Khatib, Z.; Boltena, M.T.; Andualem, A.T.; Asamoah, B.O.; Biru, M.; Adane, H.T. Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10678. [Google Scholar] [CrossRef] [PubMed]
- Muture, B.N.; Keraka, M.N.; Kimuu, P.K.; Kabiru, E.W.; Ombeka, V.O.; Oguya, F. Factors associated with default from treatment among tuberculosis patients in nairobi province, Kenya: A case control study. BMC Public Health 2011, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Suliman, Q.; Lim, P.Y.; Said, S.M.; Tan, K.-A.; Zulkefli, N.A.M. Risk factors for early TB treatment interruption among newly diagnosed patients in Malaysia. Sci. Rep. 2022, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.E.; Schwalbe, N.R.; Aguiar, C.A. Timing of default from tuberculosis treatment: A systematic review. Trop. Med. Int. Health 2008, 13, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Yone, E.W.P.; Kengne, A.P.; Kuaban, C. Incidence, time and determinants of tuberculosis treatment default in Yaounde, Cameroon: A retrospective hospital register-based cohort study. Br. Med. J. Open 2011, 1, e000289. [Google Scholar] [CrossRef]
- Zhang, Q.; Gaafer, M.; El Bayoumy, I. Determinants of Default from Pulmonary Tuberculosis Treatment in Kuwait. Sci. World J. 2014, 2014, 672825. [Google Scholar] [CrossRef] [PubMed]
- Cherkaoui, I.; Sabouni, R.; Ghali, I.; Kizub, D.; Billioux, A.C.; Bennani, K.; Bourkadi, J.E.; Benmamoun, A.; Lahlou, O.; Aouad, E.R.; et al. Treatment Default amongst Patients with Tuberculosis in Urban Morocco: Predicting and Explaining Default and Post-Default Sputum Smear and Drug Susceptibility Results. PLoS ONE 2014, 9, e93574. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.d.S.; Penna, M.L.; Perez-Porcuna, T.M.; de Souza, A.B.; Marreiro, L.d.S.; Albuquerque, B.C.; Martínez-Espinosa, F.E.; Bührer-Sékula, S.; Pai, M. Factors Associated with Tuberculosis Treatment Default in an Endemic Area of the Brazilian Amazon: A Case Control-Study. PLoS ONE 2012, 7, e39134. [Google Scholar] [CrossRef] [PubMed]
- Sharani, Z.Z.; Ismail, N.; Yasin, S.M.; Zakaria, Y.; Razali, A.; Demong, N.A.R.; Mohammad, M.; Ismail, Z. Characteristics and determinants of loss to follow-up among tuberculosis (TB) patients who smoke in an industrial state of Malaysia: A registry-based study of the years 2013–2017. BMC Public Health 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Dupont, W.D.; Plummer, W.D. Power and sample size calculations. Control Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. An in-Depth Analysis of the Health-Seeking Behaviour of Patients and Health System Response in Seven Countries of the Eastern Mediterranean Region. In Diagnostic and Treatment Delay in Tuberculosis; World Health Organization (WHO): Geneva, Switzerland, 2006. [Google Scholar]
- Cheong, K.C.; Ghazali, S.M.; Zamri, A.S.S.M.; Cheong, Y.L.; Iderus, N.H.M.; Nagalingam, T.; Ruslan, Q.; Omar, M.A.; Yusoff, A.F. Gender Differences in Factors Associated with the Total Delay in Treatment of Pulmonary Tuberculosis Patients: A Cross-Sectional Study in Selangor, Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 6258. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Definitions of Tuberculosis Cases and Treatment Outcomes; World Health Organization (WHO): Geneva, Switzerland, 2013. [Google Scholar]
- Jakubowiak, W.; Bogorodskaya, E.; Borisov, S.; Danilova, I.; Kourbatova, E. Treatment interruptions and duration associated with default among new patients with tuberculosis in six regions of Russia. Int. J. Infect. Dis. 2009, 13, 362–368. [Google Scholar] [CrossRef] [PubMed]
- AlOsaimi, H.M.; Alshammari, M.K.; Almijlad, G.K.; Alotaibi, N.M.; Alqahtani, D.A.; Alshamrani, M.M.; Shutur, T.A.; Alhazmi, M.F.; Hurubi, M.A.; Alshammari, K.S.; et al. Prevalence, Clinical Characteristics and Determinants of Unsuccessful Treatment Outcomes Among Pulmonary Tuberculosis Patients: A 5-Year Registry-Based Retrospective Cohort Study. Patient Relat. Outcome Meas. 2024, 15, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Duarte, R.; Veiga, A.M.; Taylor, B. Who are the patients that default tuberculosis treatment?—Space matters! Epidemiol. Infect. 2017, 145, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Adamashvili, N.; Akopyan, K.; Tukvadze, N.; Dumchev, K.; Sereda, Y.; Khonelidze, I.; Kuchukhidze, G. Factors associated with loss to follow-up among people with tuberculosis in the country of Georgia: A cohort study. Monaldi Arch. Chest Dis. 2021, 91, 10–4081. [Google Scholar] [CrossRef] [PubMed]
- Chida, N.; Ansari, Z.; Hussain, H.; Jaswal, M.; Symes, S.; Khan, A.J.; Mohammed, S.; García-García, J.-M. Determinants of Default from Tuberculosis Treatment among Patients with Drug-Susceptible Tuberculosis in Karachi, Pakistan: A Mixed Methods Study. PLoS ONE 2015, 10, e0142384. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, G.; Heunis, C.; Chikobvu, P.; Botha, S.; van Rensburg, D. Factors influencing treatment default among tuberculosis patients in a high burden province of South Africa. Int. J. Infect. Dis. 2017, 54, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Chung, H.; Muntaner, C.; Lee, M.; Kim, Y.; Barry, C.E.; Cho, S.-N. The impact of social conditions on patient adherence to pulmonary tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2016, 20, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Wilder, M.E.; Kulie, P.; Jensen, C.; Levett, P.; Blanchard, J.; Dominguez, L.W.; Portela, M.; Srivastava, A.; Li, Y.; McCarthy, M.L. The Impact of Social Determinants of Health on Medication Adherence: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2021, 36, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Xiong, J.; Wang, Y.; Wang, W.; Wang, J.; Lin, Y.; Zhang, P. Factors Associated with Medical Follow-Up Adherence for Patients on All-Oral Regimen for Multidrug-Resistant Tuberculosis in Shenzhen, China. Patient Prefer. Adherence 2021, 15, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R. Social Support and Patient Adherence to Medical Treatment: A Meta-Analysis. Health Psychol. 2004, 23, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Sulaiman, S.A.S.; Hassali, M.A.; Khan, K.U.; Ming, L.C.; Mateen, O.; Ullah, M.O. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health 2020, 20, 854. [Google Scholar] [CrossRef] [PubMed]
- Dujaili, J.A.; Sulaiman, S.A.S.; Awaisu, A.; Muttalif, A.R.; Blebil, A.Q. Outcomes of tuberculosis treatment: A retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J. Public Health 2010, 19, 183–189. [Google Scholar] [CrossRef]
- Sherman, B.W.; Lynch, W.D. The association of smoking with medical treatment adherence in the workforce of a large employer. Patient Prefer. Adherence 2014, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Netto, T.A.L.; Diniz, B.D.; Odutola, P.; Dantas, C.R.; de Freitas, M.C.F.L.C.; Hefford, P.M.; Bes, T.M.; Pappas, G. Video-observed therapy (VOT) vs directly observed therapy (DOT) for tuberculosis treatment: A systematic review on adherence, cost of treatment observation, time spent observing treatment and patient satisfaction. PLoS Negl. Trop. Dis. 2024, 18, e0012565. [Google Scholar] [CrossRef]
- Government of Malaysia. Section 1. Short Title, Application and Commencement. In Laws of Malaysia Act 342 Prevention and Control of Infectious Diseases Act 1988; Arrangement of Sections Part I-Preliminary; The Commissioner of Law Revision: Putrajaya, Malaysia, 1988. [Google Scholar]
| Variable | All Patients (n = 699) | Lost to Follow-Up (n = 55) | Chi-Square (p-Value) |
|---|---|---|---|
| n (%) a | n (%) b | ||
| Gender | 2.87 (0.900) | ||
| Male | 434 (62.1) | 40 (9.2) | |
| Female | 265 (37.9) | 15 (5.7) | |
| Age | 11.93 (0.008) ** | ||
| 18–24 (young adults) | 119 (17.0) | 9 (7.6) | |
| 25–44 (adults) | 274 (39.2) | 32 (11.7) | |
| 45–64 (middle age) | 253 (36.2) | 14 (5.5) | |
| 65 and above (seniors) | 53 (7.6) | 0 (0.0) | |
| Ethnicity | 7.00 (0.136) | ||
| Malay | 404 (57.8) | 30 (7.4) | |
| Chinese | 71 (10.2) | 5 (7.0) | |
| Indian | 87 (12.4) | 6 (6.9) | |
| Other indigenous peoples (Sabah/Sarawak/Orang Asli) | 44 (6.3) | 8 (18.2) | |
| Others | 93 (13.3) | 6 (6.5) | |
| Educational level | 18.82 (<0.001) *** | ||
| No formal education | 32 (4.6) | 0 (0.0) | |
| Primary school | 148 (21.2) | 7 (4.7) | |
| Secondary school | 355 (50.8) | 43 (12.1) | |
| Tertiary | 164 (23.5) | 5 (3.0) | |
| Nationality | 0.297 (0.586) | ||
| Malaysian | 606 (86.7) | 49 (8.1) | |
| Non-Malaysian | 93 (13.3) | 6 (6.5) | |
| Marital status | 10.58 (0.001) ** | ||
| Married | 435 (62.2) | 23 (5.3) | |
| Single/Divorced/Widowed | 264 (37.8) | 32 (12.1) | |
| Employment status | 8.22 (0.004) ** | ||
| Unemployed | 192 (27.5) | 6 (3.1) | |
| Employed | 507 (72.5) | 49 (9.7) | |
| Presence of comorbidities | 0.001 (0.977) | ||
| Yes (≥1 comorbidity) | 319 (45.6) | 25 (7.8) | |
| No | 380 (54.4) | 30 (7.9) |
| Variable | All Patients (n = 699) | Lost to Follow-Up (n = 55) | Chi-Square (p-Value) c |
|---|---|---|---|
| n (%) a | n (%) b | ||
| Ever smoked | 12.22 (<0.001) *** | ||
| No | 386 (55.2) | 18 (4.7) | |
| Yes | 313 (44.8) | 37 (11.8) | |
| Alcohol drinker | 0.45 (0.336) c | ||
| No | 673 (96.3) | 52 (7.7) | |
| Yes | 26 (3.7) | 3 (11.5) | |
| Total delay in treatment | 0.065 (0.798) | ||
| Low | 357 (51.1) | 29 (8.1) | |
| High | 342 (48.9) | 26 (7.6) | |
| Stigma score | 3.32 (0.068) | ||
| Low | 362 (51.8) | 22 (6.1) | |
| High | 337 (48.2) | 33 (9.8) | |
| TB knowledge score | 1.40 (0.237) d | ||
| 0 | 7 (1.0) | 2 (28.6) | |
| 1 | 7 (1.0) | 0 (0) | |
| 2 | 12 (1.8) | 0 (0) | |
| 3 | 50 (7.3) | 4 (8.0) | |
| 4 | 110 (16.1) | 11 (10.0) | |
| 5 | 202 (29.6) | 19 (9.4) | |
| 6 | 213 (31.2) | 13 (6.1) | |
| 7 | 81 (11.9) | 5 (6.2) | |
| Aware of TB diagnosis | 0.013 *c | ||
| Yes | 656 (94.0) | 47 (7.2) | |
| No | 42 (6.0) | 8 (19.0) |
| Variable | All Patients (n = 699) | Lost to Follow-Up (n = 55) | Chi-Square (p-Value) |
|---|---|---|---|
| n (%) a | n (%) b | ||
| TB drug side effects | |||
| Nausea | 157 (22.5) | 11 (7.0) | 0.213 (0.645) |
| Abdominal pain | 80 (11.5) | 9 (11.3) | 1.414 (0.234) |
| Headache | 167 (23.9) | 11 (6.6) | 0.505 (0.477) |
| Loss of appetite | 145 (20.8) | 9 (6.2) | 0.706 (0.401) |
| Jaundice | 20 (2.9) | 2 (10.0) | 0.128 (0.721) |
| Numbness/Tingling | 83 (11.9) | 7 (8.4) | 0.040 (0.842) |
| Rash | 245 (35.1) | 22 (9.0) | 0.629 (0.428) |
| Fatigue | 174 (24.9) | 14 (8.0) | 0.009 (0.925) |
| Joint pain | 174 (24.9) | 19 (10.9) | 2.951 (0.086) |
| Visual changes | 70 (10.0) | 5 (7.1) | 0.058 (0.809) |
| Hearing change | 37 (5.3) | 2 (5.4) | 0.760 c |
| Others | 168 (24.1) | 16 (9.5) | 0.824 (0.364) |
| Cumulative side effects | 548 (78.4) | 46 (8.4) | 0.967 (0.325) |
| Healthcare provider explained regarding: | |||
| TB treatment regime | 630 (91.0) | 49 (7.8) | 1.000 c |
| Treatment duration | 638 (92.1) | 48 (7.5) | 0.426 c |
| Treatment side effects | 570 (82.3) | 42 (7.4) | 0.803 (0.370) |
| Prognosis | 622 (90.0) | 49 (7.9) | 0.034 (0.853) |
| Satisfaction with healthcare services | |||
| Clinic hours are convenient | 667 (96.0) | 55 (8.2) | 0.157 c |
| Waiting time is reasonable (<1 h) | 574 (82.5) | 47 (8.2) | 0.368 (0.544) |
| Healthcare workers treat me with respect | 694 (99.6) | 55 (7.9) | 1.000 c |
| Healthcare workers show a good attitude towards me | 691 (99.3) | 55 (8.0) | 1.000 c |
| I trust the healthcare workers | 694 (99.6) | 55 (7.9) | 1.000 c |
| I am currently feeling better | 677 (97.1) | 53 (7.8) | 0.667 c |
| I am satisfied with the services provided | 688 (98.9) | 55 (8.0) | 1.000 c |
| I am currently taking herbal medication/supplements | 153 (22.0) | 7 (4.6) | 2.965 (0.085) |
| Factor | p-Value | aOR | 95% CI |
|---|---|---|---|
| Age (years) | |||
| 18–24 (young adults) | 0.249 | 1.79 | 0.66, 4.86 |
| 25–44 (adults) | 0.004 | 2.83 | 1.40, 5.72 |
| 45 and above (middle age and older) | Ref. | ||
| Marital status | |||
| Single/divorced/widowed | 0.019 | 2.17 | 1.14, 4.14 |
| Married | Ref. | ||
| Education | |||
| Secondary or less | 0.003 | 6.13 | 1.83, 20.53 |
| Tertiary | Ref. | ||
| Ever smoked | |||
| Yes | 0.002 | 2.65 | 1.43, 4.92 |
| No | Ref. | ||
| Ignorant of TB diagnosis | |||
| Yes | 0.001 | 38.14 | 4.10, 354.89 |
| No | Ref. | ||
| Education level × ignorant of TB diagnosis * | 0.017 | 0.05 | 0.004, 0.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Ghazali, S.; Cheong, K.C.; Md Nadzri, M.N.; Mohd Ghazali, N.; Cheng, L.M.; Ahmad, L.C.R.Q.; Kamarudin, M.K.; Ahmad, N.A.R.; Zulkifli, A.A.; Ling, C.Y.; et al. Unmasking the Determinants of Loss to Follow-Up in Pulmonary Tuberculosis: A Study in Selangor, Malaysia. Trop. Med. Infect. Dis. 2025, 10, 226. https://doi.org/10.3390/tropicalmed10080226
Mohd Ghazali S, Cheong KC, Md Nadzri MN, Mohd Ghazali N, Cheng LM, Ahmad LCRQ, Kamarudin MK, Ahmad NAR, Zulkifli AA, Ling CY, et al. Unmasking the Determinants of Loss to Follow-Up in Pulmonary Tuberculosis: A Study in Selangor, Malaysia. Tropical Medicine and Infectious Disease. 2025; 10(8):226. https://doi.org/10.3390/tropicalmed10080226
Chicago/Turabian StyleMohd Ghazali, Sumarni, Kee Chee Cheong, Mohamad Nadzmi Md Nadzri, Nur’Ain Mohd Ghazali, Lim Mei Cheng, Lonny Chen Rong Qi Ahmad, Mohd Kamarulariffin Kamarudin, Nur Ar Rabiah Ahmad, Asrul Anuar Zulkifli, Cheong Yoon Ling, and et al. 2025. "Unmasking the Determinants of Loss to Follow-Up in Pulmonary Tuberculosis: A Study in Selangor, Malaysia" Tropical Medicine and Infectious Disease 10, no. 8: 226. https://doi.org/10.3390/tropicalmed10080226
APA StyleMohd Ghazali, S., Cheong, K. C., Md Nadzri, M. N., Mohd Ghazali, N., Cheng, L. M., Ahmad, L. C. R. Q., Kamarudin, M. K., Ahmad, N. A. R., Zulkifli, A. A., Ling, C. Y., Ruslan, Q., Singh, S., Gill, B. S., Razali, A., & Md Iderus, N. H. (2025). Unmasking the Determinants of Loss to Follow-Up in Pulmonary Tuberculosis: A Study in Selangor, Malaysia. Tropical Medicine and Infectious Disease, 10(8), 226. https://doi.org/10.3390/tropicalmed10080226







