Prevalence and Risk of Meningococcal Disease or Carriage During Mass Gatherings and Associated Travel: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias
2.5. Strategy for Data Synthesis
3. Results
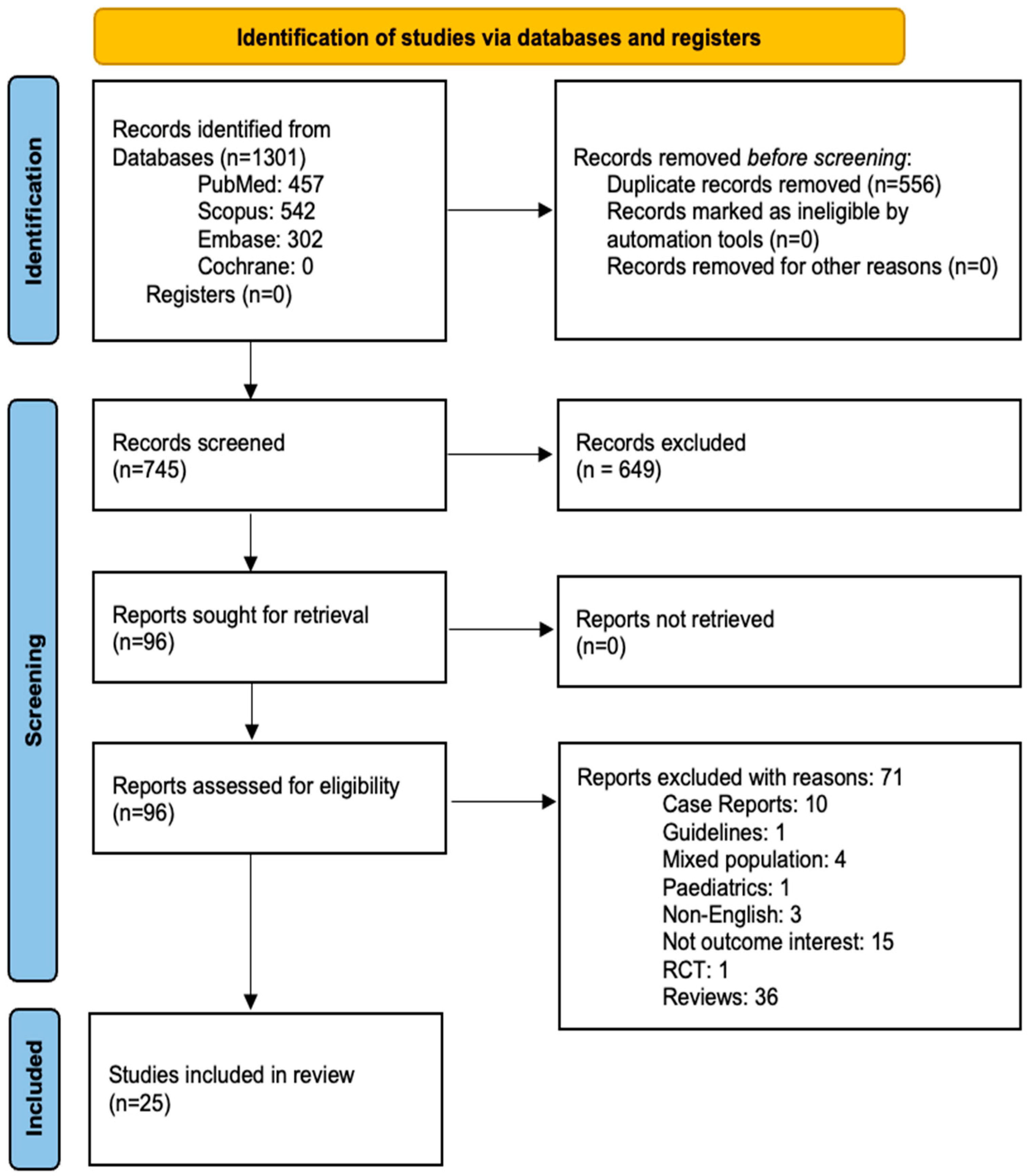
3.1. Study Selection Process
3.2. Study Characteristics
3.3. Characteristics of the Included Participants
3.4. Methodological Quality of Included Studies
3.5. Primary Outcomes
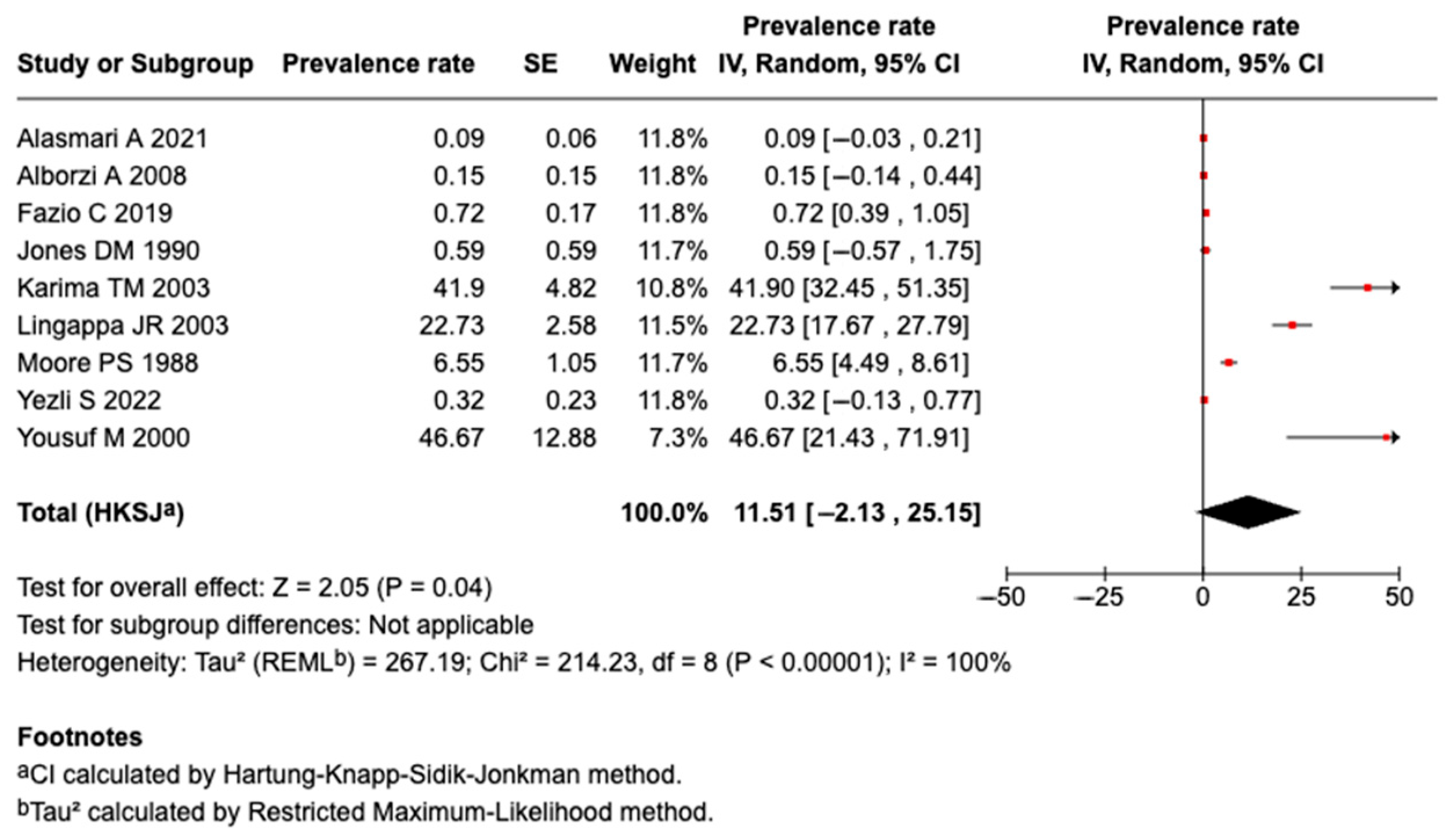
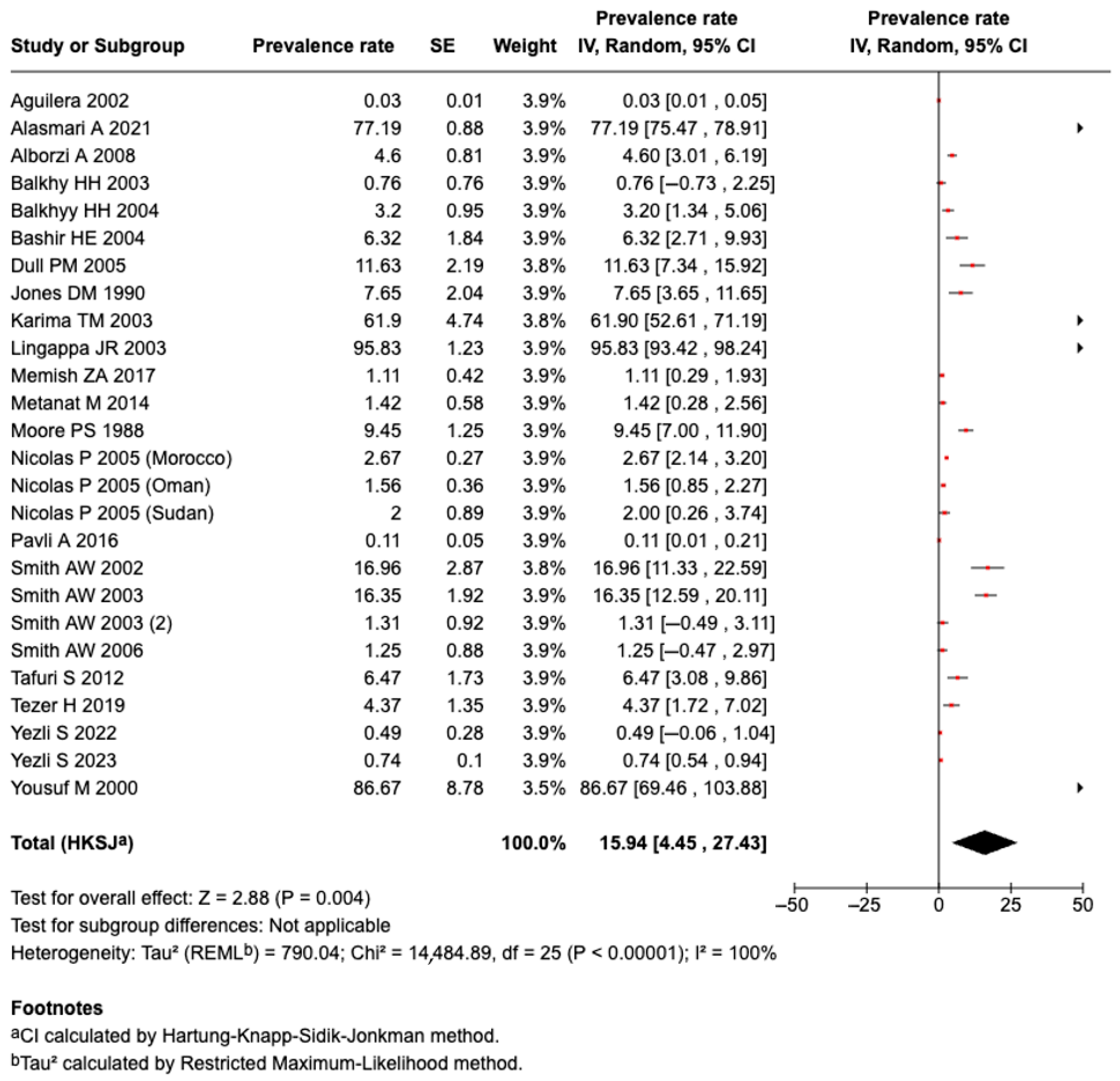
3.5.1. Prevalence of Meningococcal Disease
3.5.2. Exploration of Heterogeneity Through Subgroup Analysis
3.5.3. Results of Meta-Regression Analysis
3.5.4. Prevalence of Serogroup a Meningococcal Disease or Carriage
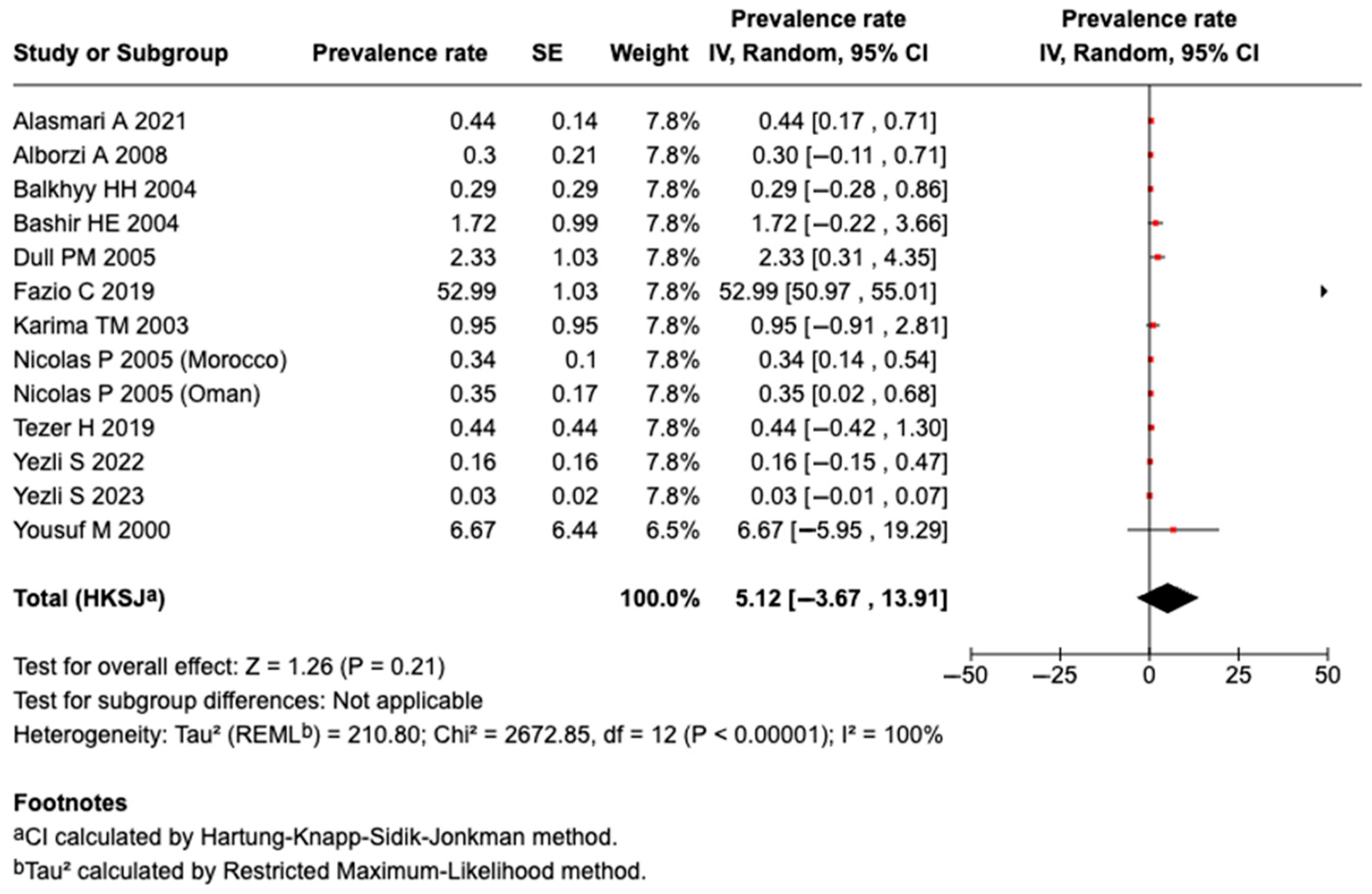
3.5.5. Prevalence of Serogroup B Meningococcal Disease or Carriage
3.5.6. Prevalence of Serogroup W135 Meningococcal Disease or Carriage
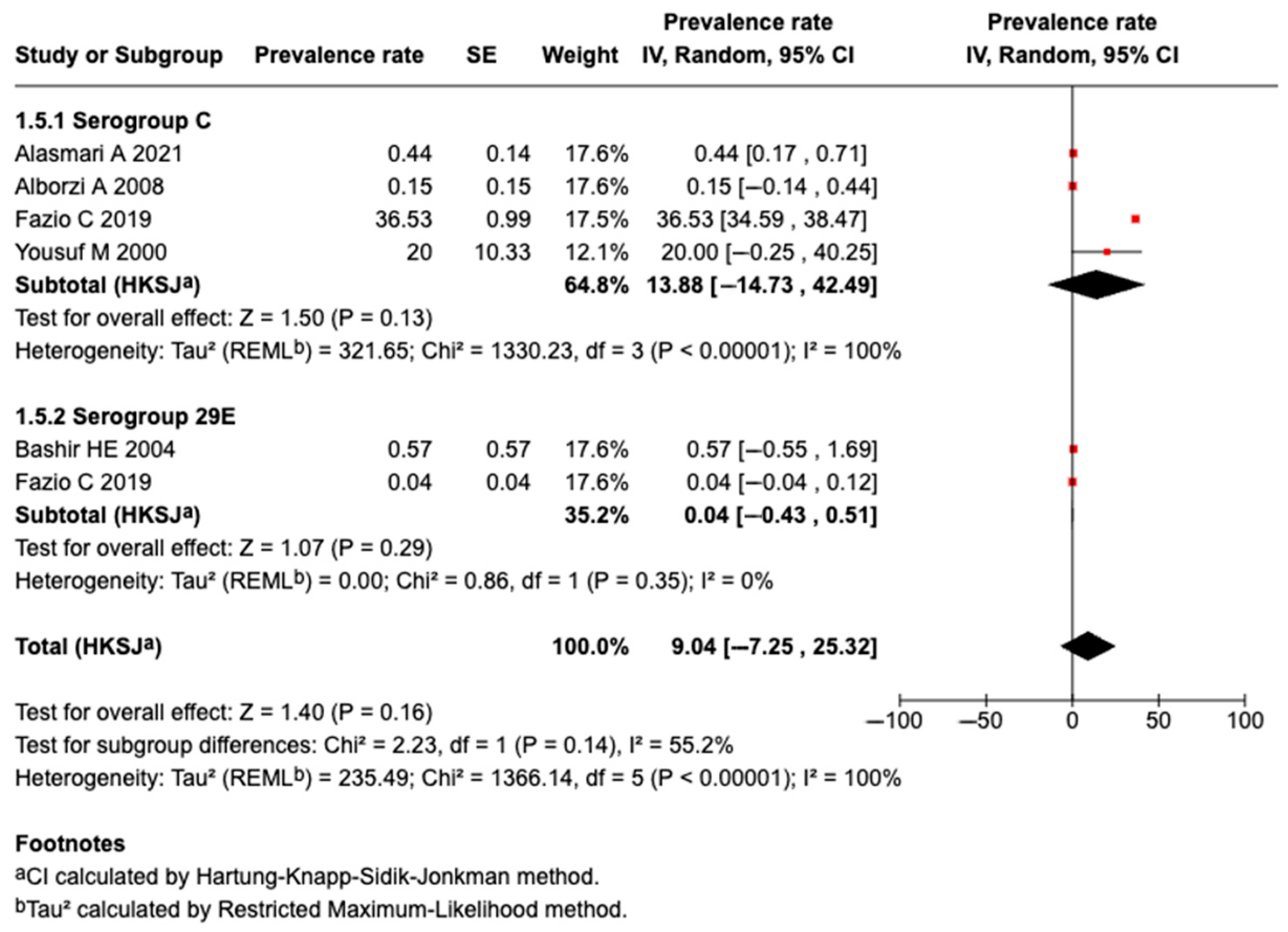
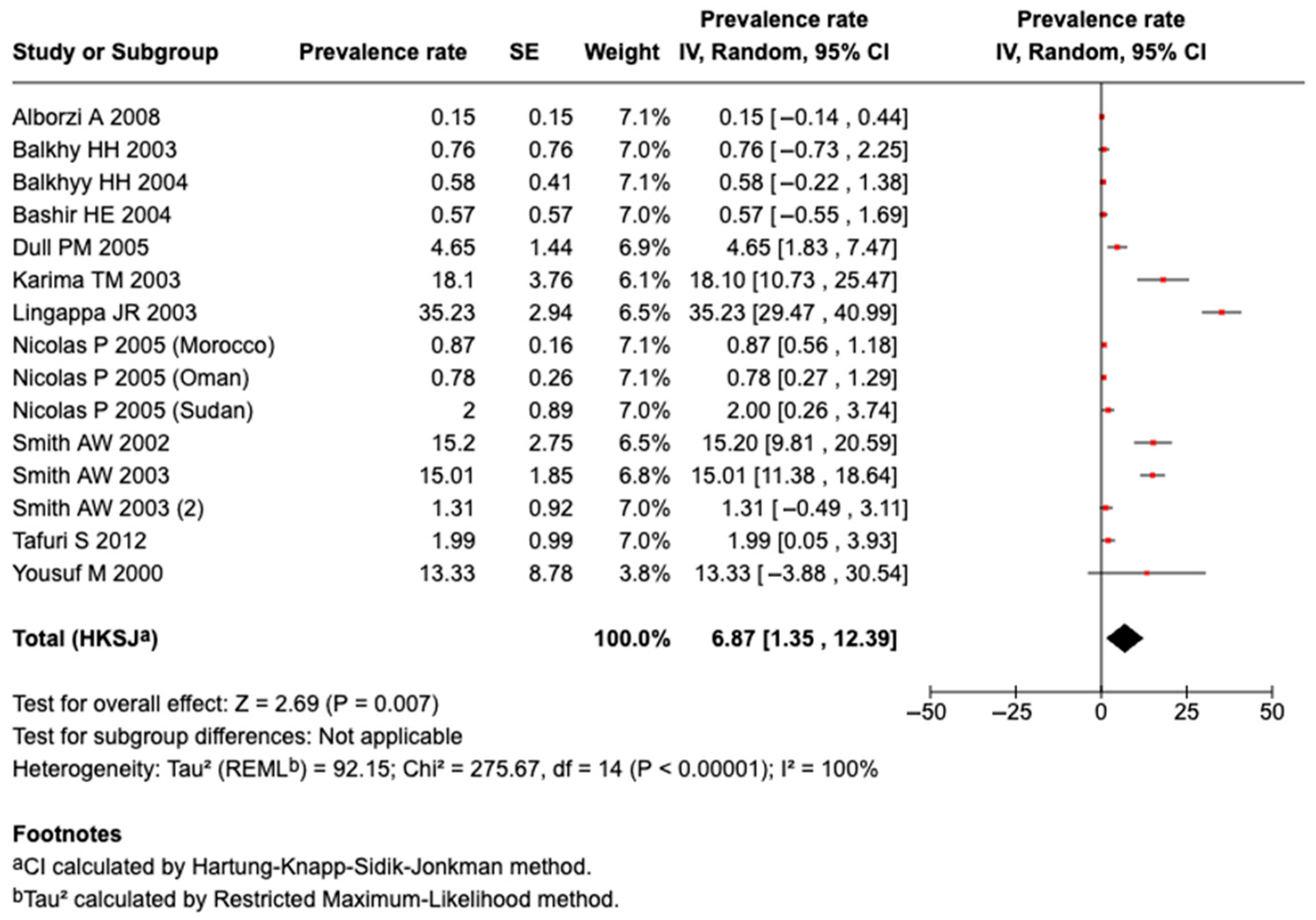
3.5.7. Prevalence of Serogroup C Meningococcal Disease or Carriage
3.5.8. Prevalence of Serogroup 29E Meningococcal Disease or Carriage
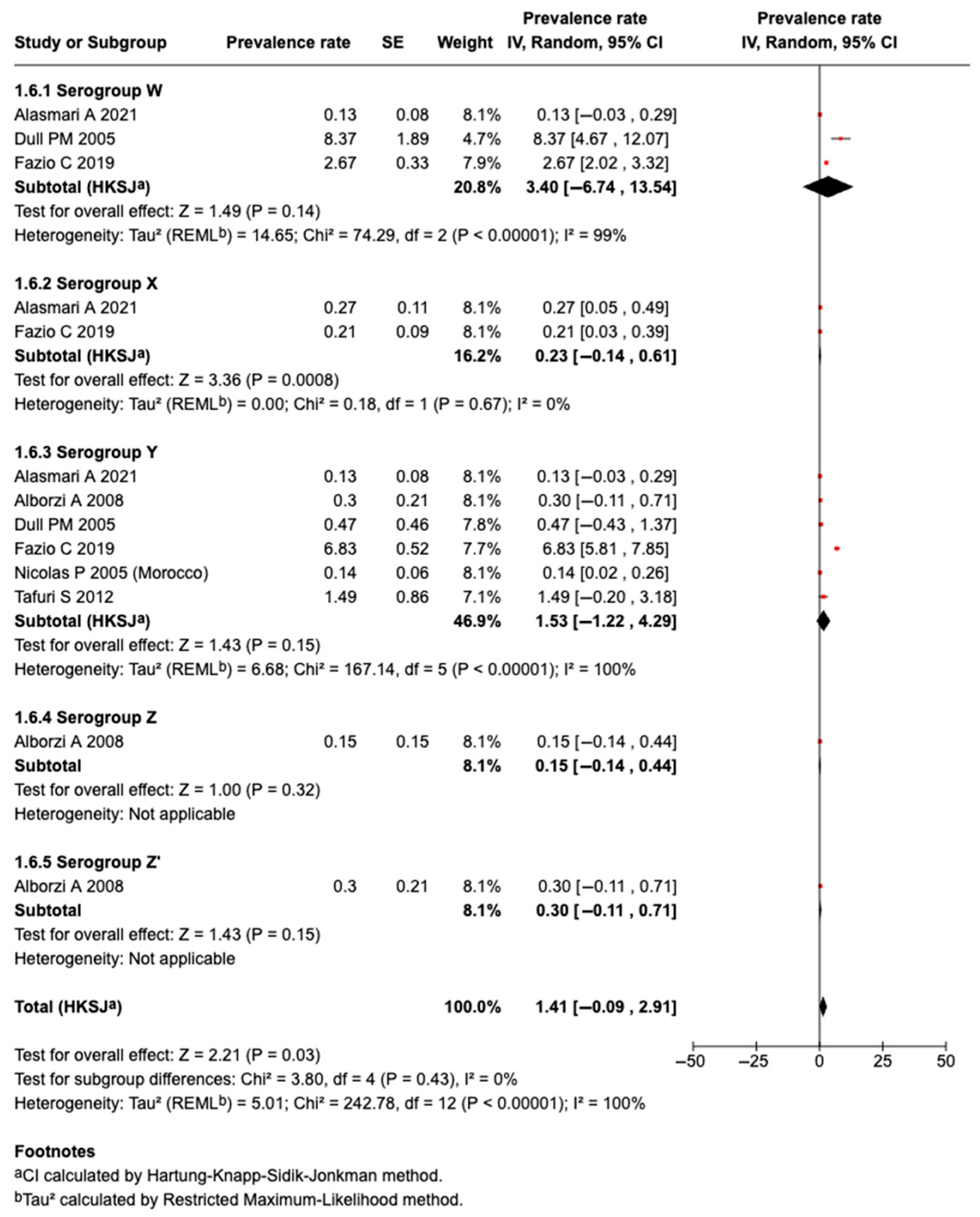
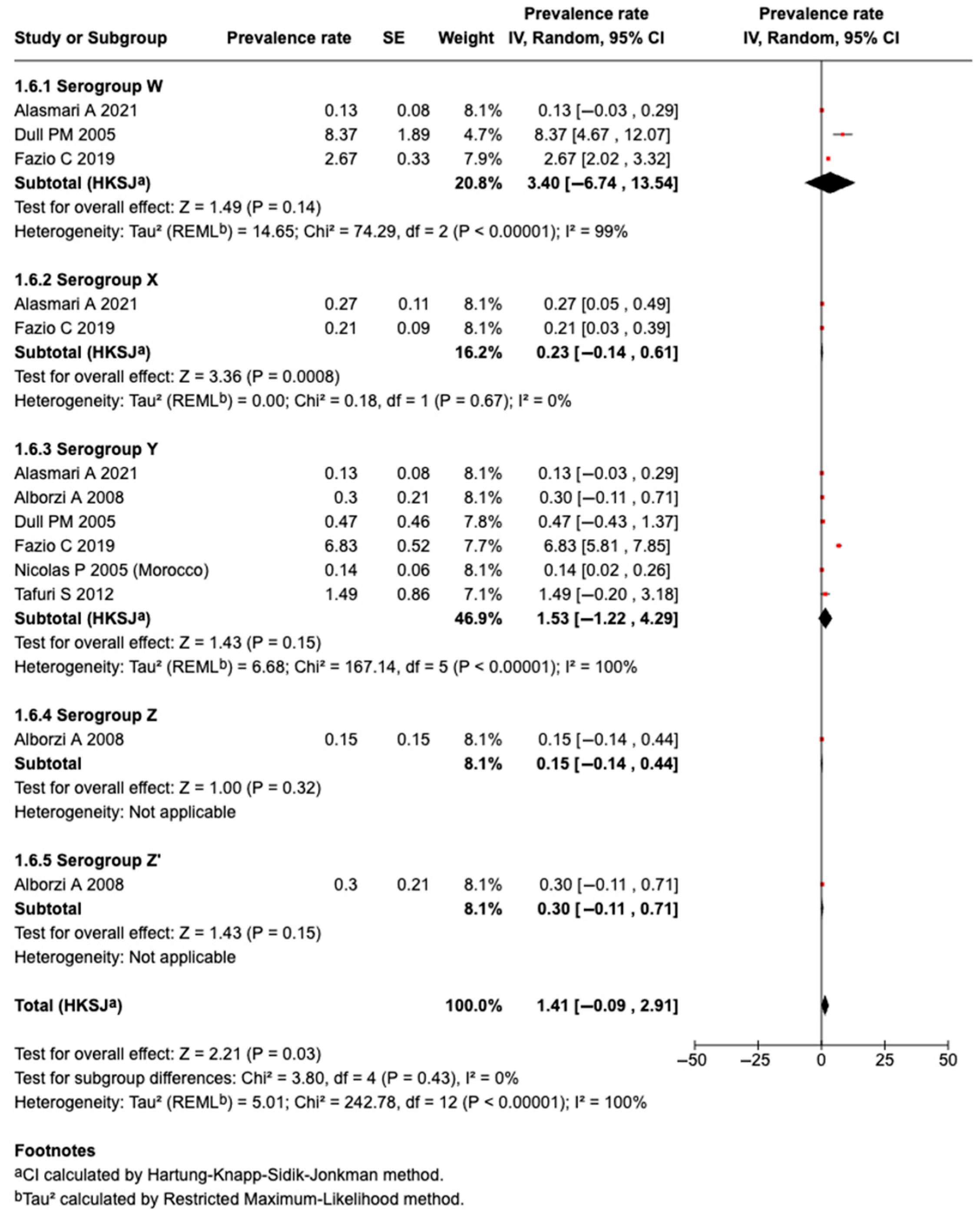
3.5.9. Prevalence of Serogroup W Meningococcal Disease or Carriage
3.5.10. Prevalence of Serogroup X Meningococcal Disease or Carriage
3.5.11. Prevalence of Serogroup Y Meningococcal Disease or Carriage
3.5.12. Prevalence of Serogroup Z Meningococcal Disease or Carriage
3.5.13. Prevalence of Non-Groupable Meningococcal Disease or Carriage
3.5.14. Prevalence of Auto-Agglutinable Meningococcal Disease or Carriage
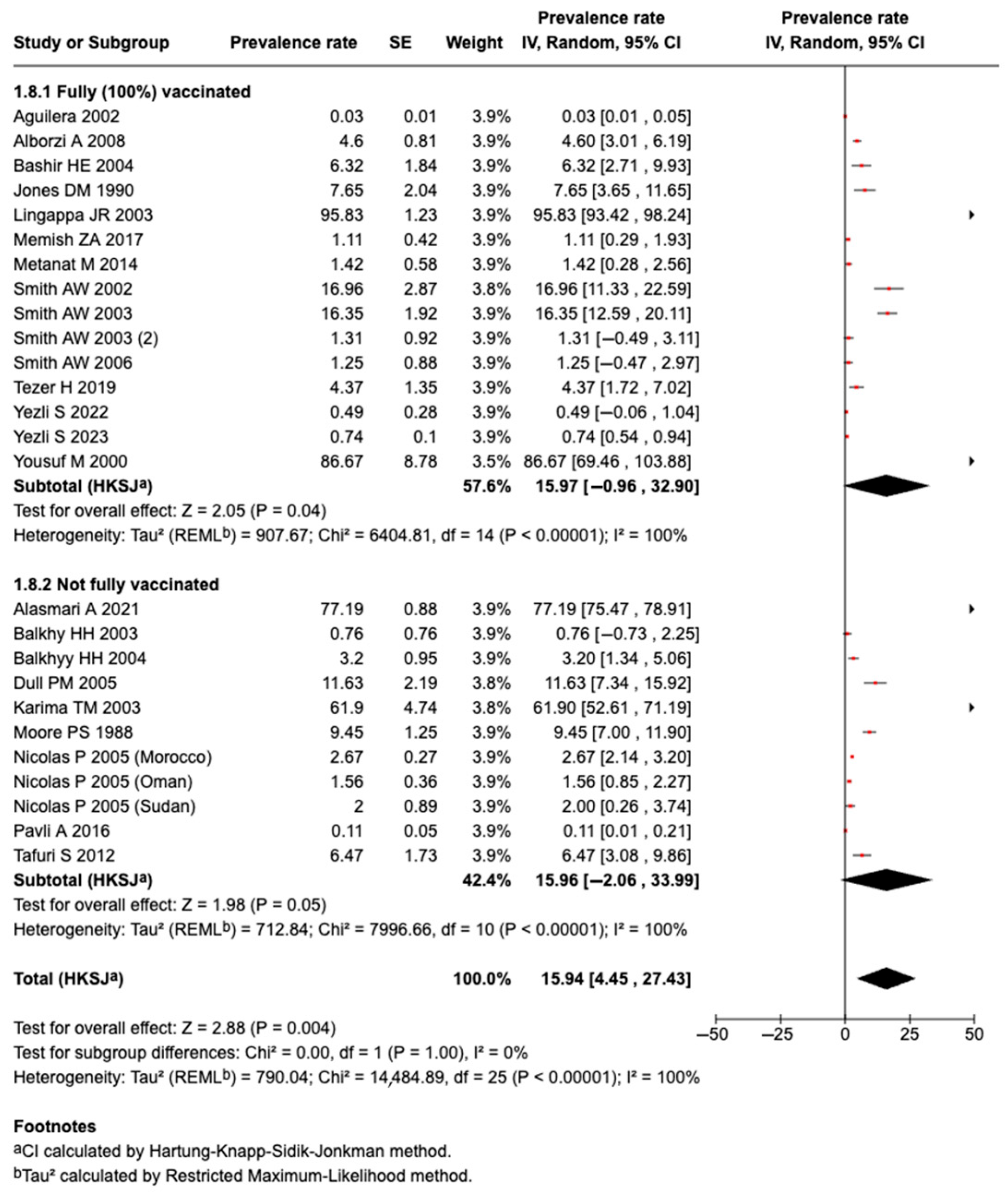
3.5.15. Effect of Vaccination on Prevalence of Meningococcal Diseases or Carriage
3.5.16. Risk Factors Associated with the Prevalence of Meningococcal Disease or Carriage
3.6. Secondary Outcomes
3.6.1. Mortality
3.6.2. Other Outcomes
3.7. Publication Bias
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| PROSPERO | International Prospective Register of Systematic Reviews |
| CRD | Part of the PROSPERO Registration Number |
| PECOS | Participant, Exposure, Comparator, Outcome, and Study |
| MeSH | Medical Subject Heading |
| IQR | Interquartile Range |
| CSF | Cerebrospinal Fluid |
| RR | Risk Ratio |
| RevMan | Review Manager Software |
| MGs | Mass Gatherings |
References
- Pardo de Santayana, C.; Htar, M.T.T.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.S. Global Control of Meningococcal Disease. N. Engl. J. Med. 2023, 388, 2003–2005. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Findlow, J.; Peyrani, P. Global Epidemiology of Meningococcal Disease-Causing Serogroups Before and After the COVID-19 Pandemic: A Narrative Review. Infect. Dis. Ther. 2024, 13, 2489–2507. [Google Scholar] [CrossRef] [PubMed]
- CDC. Increase in Invasive Serogroup Y Meningococcal Disease in the United States; CDC Health Alert Network; Centre for Disease Control: Atlanta, GA, USA, 2024.
- World Health Organization. Public Health for Mass Gatherings: Key Considerations; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Halsey, E. CDC Yellow Book 2026: Health Information for International Travel; Oxford University Press: Oxford, UK, 2025. [Google Scholar]
- Burki, T.K. Mass gatherings and respiratory disease. Lancet Respir. Med. 2013, 1, 598. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.P.; Hazarika, R.D.; Abitbol, V.; Kolhapure, S.; Agrawal, S. Mass gatherings: A review of the scope for meningococcal vaccination in the Indian context. Hum. Vaccines Immunother. 2021, 17, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Steffen, R.; White, P.; Dar, O.; Azhar, E.I.; Sharma, A.; Zumla, A. Mass gatherings medicine: Public health issues arising from mass gathering religious and sporting events. Lancet 2019, 393, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Stephens, D.S. Neisseria meningitidis: Biology, microbiology, and epidemiology. Methods Mol. Biol. 2012, 799, 1–20. [Google Scholar] [PubMed]
- Alshamrani, M.; Farahat, F.; Alzunitan, M.; Hasan, M.A.; Alsherbini, N.; Albarrak, A.; Al Johani, S.M.; Al-Tawfiq, J.A.; Zumla, A.; Memish, Z.A. Hajj vaccination strategies: Preparedness for risk mitigation. J. Infect. Public Health 2024, 17, 102547. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Memish, Z.A. The Hajj 2019 Vaccine Requirements and Possible New Challenges. J. Epidemiol. Glob. Health 2019, 9, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Gautret, P.; Memish, Z.A. Expected immunizations and health protection for Hajj and Umrah 2018—An overview. Travel. Med. Infect. Dis. 2017, 19, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S. The threat of meningococcal disease during the Hajj and Umrah mass gatherings: A comprehensive review. Travel Med. Infect. Dis. 2018, 24, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Rousseau, A.; Piednoir, E.; Cattoir, V.; de La Blanchardière, A. Hajj-associated infections. Med. Mal. Infect. 2016, 46, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Vuocolo, S.; Balmer, P.; Gruber, W.C.; Jansen, K.U.; Anderson, A.S.; Perez, J.L.; York, L.J. Vaccination strategies for the prevention of meningococcal disease. Hum. Vaccines Immunother. 2018, 14, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Almoallim, H.A.H.; Samannodi, M. Meningococcal Disease Burden and Transmission Related to Mass Gatherings and Travel: A Systematic Review and Meta-Analysis; York University: North York, ON, Canada, 2025. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.R.S.; Law, C. The Effectiveness, Cost-Effectiveness and Policy Processes of Regulatory, Voluntary and Partnership Policies to Improve Food Environments: An Evidence Synthesis; Appendix 4, Modifications to the Newcastle–Ottawa Scale for cross-sectional studies; National Institute for Health and Care Research: Southampton, UK, 2024. [Google Scholar]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- RevMan. Review Manager (RevMan) [Computer Program]; Version 5.4; The Cochrane Collaboration: Oxford, UK, 2020. [Google Scholar]
- StataCorp. Stata Statistical Software Release, 16th ed.; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Rashid, M.; Chhabra, M.; Kashyap, A.; Undela, K.; Gudi, S.K. Prevalence and Predictors of Self-Medication Practices in India: A Systematic Literature Review and Meta-Analysis. Curr. Clin. Pharmacol. 2020, 15, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Suhita, R.; Begum, I.; Rashid, M.; Chandran, V.P.; Shastri, S.A.; Kantamneni, R.; Rajan, A.K.; Thunga, G. Systematic review and meta-analysis of global prevalence of neurotoxic and hemotoxic snakebite envenomation. East Mediterr. Health J. 2022, 28, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Nadeem, A. Meningococcal infection among pilgrims visiting Madinah Al-Munawarah despite prior A-C vaccination. J. Pak. Med. Assoc. 2000, 50, 184–186. [Google Scholar] [PubMed]
- Balkhy, H.H.; Memish, Z.A.; Osoba, A.O. Meningococcal carriage among local inhabitants during the pilgrimage 2000–2001. Int. J. Antimicrob. Agents 2003, 21, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Balkhy, H.H.; Memish, Z.A.; Almuneef, M.A.; Osoba, A.O. Neisseria meningitidis W-135 carriage during the Hajj season 2003. Scand. J. Infect. Dis. 2004, 36, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S.; Yassin, Y.; Mushi, A.; Alabdullatif, L.; Alburayh, M.; Alotaibi, B.M.; Khan, A.; Walsh, L.; Lekshmi, A.; Walker, A.; et al. Carriage of Neisseria meningitidis among travelers attending the Hajj pilgrimage, circulating serogroups, sequence types and antimicrobial susceptibility: A multinational longitudinal cohort study. Travel Med. Infect. Dis. 2023, 53, 102581. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, A.; Houghton, J.; Greenwood, B.; Heymann, D.; Edwards, P.; Larson, H.; Assiri, A.; Ben-Rached, F.; Pain, A.; Behrens, R.; et al. Meningococcal carriage among Hajj pilgrims, risk factors for carriage and records of vaccination: A study of pilgrims to Mecca. Trop. Med. Int. Health 2021, 26, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Fazio, C.; Neri, A.; Vacca, P.; Ciammaruconi, A.; Arghittu, M.; Barbui, A.M.; Vocale, C.; Bernaschi, P.; Isola, P.; Galanti, I.A.; et al. Cocirculation of Hajj and non-Hajj strains among serogroup W meningococci in Italy, 2000 to 2016. Eurosurveillance 2019, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Pavli, A.; Katerelos, P.; Smeti, P.; Maltezou, H.C. Meningococcal vaccination for international travellers from Greece visiting developing countries. Travel Med. Infect. Dis. 2016, 14, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Metanat, M.; Sharifi-Mood, B.; Sanei-Moghaddam, S.; Rad, N.S. Pharyngeal carriage rate of Neisseria meningitidis before and after the Hajj pilgrimage, in Zahedan (southeastern Iran), 2012. Turk. J. Med. Sci. 2015, 45, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S.; Yassin, Y.; Mushi, A.; Bukhari, M.; Banasser, T.; Khan, A. Carriage of Neisseria meningitidis Among Umrah Pilgrims: Circulating Serogroups and Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Tezer, H.; Gülhan, B.; Simge Gişi, A.; Nar Ötgün, S.; Kanık-Yüksek, S.; Özkaya-Parlakay, A.; Kılıç, S.; Şahin, N.Ü.; Şimşek, A.Ç.; Kara, A. The impact of meningococcal conjugate vaccine (MenACWY-TT) on meningococcal carriage in Hajj Pilgrims returning to Turkey. Hum. Vaccines Immunother. 2020, 16, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Prato, R.; Martinelli, D.; Germinario, C. Prevalence of carriers of Neisseria meningitidis among migrants: Is migration changing the pattern of circulating meningococci? J. Travel Med. 2012, 19, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Al-Tawfiq, J.A.; Almasri, M.; Azhar, E.I.; Yasir, M.; Al-Saeed, M.S.; Ben Helaby, H.; Borrow, R.; Turkistani, A.; Assiri, A. Neisseria meningitidis nasopharyngeal carriage during the Hajj: A cohort study evaluating the need for ciprofloxacin prophylaxis. Vaccine 2017, 35, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Dull, P.M.; Abdelwahab, J.; Sacchi, C.T.; Becker, M.; Noble, C.A.; Barnett, G.A.; Kaiser, R.M.; Mayer, L.W.; Whitney, A.M.; Schmink, S.; et al. Neisseria meningitidis serogroup W-135 carriage among US travelers to the 2001 Hajj. J. Infect. Dis. 2005, 191, 33–39. [Google Scholar] [CrossRef] [PubMed]
- El Bashir, H.; Coen, P.G.; Haworth, E.; Taylor, S.; Mifsud, A.; El Baki, A.; Zuckerman, J.; Gray, S.J.; Booy, R. Meningococcal W135 carriage; enhanced surveillance amongst east London Muslim pilgrims and their household contacts before and after attending the 2002 Hajj. Travel Med. Infect. Dis. 2004, 2, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Barkham, T.M.; Ravindran, S.; Earnest, A.; Paton, N.I. Persistence of W135 Neisseria meningitidis carriage in returning Hajj pilgrims: Risk for early and late transmission to household contacts. Emerg. Infect. Dis. 2003, 9, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Paton, N.I.; Barkham, T.M.; Earnest, A. Meningococcal carriage in Umra pilgrims returning from Saudi Arabia. J. Travel Med. 2003, 10, 147–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karima, T.M.; Bukhari, S.Z.; Fatani, M.I.; Yasin, K.A.; Al-Afif, K.A.; Hafiz, F.H. Clinical and microbiological spectrum of meningococcal disease in adults during Hajj 2000: An implication of quadrivalent vaccination policy. J. Pak. Med. Assoc. 2003, 53, 3–7. [Google Scholar] [PubMed]
- Lingappa, J.R.; Al-Rabeah, A.M.; Hajjeh, R.; Mustafa, T.; Fatani, A.; Al-Bassam, T.; Badukhan, A.; Turkistani, A.; Makki, S.; Al-Hamdan, N.; et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg. Infect. Dis. 2003, 9, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Barkham, T.M.; Chew, S.K.; Paton, N.I. Absence of Neisseria meningitidis W-135 electrophoretic Type 37 during the Hajj, 2002. Emerg. Infect. Dis. 2003, 9, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; Ait M’barek, N.; Al-Awaidy, S.; Al Busaidy, S.; Sulaiman, N.; Issa, M.; Mahjour, J.; Mölling, P.; Caugant, D.A.; Olcén, P.; et al. Pharyngeal carriage of serogroup W135 Neisseria meningitidis in Hajjees and their family contacts in Morocco, Oman and Sudan. Apmis 2005, 113, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Barkham, T.M.; Earnest, A.; Paton, N.I. Acquisition of W135 meningococcal carriage in Hajj pilgrims and transmission to household contacts: Prospective study. BMJ 2002, 325, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.F.; Perrocheau, A.; Meffre, C.; Hahné, S. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg. Infect. Dis. 2002, 8, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Harrison, L.H.; Telzak, E.E.; Ajello, G.W.; Broome, C.V. Group A meningococcal carriage in travelers returning from Saudi Arabia. JAMA 1988, 260, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Sutcliffe, E.M. Group A meningococcal disease in England associated with the Haj. J. Infect. 1990, 21, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, A.; Oskoee, S.; Pourabbas, B.; Alborzi, S.; Astaneh, B.; Gooya, M.M.; Kaviani, M.J. Meningococcal carrier rate before and after hajj pilgrimage: Effect of single dose ciprofloxacin on carriage. East Mediterr. Health J. 2008, 14, 277–282. [Google Scholar] [PubMed]
- Almehmadi, M.; Alqahtani, J.S. Healthcare Research in Mass Religious Gatherings and Emergency Management: A Comprehensive Narrative Review. Healthcare 2023, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Gautret, P.; Benkouiten, S.; Memish, Z.A. Mass Gatherings and the Spread of Respiratory Infections. Lessons from the Hajj. Ann. Am. Thorac. Soc. 2016, 13, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Zumla, A.; Alhakeem, R.F.; Assiri, A.; Turkestani, A.; Al Harby, K.D.; Alyemni, M.; Dhafar, K.; Gautret, P.; Barbeschi, M.; et al. Hajj: Infectious disease surveillance and control. Lancet 2014, 383, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Al Awaidy, S.; Ozudogru, O.; Badur, S. Meningococcal disease within the Gulf Cooperation Council Countries. Hum. Vaccines Immunother. 2023, 19, 2193120. [Google Scholar] [CrossRef] [PubMed]
- Badur, S.; Khalaf, M.; Öztürk, S.; Al-Raddadi, R.; Amir, A.; Farahat, F.; Shibl, A. Meningococcal Disease and Immunization Activities in Hajj and Umrah Pilgrimage: A review. Infect. Dis. Ther. 2022, 11, 1343–1369. [Google Scholar] [CrossRef] [PubMed]
- Morello, B.R.; Milazzo, A.; Marshall, H.S.; Giles, L.C. Public health management of invasive meningococcal disease outbreaks: Worldwide 1973–2018, a systematic review. BMC Public Health 2024, 24, 2254. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C. Large outbreak of Neisseria meningitidis serogroup C—Nigeria, December 2016–June 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.M.; Harrison, L.H. Vaccine prevention of meningococcal disease in Africa: Major advances, remaining challenges. Hum. Vaccines Immunother. 2018, 14, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, S.A. Meningococcal vaccination: Recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Mauskopf, J.; Masaquel, C.; Huang, L. Evaluating Vaccination Programs That Prevent Diseases With Potentially Catastrophic Health Outcomes: How Can We Capture the Value of Risk Reduction? Value Health 2021, 24, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Herrera-Restrepo, O.; Rolland, C.; Purushotham, S.; Andani, A.; Shah, H.; Kocaata, Z. Inequalities in the risk and prevention of invasive meningococcal disease in the United States—A systematic literature review. Hum. Vaccines Immunother. 2024, 20, 2406613. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.K.; Borrow, R. Serogroup B meningococcal disease or carriageduring Hajj: Preparing for the worst scenario. Travel. Med. Infect. Dis. 2009, 7, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Soeters, H.M.; McNamara, L.A.; Blain, A.E.; Whaley, M.; MacNeil, J.R.; Hariri, S.; Mbaeyi, S.A. Serogroup B Meningococcal disease or carriageUniversity Outbreak Group. University-Based Outbreaks of Meningococcal disease or carriageCaused by Serogroup B, United States, 2013–2018. Emerg. Infect. Dis. 2019, 25, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.M. Impact of serogroup A meningococcal conjugate vaccine for Africa. Hum. Vaccines Immunother. 2018, 14, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Fergie, J.; Presa, J.; Peyrani, P. Rationale for the Development of a Pentavalent Meningococcal Vaccine: A US-Focused Review. Infect. Dis. Ther. 2022, 11, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kale, S.; Phugare, S.; Goel, S.K.; Gairola, S. Analytical Challenges in Novel Pentavalent Meningococcal Conjugate Vaccine (A, C, Y, W, X). Vaccines 2024, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Badahdah, A.M.; Alghabban, F.; Falemban, W.; Albishri, A.; Rani Banik, G.; Alhawassi, T.; Abuelizz, H.; Bakarman, M.A.; Khatami, A.; Booy, R.; et al. Meningococcal Vaccine for Hajj Pilgrims: Compliance, Predictors, and Barriers. Trop. Med. Infect. Dis. 2019, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S.; Bin Saeed, A.A.; Assiri, A.M.; Alhakeem, R.F.; Yunus, M.A.; Turkistani, A.M.; Booy, R.; Alotaibi, B.M. Prevention of meningococcal disease during the Hajj and Umrah mass gatherings: Past and current measures and future prospects. Int. J. Infect. Dis. 2016, 47, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Caugant, D.A.; Ceyhan, M.; Christensen, H.; Dinleyici, E.C.; Findlow, J.; Glennie, L.; Von Gottberg, A.; Kechrid, A.; Moreno, J.V.; et al. Meningococcal disease in the Middle East and Africa: Findings and updates from the Global Meningococcal Initiative. J. Infect. 2017, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.C.; MacNeil, J.R.; Clark, T.A.; Ortega-Sanchez, I.R.; Briere, E.Z.; Meissner, H.C.; Baker, C.J.; Messonnier, N.E. Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2013, 62, 1–22. [Google Scholar]
- MacNeil, J.R.; Rubin, L.; Folaranmi, T.; Ortega-Sanchez, I.R.; Patel, M.; Martin, S.W. Use of serogroup B meningococcal vaccines in adolescents and young adults: Recommendations of the Advisory Committee on Immunization Practices, 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Booy, R.; Haworth, E.; Rashid, H.; Memish, Z.A. Hajj: Health lessons for mass gatherings. J. Infect. Public Health 2008, 1, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Balkhi, B.S.; Alamri, F.A.; Alsaleh, G.S.; Al-Tawfiq, J.A.; Jokhdar, H. Vaccinations for Hajj: Enhancing health and global health security. Travel Med. Infect. Dis. 2025, 63, 102784. [Google Scholar] [CrossRef] [PubMed]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Latif, A.; Timmons, S.; Nkhoma, K.; Nellums, L.B. Overcoming COVID-19 vaccine hesitancy among ethnic minorities: A systematic review of UK studies. Vaccine 2022, 40, 3413–3432. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.; Forbes, C.; Wittkopf, P.; Pandey, A.; Mendes, D.; Kowalik, J.; Czudek, C.; Mugwagwa, T. Impact of pandemics and disruptions to vaccination on infectious diseases epidemiology past and present. Hum. Vaccines Immunother. 2023, 19, 2219577. [Google Scholar] [CrossRef] [PubMed]
- Ibarz-Pavón, A.B.; Maclennan, J.; Andrews, N.J.; Gray, S.J.; Urwin, R.; Clarke, S.C.; Walker, A.M.; Evans, M.R.; Kroll, J.S.; Neal, K.R.; et al. Changes in serogroup and genotype prevalence among carried meningococci in the United Kingdom during vaccine implementation. J. Infect. Dis. 2011, 204, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Toneatto, D.; Pizza, M.; Masignani, V.; Rappuoli, R. Emerging experience with meningococcal serogroup B protein vaccines. Expert. Rev. Vaccines 2017, 16, 433–451. [Google Scholar]
| Author, Year | Study Country | Study Design | Place of Data Collection | Type of Exposure | Type of Gathering | Country of Participants | Total Number of Participants | Male (%) | Age | Vaccinated (n) | Vaccination (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Balkhy HH, 2004 [29] | Saudi Arabia | Cross-sectional | Clinic/hospital | Mass Gathering | Hajj | Multi-national (n = 29) | 344 | 45% | 0–50 *** | 245 | 71.2 |
| Yezli S, 2023 [30] | Saudi Arabia | Prospective | Place of residence | Mass Gathering | Hajj | Multi-national (n = 13) | 3921 | 52.4% | 55 (13.4) * | 3921 | 100.0 |
| Alasmari A, 2021 [31] | Saudi Arabia | Cross-sectional | Airport | Mass Gathering | Hajj | Multi-national (n = 11) | 2973 | 68% | 11–65 *** | 2117 | 71.2 |
| Fazio C, 2019 [32] | Italy | Retrospective | Public database | Mass Gathering | Hajj | Italy | 2357 | NR | 26 * | NR | N/A |
| Pavli A, 2016 [33] | Greece | Cross-sectional | Public database | Travel | World Travel | Greece | 5283 | 64.30% | 39.2 * | 1150 | 21.8 |
| Metanat M, 2015 [34] | Iran | Cross-sectional | Airport | Mass Gathering | Hajj | Iran | 422 | 42.20% | 21–95 *** | 422 | 100.0 |
| Yezli S, 2022 [35] | Saudi Arabia | Cross-sectional | Place of residence | Mass Gathering | Umrah | Multi-national (n = 17) | 616 | 50.6% | 19–91 *** (53.8) * | 56 | 9.1 |
| Tezer H, 2020 [36] | Turkey | Prospective | Airport | Mass Gathering | Hajj | Turkey | 229 | 48.91% | 23–71 *** 56 * | 229 | 100.0 |
| Tafuri S, 2012 [37] | Italy | Cross-sectional | Place of residence | Travel | Refugee Migration | Africa | 253 | 88.50% | 19.8 (6) * | NR | N/A |
| Memish ZA, 2017 [38] | Saudi Arabia | Prospective | Airport | Mass Gathering | Hajj | Multi-national (n = 10) | 628 | 63.70% | 18–65 *** | 628 | 100.0 |
| Dull PM, 2005 [39] | USA | Retrospective | Airport | Both | World Travel | USA | 844 | 61.5% | Pilgrim: 47; non-pilgrim: 35 ** | 840 | 99.5 |
| El-Bashir HE, 2004 [40] | UK | Ambispective | Muslim center | Mass Gathering | Hajj | UK | 253 | 70% | 42 (2–82) ** | 253 | 100.0 |
| Wilder-Smith A, 2003 [41] | Singapore | Prospective | Muslim center | Mass Gathering | Hajj | Singapore | 373 | 43% | 47 (3–78) ** | 373 | 100.0 |
| Wilder-Smith A, 2003 (2) [45] | Singapore | Prospective | Muslim Center | Mass Gathering | Hajj | Singapore | 153 | 48% | 48 (8.07) * | 153 | 100.0 |
| Wilder-Smith A, 2003 (3) [42] | Singapore | Prospective | Place of residence | Mass Gathering | Umrah | Singapore | 160 | 36% | 40 (2–80) ** | 160 | 100.0 |
| Yousuf M, 2000 [27] | Saudi Arabia | Prospective | Holy Masjid | Mass Gathering | Hajj and Umrah | Multi-national (n = 7) | 15 | 11% | NR | 15 | 100.0 |
| Karima TM, 2003 [43] | Saudi Arabia | Prospective | Clinic/hospital | Mass Gathering | Hajj | Multi-national (n = 26) | 105 | 56.2% | 20–80 *** | NR | N/A |
| Lingappa JR, 2003 [44] | Saudi Arabia | Retrospective | Public database | Mass Gathering | Hajj | Multi-national | 264 | 53% | 40 (0.2–80) ** | 264 | 100.0 |
| Alborzi A, 2008 [51] | Iran | Prospective | Airport | Mass Gathering | Hajj | Iran | 674 | 58.20% | 52 * | 674 | 100.0 |
| Nicolas P, 2005 [46] | Morocco, Oman, and Sudan | Prospective | Place of residence | Mass Gathering | Hajj | Morocco | 1186 | NR | NR | NR | N/A |
| Oman | 399 | NR | NR | NR | N/A | ||||||
| Sudan | 250 | NR | NR | NR | N/A | ||||||
| Balkhy HH, 2003 [28] | Saudi Arabia | Prospective | Clinic/hospital | Mass Gathering | Hajj | Saudi Arabia | 28 | 75% | 18–61 *** | 1 | 3.6 |
| Wilder-Smith A, 2002 [47] | Singapore | Prospective | Place of residence | Mass Gathering | Hajj | Singapore | 204 | 45% | 48 (24–74) ** | 204 | 100.0 |
| Aguilera, 2002 [48] | Europe | Prospective | Place of residence | Mass Gathering | Hajj | UK and France | 38,849 | NR | 50 | 38849 | 100.0 |
| Moore PS, 1988 [49] | USA | Cross-sectional | Airport | Both | World Travel | USA | 550 | Pilgrimage: 173 | 43.9 (19.1) * | Pilgrimage: 33/192 | Pilgrimage: 17 |
| Jones DM, 1990 [50] | UK | Prospective | Airport | Travel | Hajj | England and Wales | 176 | NR | NR | 176 | 100 |
| Author, Year | Study Duration | Test Sample | No. of People Tested (n) | Number Infected (n) | Serogroup (n) |
|---|---|---|---|---|---|
| Balkhy HH, 2004 [29] | 2003 | TS | 344 | 11 | W-135: 2; B: 1; NG: 8 |
| Yezli S, 2023 [30] | 2019 | OPS | 7842 | 58 | B: 2; NG: 2 |
| Alasmari A, 2021 [31] | 2017 | OPS | 2249 | 130 | A: 2; B: 10; C: 10; W: 3; X: 6; Y: 3 |
| Fazio C, 2019 [32] | 2000–2016 | BIs and/or CS | 2357 | 2357 | 29E: 1; A: 17; B: 1249; C: 861; W: 63; X: 5; Y: 161 |
| Pavli A, 2016 [33] | 2009–2013 | TS | 5283 | 6 | NR |
| Metanat M, 2015 [34] | 2012 | OPS | 422 | 6 | NR |
| Yezli S, 2022 [35] | 2019 | OPS | 616 | 3 | A: 2; B: 1 |
| Tezer H, 2020 [36] | 2018 | OPS | 229 | 10 | B: 1 |
| Tafuri S, 2012 [37] | NR | NPS | 201 | 13 | W-135: 4; Y: 3; Autoagglutinable: 6 |
| Memish ZA, 2017 [38] | 2014 | NPS | 628 | 7 | NR |
| Dull PM, 2005 [39] | 2001 | OPS | 215 | 25 | W-135: 10; B: 5; W: 18; Y: 1; NG: 15 |
| El-Bashir HE, 2004 [40] | 2000–2001 | TS | 174 | 11 | 29E: 1; W-135: 1; B: 3; NG: 6 |
| Wilder-Smith A, 2003 [41] | 2001 | TS | 373 | 61 | W-135: 56 |
| Wilder-Smith A, 2003 (2) [45] | 2002 | OPS | 153 | 2 | W-135: 2 |
| Wilder-Smith A, 2003 (3) [42] | 2001 | TS | 160 | 2 | Autoagglutinable: 1; NG: 1 |
| Yousuf M, 2000 [27] | 1992–1993 | CSF | 15 | 13 | W-135: 2; A: 7; B: 1; C: 3 |
| Karima TM, 2003 [43] | 2000 | CSF | 105 | 65 | W-135: 19; A: 44; B: 1; NG: 1 |
| Lingappa JR, 2003 [44] | 2000 | Blood and CSF | 264 | 253 | W-135: 93; A: 60 |
| Alborzi A, 2008 [51] | 2003 | TS | 674 | 31 | W-135: 1; A: 1; B: 2; C: 1; Y: 2; Z: 1; Z’: 2; NG: 21 |
| Nicolas P, 2005 [46] | 2000–2001 | TS | 3558 | 95 | W-135: 31; B: 12; Y: 5; NG: 47 |
| Nicolas P. 2005 (Oman) * | 2000–2001 | TS | 1157 | 18 | W-135: 9; B: 4; NG: 5 |
| Nicolas P. 2005 (Sudan) * | 2000–2001 | TS | 250 | 5 | W-135: 5 |
| Balkhy HH, 2003 [28] | 2001 | TS | 131 | 1 | W-135: 1; NG: 5 |
| Wilder-Smith A, 2002 [47] | 2001 | NPS | 171 | 29 | W-135: 26 |
| Aguilera, 2002 [48] | 2000 | BI and/or CSs | 38,849 | 12 | NR |
| Moore PS, 1988 [49] | 1987 | TS | 550 | 52 | A: 36; NG: 16 |
| Jones DM, 1990 [50] | 1987 | TS | 170 | 13 | A: 1; NG: 11 |
| Author, Year | Study Design | Selection (5 Score) | Comparability (2 Score) | Outcome (3 Score) | Total (10 Score) |
|---|---|---|---|---|---|
| Balkhy HH, 2004 [29] | Cross-sectional | 5 | 1 | 3 | 9 |
| Yezli S, 2023 [30] | Prospective | 5 | 2 | 3 | 10 |
| Alasmari A, 2021 [31] | Cross-sectional | 5 | 1 | 2 | 8 |
| Fazio C, 2019 [32] | Retrospective | 5 | 1 | 3 | 9 |
| Pavli A, 2016 [33] | Cross-sectional | 5 | 2 | 2 | 9 |
| Metanat M, 2015 [34] | Cross-sectional | 5 | 2 | 3 | 10 |
| Yezli S, 2022 [35] | Cross-sectional | 5 | 2 | 3 | 10 |
| Tezer H, 2020 [36] | Prospective | 5 | 1 | 3 | 9 |
| Tafuri S, 2012 [37] | Cross-sectional | 5 | 1 | 2 | 8 |
| Memish ZA, 2017 [38] | Prospective | 5 | 2 | 3 | 10 |
| Dull PM, 2005 [39] | Retrospective | 5 | 2 | 2 | 9 |
| El-Bashir HE, 2004 [40] | Ambispective | 5 | 2 | 2 | 9 |
| Wilder-Smith A, 2003 [41] | Prospective | 5 | 2 | 3 | 10 |
| Wilder-Smith A, 2003 (2) [45] | Prospective | 4 | 2 | 3 | 9 |
| Wilder-Smith A, 2003 (3) [42] | Prospective | 5 | 2 | 3 | 10 |
| Yousuf M, 2000 [27] | Prospective | 5 | 1 | 3 | 9 |
| Karima TM, 2003 [43] | Prospective | 5 | 0 | 3 | 8 |
| Lingappa JR, 2003 [44] | Retrospective | 5 | 1 | 3 | 9 |
| Alborzi A, 2008 [51] | Prospective | 5 | 2 | 2 | 9 |
| Nicolas P, 2005 [46] | Prospective | 5 | 1 | 2 | 8 |
| Nicolas P. 2005 (Oman) * | Prospective | 5 | 1 | 3 | 9 |
| Nicolas P. 2005 (Sudan) * | Prospective | 5 | 1 | 3 | 9 |
| Balkhy HH, 2003 [28] | Prospective | 5 | 2 | 3 | 10 |
| Wilder-Smith A, 2002 [47] | Cross-sectional | 4 | 1 | 3 | 8 |
| Aguilera, 2002 [48] | Prospective | 5 | 0 | 3 | 8 |
| Variable | Meta-Regression Coefficient | Standard Error | p-Value | 95%CI |
|---|---|---|---|---|
| Study duration/time | −0.503 | 0.755 | 0.512 | −2.071 to 1.063 |
| Test sample | 0.241 | 0.326 | 0.469 | −0.436 to 0.917 |
| Study design | 0.332 | 0.741 | 0.658 | 1.205 to 1.870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samannodi, M.; Alwafi, H.; Muglan, J.; Tawakul, A.; Algahtani, R.M.; Almoallim, H.M.; Alghamdi, I.A.; Obaid, M.S.; Alkhotani, A.M.A.; Alhazmi, A.S.H.; et al. Prevalence and Risk of Meningococcal Disease or Carriage During Mass Gatherings and Associated Travel: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2025, 10, 207. https://doi.org/10.3390/tropicalmed10080207
Samannodi M, Alwafi H, Muglan J, Tawakul A, Algahtani RM, Almoallim HM, Alghamdi IA, Obaid MS, Alkhotani AMA, Alhazmi ASH, et al. Prevalence and Risk of Meningococcal Disease or Carriage During Mass Gatherings and Associated Travel: Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease. 2025; 10(8):207. https://doi.org/10.3390/tropicalmed10080207
Chicago/Turabian StyleSamannodi, Mohammed, Hassan Alwafi, Jihad Muglan, Abdullah Tawakul, Rami M. Algahtani, Hani M. Almoallim, Ismail Ahmad Alghamdi, Majed Sameer Obaid, Amar Mohammad A. Alkhotani, Aous Sami Hayat Alhazmi, and et al. 2025. "Prevalence and Risk of Meningococcal Disease or Carriage During Mass Gatherings and Associated Travel: Systematic Review and Meta-Analysis" Tropical Medicine and Infectious Disease 10, no. 8: 207. https://doi.org/10.3390/tropicalmed10080207
APA StyleSamannodi, M., Alwafi, H., Muglan, J., Tawakul, A., Algahtani, R. M., Almoallim, H. M., Alghamdi, I. A., Obaid, M. S., Alkhotani, A. M. A., Alhazmi, A. S. H., Adly, H. M., Khan, A. A., Alamri, F. A., & Garout, M. A. (2025). Prevalence and Risk of Meningococcal Disease or Carriage During Mass Gatherings and Associated Travel: Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease, 10(8), 207. https://doi.org/10.3390/tropicalmed10080207