Anti-IFN-γ Autoantibody Syndrome Presenting with Disseminated Nontuberculous Mycobacteria Infections: A Case Series of Therapeutic Implications and Review of Literature
Abstract
1. Introduction
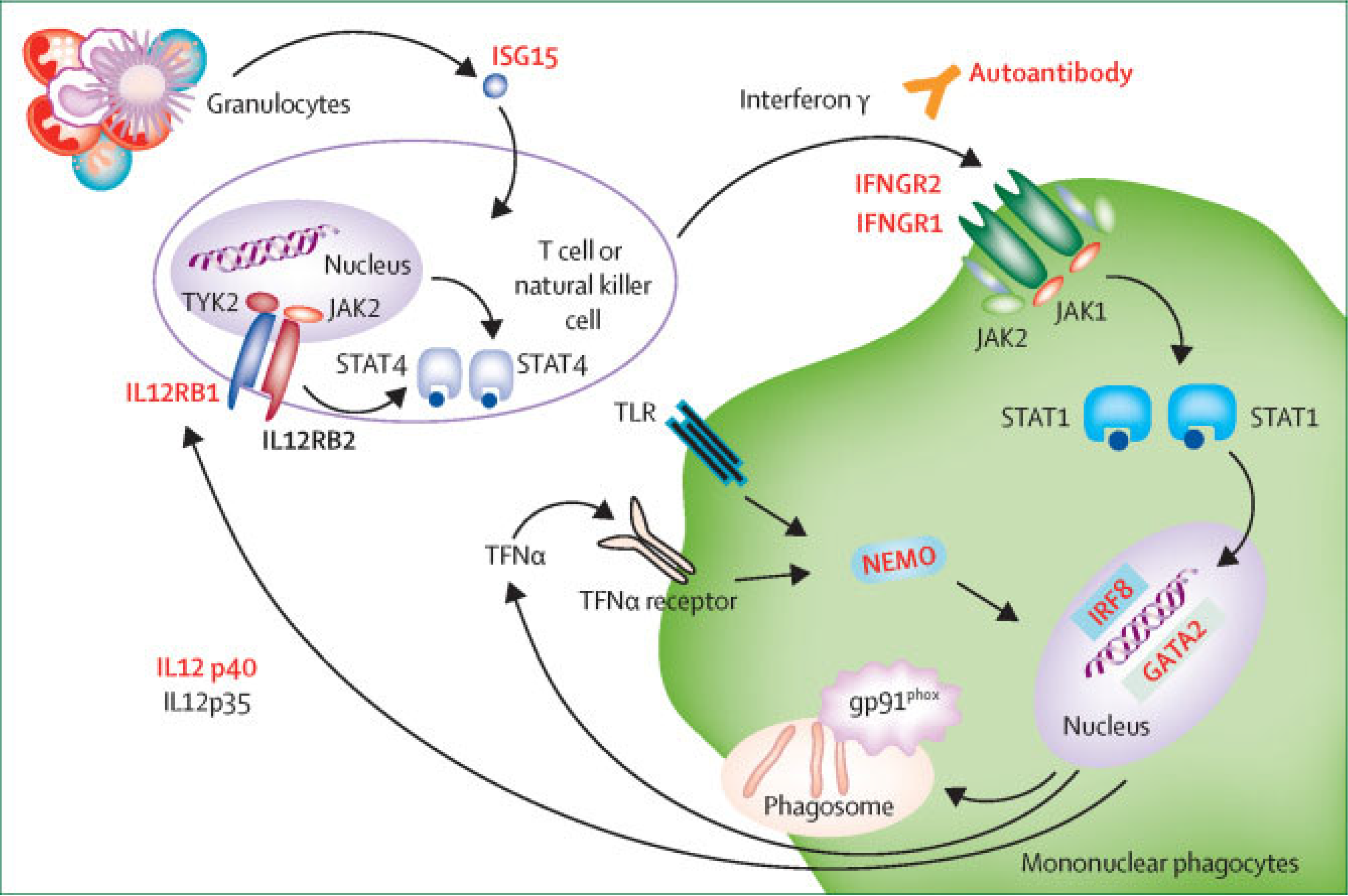
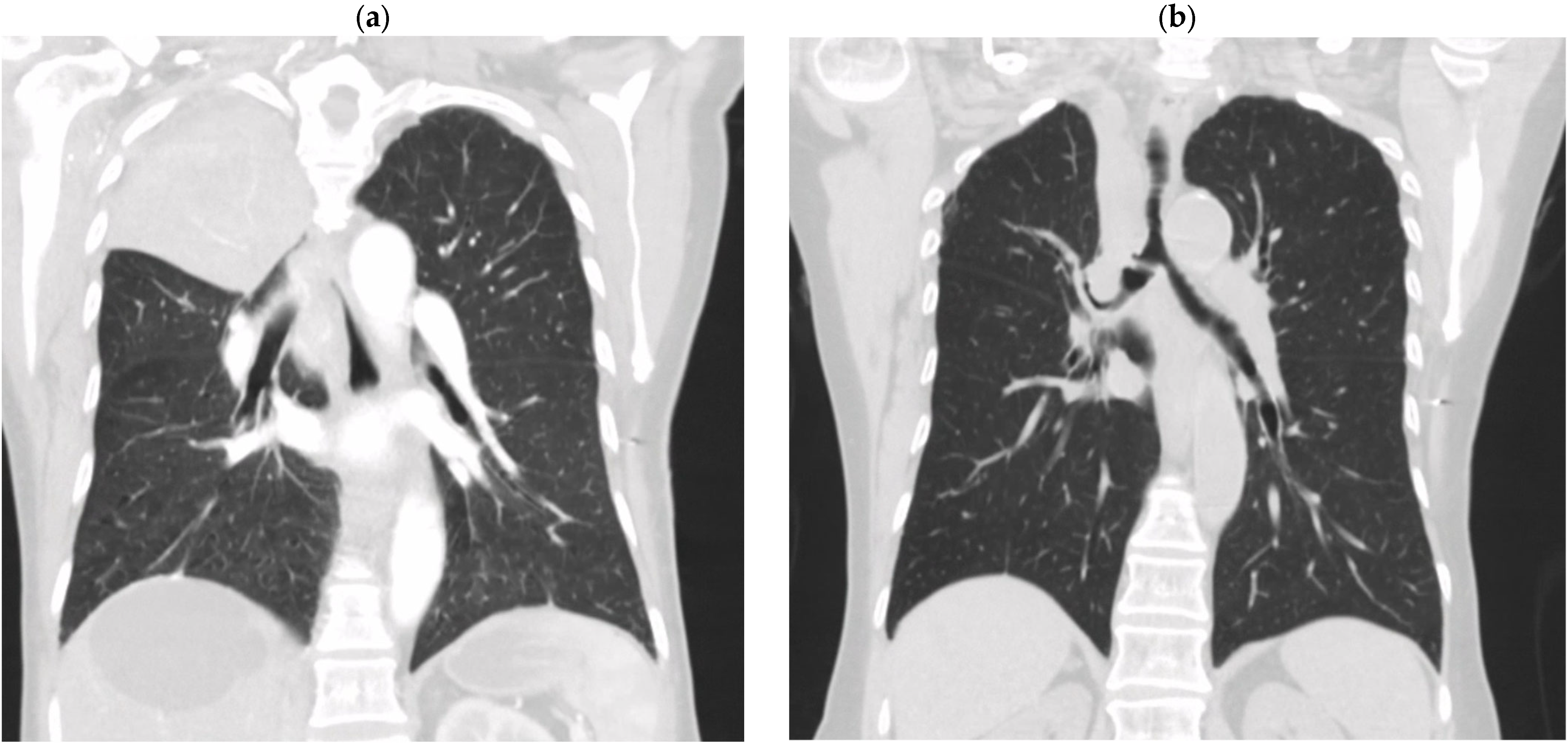
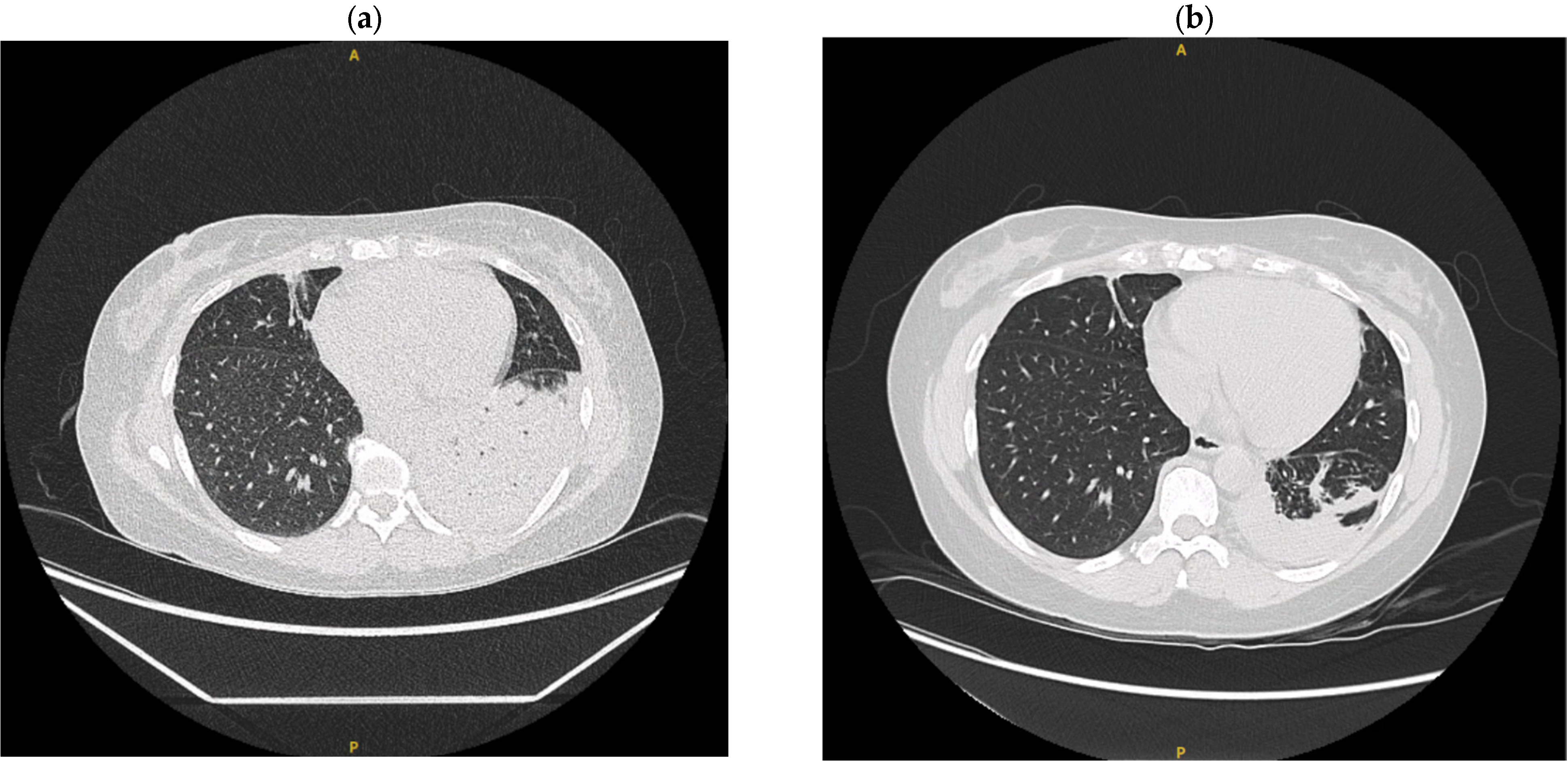
2. Clinical Case Summaries
3. Discussion
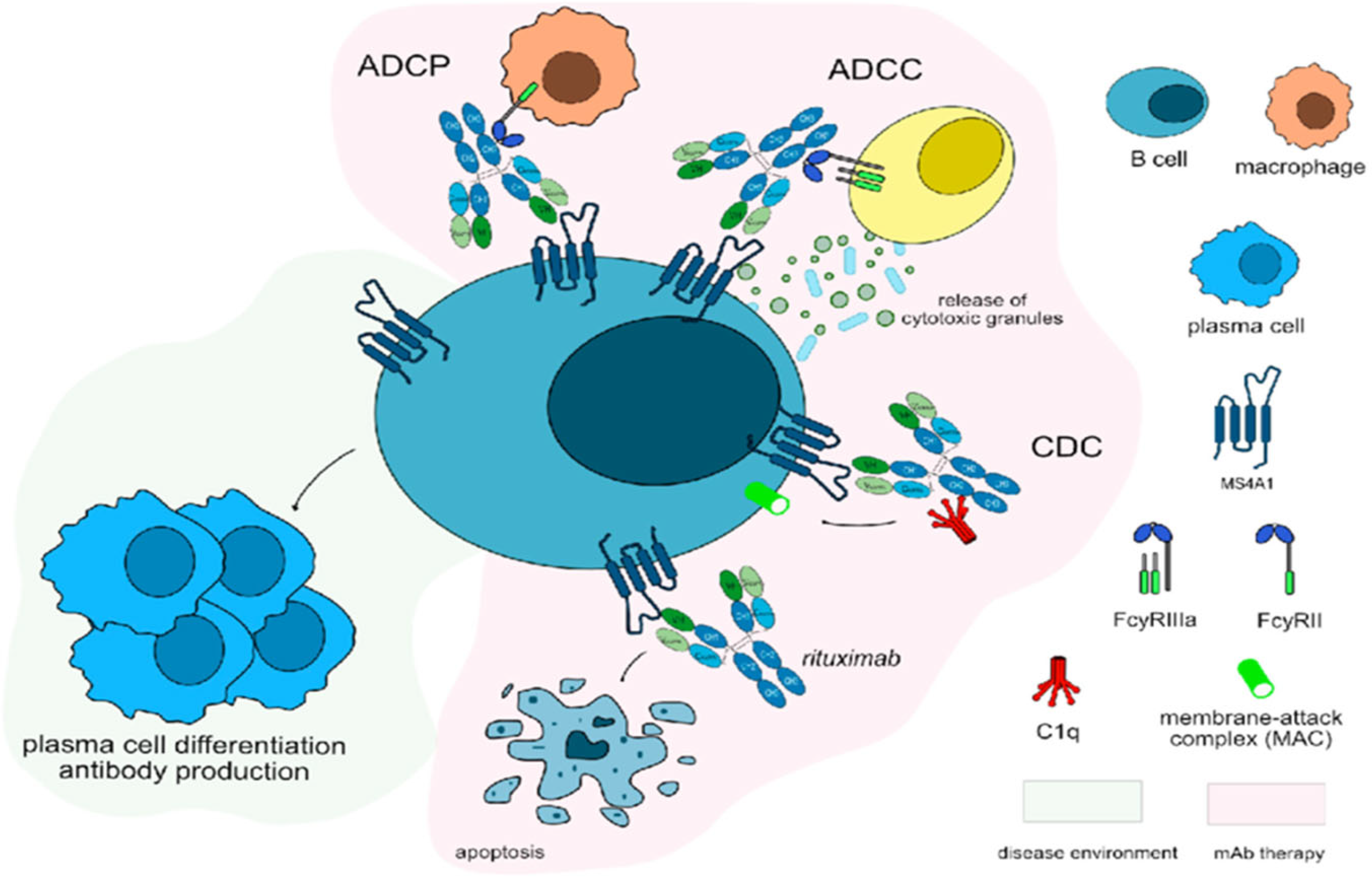
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arts, R.J.W.; Janssen, N.A.F.; van de Veerdonk, F.L. Anticytokine Autoantibodies in Infectious Diseases: A Practical Overview. Int. J. Mol. Sci. 2024, 25, 515. [Google Scholar] [CrossRef]
- Car, B.D.; Eng, V.M.; Schnyder, B.; Ozmen, L.; Huang, S.; Gallay, P.; Heumann, D.; Aguet, M.; Ryffel, B. Interferon Gamma Receptor Deficient Mice Are Resistant to Endotoxic Shock. J. Exp. Med. 1994, 179, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Sakagami, T.; Yoshizawa, K.; Shima, K.; Toyama, M.; Tanabe, Y.; Moro, H.; Aoki, N.; Watanabe, S.; Koya, T.; et al. Clinical Significance of Interferon-γ Neutralizing Autoantibodies Against Disseminated Nontuberculous Mycobacterial Disease. Clin. Infect. Dis. 2018, 66, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-Onset Immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Chawansuntati, K.; Rattanathammethee, K.; Wipasa, J. Minireview: Insights into Anti-Interferon-γ Autoantibodies. Exp. Biol. Med. 2021, 246, 790–795. [Google Scholar] [CrossRef]
- Dorman, S.E.; Holland, S.M. Mutation in the Signal-Transducing Chain of the Interferon-Gamma Receptor and Susceptibility to Mycobacterial Infection. J. Clin. Investig. 1998, 101, 2364–2369. [Google Scholar] [CrossRef]
- King, E.M.; Weaver, V.K.; Kestler, M.H. Treatment Dilemmas in Disseminated Nontuberculous Mycobacterial Infections with Interferon-Gamma Autoantibodies. Open Forum Infect. Dis. 2021, 8, ofab253. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, J.; Huang, C.; Fan, H.; Chen, J.; Huang, X.; Zeng, X. Characteristics and Outcomes of Anti-Interferon Gamma Antibody-Associated Adult Onset Immunodeficiency. J. Clin. Immunol. 2023, 43, 1660–1670. [Google Scholar] [CrossRef]
- Wu, U.I.; Wang, J.; Sheng, W.H.; Sun, H.-Y.; Cheng, A.; Hsu, L.-Y.; Chang, S.-C.; Chen, Y.-C. Incorrect Diagnoses in Patients with Neutralizing Anti-Interferon-Gamma-Autoantibodies. Clin. Microbiol. Infect. 2020, 26, 1684.e1–1684.e6. [Google Scholar] [CrossRef]
- Hong, G.H.; Ortega-Villa, A.M.; Hunsberger, S.; Chetchotisakd, P.; Anunnatsiri, S.; Mootsikapun, P.; Rosen, L.B.; Zerbe, C.S.; Holland, S.M. Natural History and Evolution of Anti-Interferon-γ Autoantibody-Associated Immunodeficiency Syndrome in Thailand and the United States. Clin. Infect. Dis. 2020, 71, 53–62. [Google Scholar] [CrossRef]
- Cheng, A.; Holland, S.M. Anticytokine Autoantibodies: Autoimmunity Trespassing on Antimicrobial Immunity. J. Allergy Clin. Immunol. 2022, 149, 24–28. [Google Scholar] [CrossRef]
- Qiu, Y.; Fang, G.; Ye, F.; Zeng, W.; Tang, M.; Wei, X.; Yang, J.; Li, Z.; Zhang, J. Pathogen Spectrum and Immunotherapy in Patients with Anti-IFN-γ Autoantibodies: A Multicenter Retrospective Study and Systematic Review. Front. Immunol. 2022, 13, 1051673. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef]
- Chen, L.F.; Yang, C.D.; Cheng, X.B. Anti-Interferon Autoantibodies in Adult-Onset Immunodeficiency Syndrome and Severe COVID-19 Infection. Front. Immunol. 2021, 12, 788368. [Google Scholar] [CrossRef]
- Wu, U.I.; Holland, S.M. Host Susceptibility to Nontuberculous Mycobacterial Infections. Lancet Infect. Dis. 2015, 15, 968–980. [Google Scholar] [CrossRef]
- O’Connell, E.; Rosen, L.B.; LaRue, R.W.; Fabre, V.; Melia, M.T.; Auwaerter, P.G.; Holland, S.M.; Browne, S.K. The First U.S. Domestic Report of Disseminated Mycobacterium avium Complex and Anti-Interferon-γ Autoantibodies. J. Clin. Immunol. 2014, 34, 928–932. [Google Scholar] [CrossRef]
- Wu, U.I.; Hung, C.C.; Chang, S.Y.; Jhong, Y.-T.; Sun, H.-Y.; Wang, J.-T.; Hsieh, S.-M.; Sheng, W.-H.; Liu, W.-C.; Chang, S.-C.; et al. Neutralizing Anti-Interferon-γ Autoantibodies Causing Disseminated Mycobacterium avium Complex Infection in an HIV-Infected Patient on Successful Combination Antiretroviral Therapy. AIDS 2017, 31, 2557–2559. [Google Scholar] [CrossRef]
- Smith, M.R. Rituximab (Monoclonal Anti-CD20 Antibody): Mechanisms of Action and Resistance. Oncogene 2003, 22, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.K.; Zaman, R.; Sampaio, E.P.; Jutivorakool, K.; Rosen, L.B.; Ding, L.; Pancholi, M.J.; Yang, L.M.; Priel, D.L.; Uzel, G.; et al. Anti-CD20 (Rituximab) Therapy for Anti-IFN-γ Autoantibody-Associated Nontuberculous Mycobacterial Infection. Blood 2012, 119, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Czaja, C.A.; Merkel, P.A.; Chan, E.D.; Lenz, L.L.; Wolf, M.L.; Alam, R.; Frankel, S.K.; Fischer, A.; Gogate, S.; Perez-Velez, C.M.; et al. Rituximab as Successful Adjunct Treatment in a Patient with Disseminated Nontuberculous Mycobacterial Infection due to Acquired Anti-Interferon-γ Autoantibody. Clin. Infect. Dis. 2014, 58, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Label: Rituximab. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf (accessed on 5 November 2024).
- Koizumi, Y.; Sakagami, T.; Nishiyama, N.; Hirai, J.; Hayashi, Y.; Asai, N.; Yamagishi, Y.; Kato, H.; Hagihara, M.; Sakanashi, D.; et al. Rituximab Restores IFN-γ-STAT1 Function and Ameliorates Disseminated Mycobacterium avium Infection in a Patient with Anti-Interferon-γ Autoantibody. J. Clin. Immunol. 2017, 37, 644–649. [Google Scholar] [CrossRef]
- Koo, S.; Marty, F.M.; Baden, L.R. Infectious Complications Associated with Immunomodulating Biologic Agents. Hematol./Oncol. Clin. N. Am. 2011, 25, 117–138. [Google Scholar] [CrossRef]
- Lahiri, M.; Dixon, W.G. Risk of Infection with Biologic Antirheumatic Therapies in Patients with Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 290–305. [Google Scholar] [CrossRef]
- Holland, S.M.; Dorman, S.E.; Kwon, A.; Pitha-Rowe, I.F.; Frucht, D.M.; Gerstberger, S.M.; Noel, G.J.; Vesterhus, P.; Brown, M.R.; Fleisher, T.A. Abnormal Regulation of Interferon-γ, Interleukin-12, and Tumor Necrosis Factor-α in Human Interferon-γ Receptor 1 Deficiency. J. Infect. Dis. 1998, 178, 1095–1104. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Kalra, N.; Horwitz, A.; Nino, G. Novel Mutation of Interferon-γ Receptor 1 Gene Presenting as Early Life Mycobacterial Bronchial Disease. J. Investig. Med. High Impact Case Rep. 2016, 4, 2324709616675463. [Google Scholar] [CrossRef]
- Ram, R.; Ben-Bassat, I.; Shpilberg, O.; Polliack, A.; Raanani, P. The Late Adverse Events of Rituximab Therapy—Rare but There! Leuk. Lymphoma 2009, 50, 1083–1095. [Google Scholar] [CrossRef]
- Nissen, J.C.; Hummel, M.; Brade, J.; Kruth, J.; Hofmann, W.-K.; Buchheidt, D.; Reinwald, M. The Risk of Infections in Hematologic Patients Treated with Rituximab Is Not Influenced by Cumulative Rituximab Dosage—A Single Center Experience. BMC Infect. Dis. 2014, 14, 364. [Google Scholar] [CrossRef][Green Version]
- Kelesidis, T.; Daikos, G.; Boumpas, D.; Tsiodras, S. Does Rituximab Increase the Incidence of Infectious Complications? A Narrative Review. Int. J. Infect. Dis. 2011, 15, e2–e16. [Google Scholar] [CrossRef] [PubMed]
- Golfinopoulou, R.; Giudicelli, V.; Manso, T.; Kossida, S. Delving into Molecular Pathways: Analyzing the Mechanisms of Action of Monoclonal Antibodies Integrated in IMGT/mAb-DB for Myasthenia Gravis. Vaccines 2023, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Bajwa, B.; Choi, S.; Martin, H.; Miao, T.; Werry, D.; Perlman, M.; Mirzanejad, Y. Anti-IFN-γ Autoantibody Syndrome Presenting with Disseminated Nontuberculous Mycobacteria Infections: A Case Series of Therapeutic Implications and Review of Literature. Trop. Med. Infect. Dis. 2025, 10, 202. https://doi.org/10.3390/tropicalmed10070202
Cheng B, Bajwa B, Choi S, Martin H, Miao T, Werry D, Perlman M, Mirzanejad Y. Anti-IFN-γ Autoantibody Syndrome Presenting with Disseminated Nontuberculous Mycobacteria Infections: A Case Series of Therapeutic Implications and Review of Literature. Tropical Medicine and Infectious Disease. 2025; 10(7):202. https://doi.org/10.3390/tropicalmed10070202
Chicago/Turabian StyleCheng, Brooke, Barinder Bajwa, Seungwon Choi, Hannah Martin, Tyson Miao, Denise Werry, Michael Perlman, and Yazdan Mirzanejad. 2025. "Anti-IFN-γ Autoantibody Syndrome Presenting with Disseminated Nontuberculous Mycobacteria Infections: A Case Series of Therapeutic Implications and Review of Literature" Tropical Medicine and Infectious Disease 10, no. 7: 202. https://doi.org/10.3390/tropicalmed10070202
APA StyleCheng, B., Bajwa, B., Choi, S., Martin, H., Miao, T., Werry, D., Perlman, M., & Mirzanejad, Y. (2025). Anti-IFN-γ Autoantibody Syndrome Presenting with Disseminated Nontuberculous Mycobacteria Infections: A Case Series of Therapeutic Implications and Review of Literature. Tropical Medicine and Infectious Disease, 10(7), 202. https://doi.org/10.3390/tropicalmed10070202






