Benzalkonium Chloride Significantly Improves Environmental DNA Detection from Schistosomiasis Snail Vectors in Freshwater Samples
Abstract
1. Introduction
2. Materials and Methods
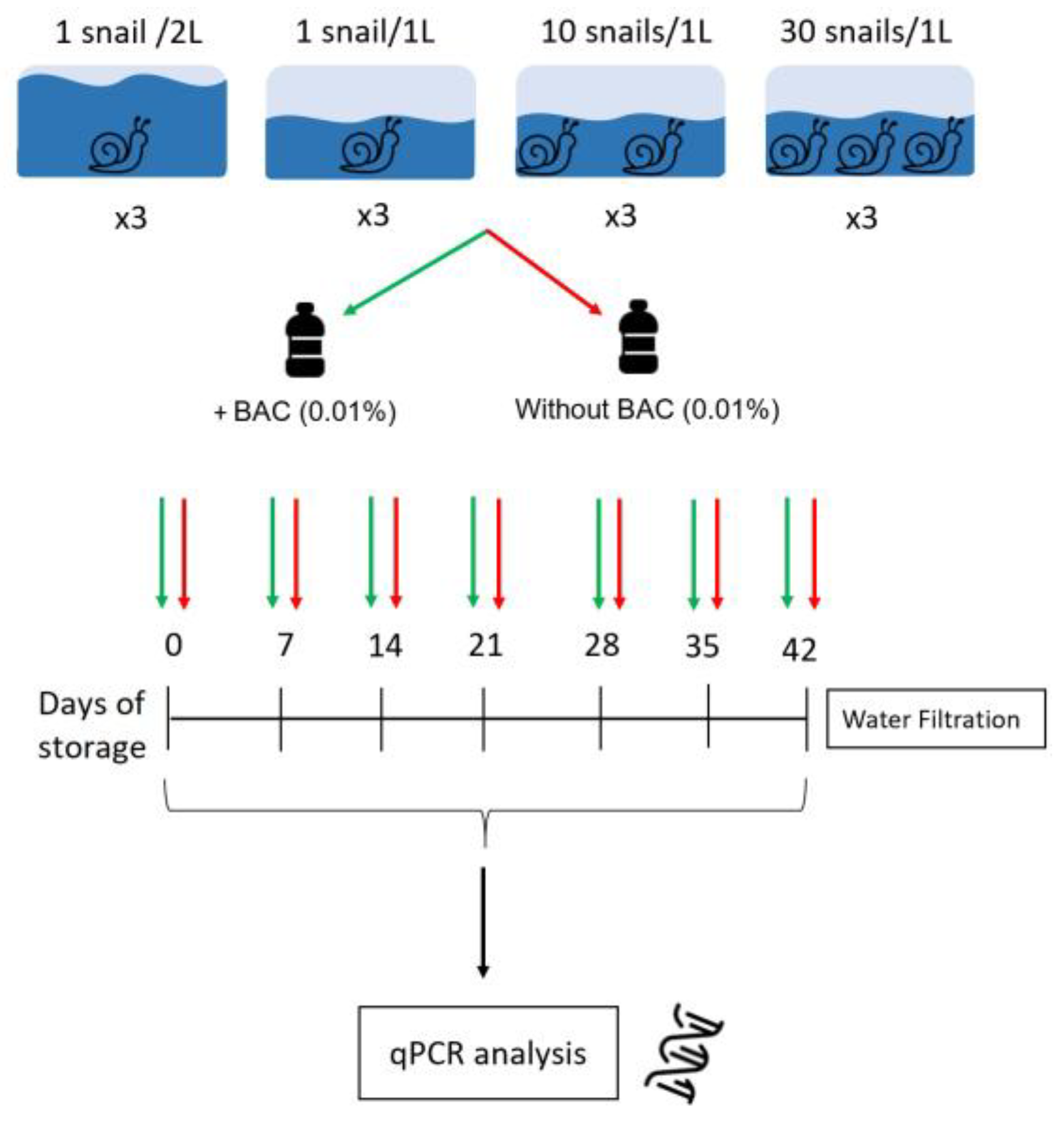
2.1. Experiment Design
2.2. Water Filtration
2.3. eDNA Extraction
2.4. qPCR Amplification
2.5. Statistical Analyses of the Laboratory Experiment
3. Results
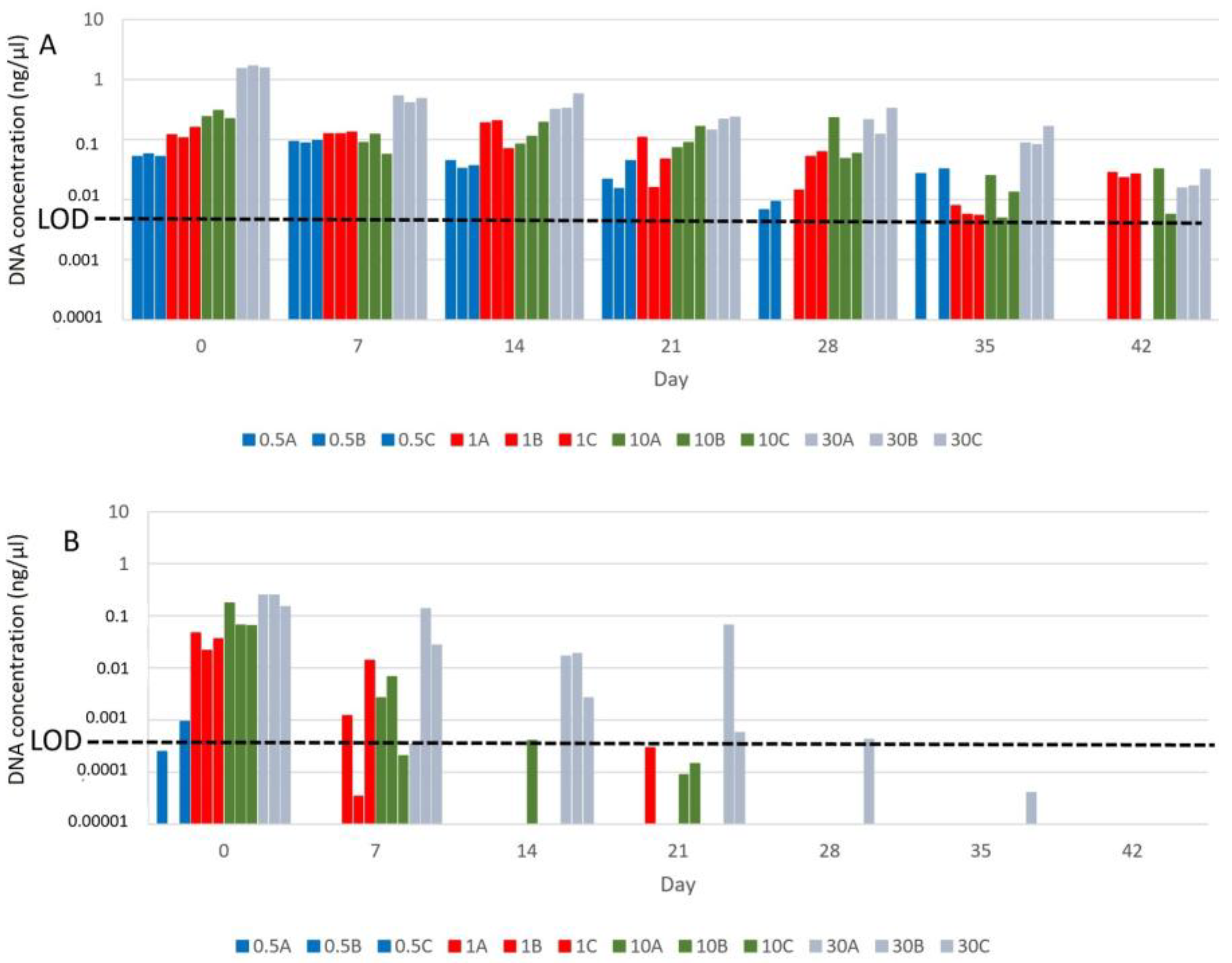
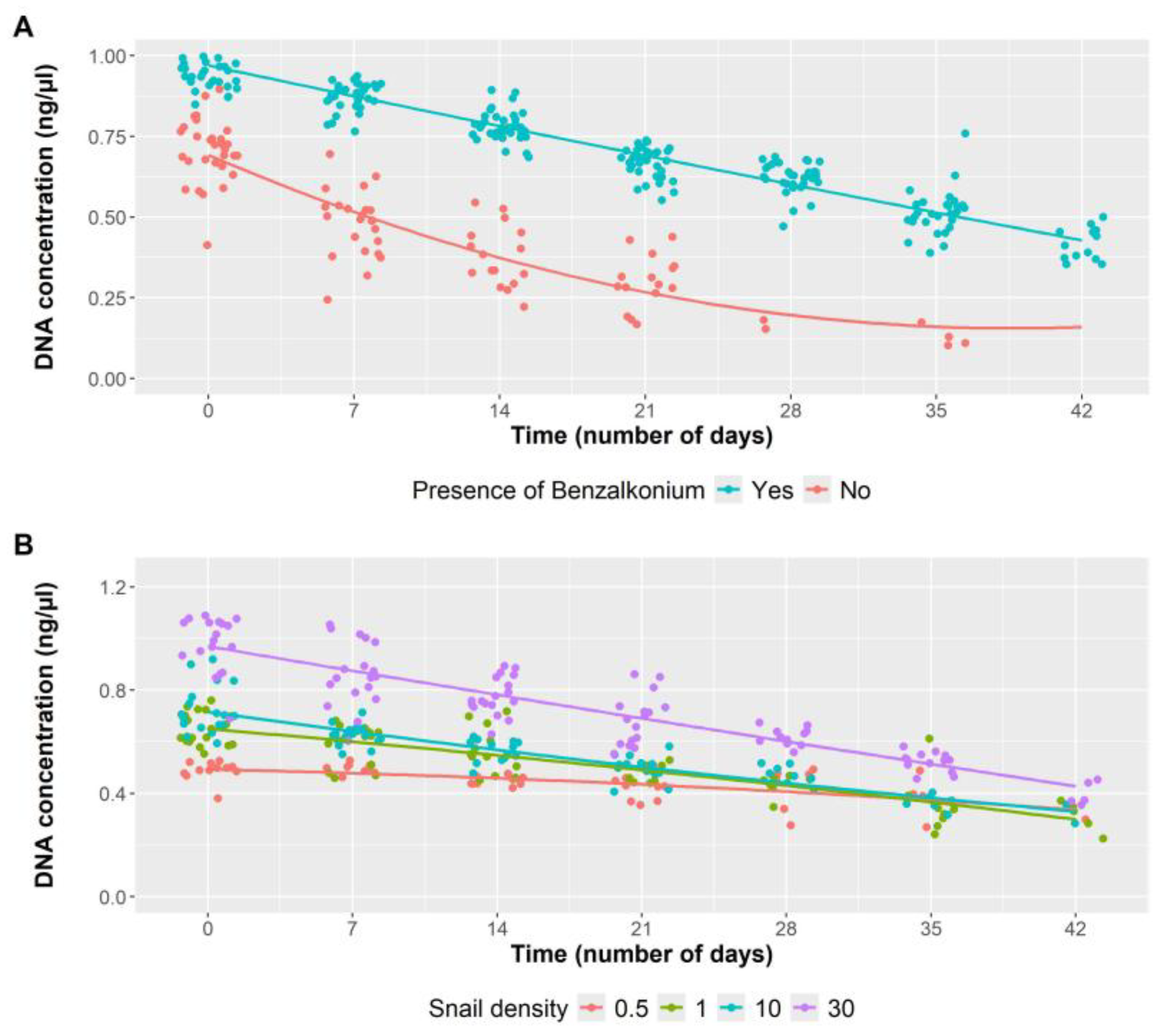
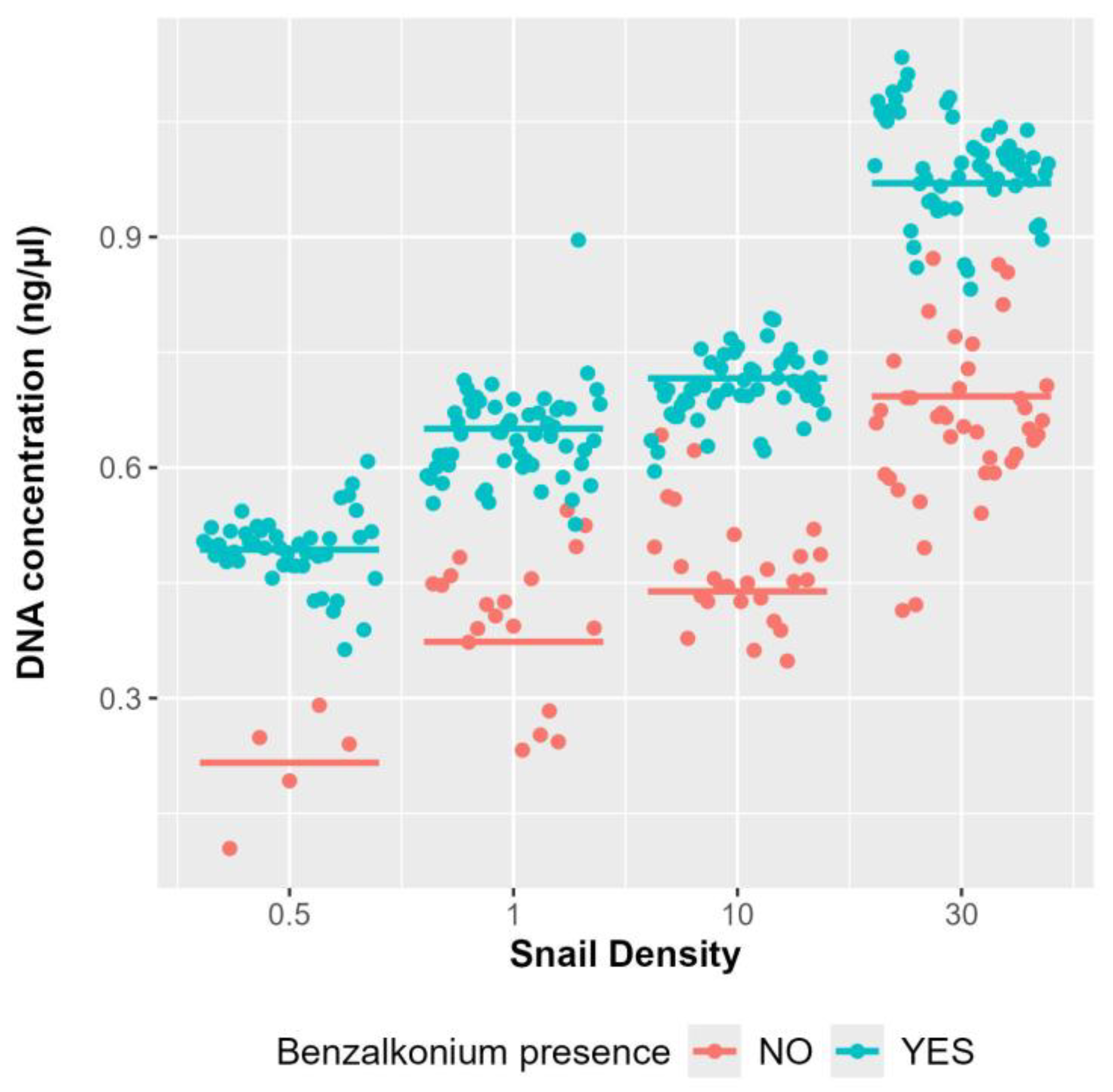
3.1. Influence of Benzalkonium Chloride on B. truncatus eDNA Decay and eDNA Detection Sensitivity
3.2. Influence of Snail Density in eDNA Detection
4. Discussion
4.1. Challenges and Advances in the Application of eDNA for Surveillance of Snail-Borne Parasitic Diseases
4.2. Improved Detection of B. truncatus eDNA Using BAC: Implications for Field Surveillance and Sample Preservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AICc | Corrected Akaike Information Criterion |
| BAC | Benzalkonium chloride |
| eDNA | Environmental DNA |
| LOD | Limit of detection |
| MDA | Mass drug administration |
| NTD | Neglected tropical diseases |
| WHO | World Health Organization |
| qPCR | Quantitative polymerase chain reaction |
| ddPCR | Digital droplet polymerase chain reaction |
| LAMP | Loop-mediated isothermal amplification |
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortí, A.; Adam, S.; Garippa, G.; Boissier, J.; Bargues, M.D.; Mas-Coma, S. Morpho-Anatomical Characterization of the Urogenital Schistosmiasis Vector Bulinus truncatus (Audouin, 1827) (Heterobranchia: Bulinidae) from Southwestern Europe. J. Conchol. 2022, 44, 355–372. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Climate Change Effects on Trematodiases, with Emphasis on Zoonotic Fascioliasis and Schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef] [PubMed]
- van der Deure, T.; Maes, T.; Huyse, T.; Stensgaard, A.-S. Climate Change Could Fuel Urinary Schistosomiasis Transmission in Africa and Europe. Glob. Change Biol. 2024, 30, e17434. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/publications-detail-redirect/9789240010352 (accessed on 20 December 2024).
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. One Health for Fascioliasis Control in Human Endemic Areas. Trends Parasitol. 2023, 39, 650–667. [Google Scholar] [CrossRef] [PubMed]
- Ramalli, L.; Mulero, S.; Noël, H.; Chiappini, J.-D.; Vincent, J.; Barré-Cardi, H.; Malfait, P.; Normand, G.; Busato, F.; Gendrin, V.; et al. Persistence of Schistosomal Transmission Linked to the Cavu River in Southern Corsica since 2013. Eurosurveillance 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronas, J.; Bargues, M.D.; Lozano-Serrano, A.B.; Artigas, P.; Martínez-Ortí, A.; Mas-Coma, S.; Merino-Salas, S.; Abad Vivas-Pérez, J.I. Evidence of Autochthonous Transmission of Urinary Schistosomiasis in Almeria (Southeast Spain): An Outbreak Analysis. Travel Med. Infect. Dis. 2021, 44, 102165. [Google Scholar] [CrossRef] [PubMed]
- Stothard, J.R.; Campbell, S.J.; Osei-Atweneboana, M.Y.; Durant, T.; Stanton, M.C.; Biritwum, N.-K.; Rollinson, D.; Ombede, D.R.E.; Tchuem-Tchuenté, L.-A. Towards Interruption of Schistosomiasis Transmission in Sub-Saharan Africa: Developing an Appropriate Environmental Surveillance Framework to Guide and to Support “end Game” Interventions. Infect. Dis. Poverty 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Selbach, C.; Jorge, F.; Dowle, E.; Bennett, J.; Chai, X.; Doherty, J.-F.; Eriksson, A.; Filion, A.; Hay, E.; Herbison, R.; et al. Parasitological Research in the Molecular Age. Parasitology 2019, 146, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Hellström, M.; Kariuki, H.C.; Olsen, A.; Thomsen, P.F.; Mejer, H.; Willerslev, E.; Mwanje, M.T.; Madsen, H.; Kristensen, T.K.; et al. Environmental DNA for Improved Detection and Environmental Surveillance of Schistosomiasis. Proc. Natl. Acad. Sci. USA 2019, 116, 8931–8940. [Google Scholar] [CrossRef] [PubMed]
- Kamel, B.; Laidemitt, M.R.; Lu, L.; Babbitt, C.; Weinbaum, O.L.; Mkoji, G.M.; Loker, E.S. Detecting and Identifying Schistosoma Infections in Snails and Aquatic Habitats: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009175. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S. Human Fascioliasis Emergence Risks in Developed Countries: From Individual Patients and Small Epidemics to Climate and Global Change Impacts. Enfermedades Infecc. Microbiol. Clín. (Engl. Ed.) 2020, 38, 253–256. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. Int. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, P.F.; Bargues, M.D.; Artigas, P.; Buchon, P.; Angles, R.; Mas-Coma, S. Global Warming Induced Spread of the Highest Human Fascioliasis Hyperendemic Area. Parasit. Vectors 2024, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.; Buonfrate, D.; Gobbi, F.; Angheben, A.; Marchese, V.; Monteiro, G.B.; Bisoffi, Z. The Hidden Epidemic of Schistosomiasis in Recent African Immigrants and Asylum Seekers to Italy. Eur. J. Epidemiol. 2017, 32, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Kincaid-Smith, J.; Rey, O.; Toulza, E.; Berry, A.; Boissier, J. Emerging Schistosomiasis in Europe: A Need to Quantify the Risks. Trends Parasitol. 2017, 33, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, A.F.; Garba Djirmay, A. Schistosomiasis in Europe. Curr. Trop. Med. Rep. 2023, 10, 79–87. [Google Scholar] [CrossRef]
- Boissier, J.; Moné, H.; Mitta, G.; Bargues, M.D.; Molyneux, D.; Mas-Coma, S. Schistosomiasis Reaches Europe. Lancet Infect. Dis. 2015, 15, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Boissier, J.; Grech-Angelini, S.; Webster, B.L.; Allienne, J.-F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barré-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of Urogenital Schistosomiasis in Corsica (France): An Epidemiological Case Study. Lancet Infect. Dis. 2016, 16, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Zimmer, T.; Schunk, M.; Wallrauch, C.; Helfrich, K.; Gültekin, F.; Bretzel, G.; Allienne, J.-F.; Boissier, J. Developing Endemicity of Schistosomiasis, Corsica, France. Emerg. Infect. Dis. 2021, 27, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Moné, H.; Mouahid, G.; Nebel, A.; Tappe, D.; Gabriel, M. A Family Cluster of Schistosomiasis Acquired in Solenzara River, Corsica (France)—Solenzara River Is Clearly a Transmission Site for Schistosomiasis in Corsica. Parasitol. Res. 2022, 121, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- De Elías-Escribano, A.; Artigas, P.; Salas-Coronas, J.; Luzon-Garcia, M.P.; Reguera-Gomez, M.; Cabeza-Barrera, M.I.; Vázquez-Villegas, J.; Boissier, J.; Mas-Coma, S.; Bargues, M.D. Schistosoma mansoni x S. haematobium Hybrids Frequently Infecting Sub-Saharan Migrants in Southeastern Europe: Egg DNA Genotyping Assessed by RD-PCR, Sequencing and Cloning. PLoS Negl. Trop. Dis. 2025, 19, e0012942. [Google Scholar] [CrossRef] [PubMed]
- De Elias-Escribano, A.; Artigas, P.; Salas-Coronas, J.; Luzon-Garcia, M.P.; Reguera-Gomez, M.; Sanchez-Marques, R.; Salvador, F.; Boissier, J.; Mas-Coma, S.; Bargues, M.D. Imported Schistosomiasis in Southwestern Europe: Wide Variation of Pure and Hybrid Genotypes Infecting Sub-Saharan Migrants. Transbound. Emerg. Dis. 2025, 2025, 6614509. [Google Scholar] [CrossRef]
- Veilleux, H.D.; Misutka, M.D.; Glover, C.N. Environmental DNA and Environmental RNA: Current and Prospective Applications for Biological Monitoring. Sci. Total Environ. 2021, 782, 146891. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.K.; Jacobsen, M.W.; Middelboe, A.L.; Preston, C.M.; Marin, R.; Bekkevold, D.; Knudsen, S.W.; Møller, P.R.; Nielsen, E.E. Remote, Autonomous Real-Time Monitoring of Environmental DNA from Commercial Fish. Sci. Rep. 2020, 10, 13272. [Google Scholar] [CrossRef] [PubMed]
- Beng, K.C.; Corlett, R.T. Applications of Environmental DNA (eDNA) in Ecology and Conservation: Opportunities, Challenges and Prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar] [CrossRef]
- Yamanaka, H.; Minamoto, T.; Matsuura, J.; Sakurai, S.; Tsuji, S.; Motozawa, H.; Hongo, M.; Sogo, Y.; Kakimi, N.; Teramura, I.; et al. A Simple Method for Preserving Environmental DNA in Water Samples at Ambient Temperature by Addition of Cationic Surfactant. Limnology 2017, 18, 233–241. [Google Scholar] [CrossRef]
- Sato, M.O.; Rafalimanantsoa, A.; Ramarokoto, C.; Rahetilahy, A.M.; Ravoniarimbinina, P.; Kawai, S.; Minamoto, T.; Sato, M.; Kirinoki, M.; Rasolofo, V.; et al. Usefulness of Environmental DNA for Detecting Schistosoma mansoni Occurrence Sites in Madagascar. Int. J. Infect. Dis. 2018, 76, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Fornillos, R.J.C.; Sato, M.O.; Tabios, I.K.B.; Sato, M.; Leonardo, L.R.; Chigusa, Y.; Minamoto, T.; Kikuchi, M.; Legaspi, E.R.; Fontanilla, I.K.C. Detection of Schistosoma japonicum and Oncomelania hupensis Quadrasi Environmental DNA and Its Potential Utility to Schistosomiasis Japonica Surveillance in the Philippines. PLoS ONE 2019, 14, e0224617. [Google Scholar] [CrossRef] [PubMed]
- Mulero, S.; Boissier, J.; Allienne, J.-F.; Quilichini, Y.; Foata, J.; Pointier, J.-P.; Rey, O. Environmental DNA for Detecting Bulinus truncatus: A New Environmental Surveillance Tool for Schistosomiasis Emergence Risk Assessment. Environ. DNA 2020, 2, 161–174. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; English, C.A.; Burns, M.J.; Keer, J.T. Routes to Improving the Reliability of Low Level DNA Analysis Using Real-Time PCR. BMC Biotechnol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Omland, K.S. Model Selection in Ecology and Evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Alzaylaee, H.; Collins, R.A.; Rinaldi, G.; Shechonge, A.; Ngatunga, B.; Morgan, E.R.; Genner, M.J. Schistosoma Species Detection by Environmental DNA Assays in African Freshwaters. PLoS Negl. Trop. Dis. 2020, 14, e0008129. [Google Scholar] [CrossRef]
- Jones, R.A.; Brophy, P.M.; Davis, C.N.; Davies, T.E.; Emberson, H.; Rees Stevens, P.; Williams, H.W. Detection of Galba truncatula, Fasciola hepatica and Calicophoron daubneyi Environmental DNA within Water Sources on Pasture Land, a Future Tool for Fluke Control? Parasit. Vectors 2018, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Rudko, S.P.; Turnbull, A.; Reimink, R.L.; Froelich, K.; Hanington, P.C. Species-Specific qPCR Assays Allow for High-Resolution Population Assessment of Four Species Avian Schistosome That Cause Swimmer’s Itch in Recreational Lakes. Int. J. Parasitol. Parasites Wildl. 2019, 9, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Sato, M.; Sato, M.O.; Ikeda, S.; Yoonuan, T.; Sanguankiat, S.; Pongvongsa, T.; Moji, K.; Minamoto, T. Application of Environmental DNA Analysis for the Detection of Opisthorchis viverrini DNA in Water Samples. Acta Trop. 2017, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Lynggaard, C.; Mukaratirwa, S.; Vennervald, B.J.; Stensgaard, A.S. Environmental DNA in Human and Veterinary Parasitology—Current Applications and Future Prospects for Monitoring and Control. Food Waterborne Parasitol. 2022, 29, e00183. [Google Scholar] [CrossRef] [PubMed]
- Krol, L.; Van der Hoorn, B.; Gorsich, E.E.; Trimbos, K.; van Bodegom, P.M.; Schrama, M. How Does eDNA Compare to Traditional Trapping? Detecting Mosquito Communities in South-African Freshwater Ponds. Front. Ecol. Evol. 2019, 7, 260. [Google Scholar] [CrossRef]
- Schneider, J.; Valentini, A.; Dejean, T.; Montarsi, F.; Taberlet, P.; Glaizot, O.; Fumagalli, L. Detection of Invasive Mosquito Vectors Using Environmental DNA (eDNA) from Water Samples. PLoS ONE 2016, 11, e0162493. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.R.; Buxton, A.S.; Rees, H.C.; Bruce, K.; Brys, R.; Halfmaerten, D.; Read, D.S.; Watson, H.V.; Sayer, C.D.; Jones, E.P.; et al. Prospects and Challenges of Environmental DNA (eDNA) Monitoring in Freshwater Ponds. Hydrobiologia 2019, 826, 25–41. [Google Scholar] [CrossRef]
- Power, H.; Takahashi, M.; Jarman, S.; Berry, O. What Is Environmental DNA? Environ. DNA 2023, 5, 1743–1758. [Google Scholar] [CrossRef]
- McManus, D.P.; Gordon, C.; Weerakoon, K.G.A.D. Testing of Water Samples for Environmental DNA as a Surveillance Tool to Assess the Risk of Schistosome Infection in a Locality. Int. J. Infect. Dis. 2018, 76, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Calata, F.I.C.; Caranguian, C.Z.; Mendoza, J.E.M.; Fornillos, R.J.C.; Tabios, I.K.B.; Fontanilla, I.K.C.; Leonardo, L.R.; Sunico, L.S.; Kawai, S.; Chigusa, Y. Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania Hupensis Quadrasi. Pathogens 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Blin, M.; Senghor, B.; Boissier, J.; Mulero, S.; Rey, O.; Portela, J. Development of Environmental Loop-Mediated Isothermal Amplification (eLAMP) Diagnostic Tool for Bulinus truncatus Field Detection. Parasit. Vectors 2023, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Schols, R.; Carolus, H.; Hammoud, C.; Mulero, S.; Mudavanhu, A.; Huyse, T. A Rapid Diagnostic Multiplex PCR Approach for Xenomonitoring of Human and Animal Schistosomiasis in a “One Health” Context. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Alzaylaee, H.; Collins, R.A.; Shechonge, A.; Ngatunga, B.P.; Morgan, E.R.; Genner, M.J. Environmental DNA-Based Xenomonitoring for Determining Schistosoma Presence in Tropical Freshwaters. Parasit. Vectors 2020, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Davis, C.N.; Jones, D.L.; Tyson, F.; Davies, E.; Cutress, D.; Brophy, P.M.; Rose, M.T.; Williams, M.; Williams, H.W. Temporal Dynamics of Trematode Intermediate Snail Host Environmental DNA in Small Water Body Habitats. Parasitology 2021, 148, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.N.; Tyson, F.; Cutress, D.; Davies, E.; Jones, D.L.; Brophy, P.M.; Prescott, A.; Rose, M.T.; Williams, M.; Williams, H.W.; et al. Rapid Detection of Galba truncatula in Water Sources on Pasture-Land Using Loop-Mediated Isothermal Amplification for Control of Trematode Infections. Parasit. Vectors 2020, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Rathinasamy, V.; Hosking, C.; Tran, L.; Kelley, J.; Williamson, G.; Swan, J.; Elliott, T.; Rawlin, G.; Beddoe, T.; Spithill, T.W. Development of a Multiplex Quantitative PCR Assay for Detection and Quantification of DNA from Fasciola hepatica and the Intermediate Snail Host, Austropeplea tomentosa, in Water Samples. Vet. Parasitol. 2018, 259, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rathinasamy, V.; Tran, L.; Swan, J.; Kelley, J.; Hosking, C.; Williamson, G.; Knowles, M.; Elliott, T.; Rawlin, G.; Spithill, T.W.; et al. Towards Understanding the Liver Fluke Transmission Dynamics on Farms: Detection of Liver Fluke Transmitting Snail and Liver Fluke-Specific Environmental DNA in Water Samples from an Irrigated Dairy Farm in Southeast Australia. Vet. Parasitol. 2021, 291, 109373. [Google Scholar] [CrossRef] [PubMed]
- Mulero, S.; Toulza, E.; Loisier, A.; Zimmerman, M.; Allienne, J.-F.; Foata, J.; Quilichini, Y.; Pointier, J.-P.; Rey, O.; Boissier, J. Malacological Survey in a Bottle of Water: A Comparative Study between Manual Sampling and Environmental DNA Metabarcoding Approaches. Glob. Ecol. Conserv. 2021, 25, e01428. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species Detection Using Environmental DNA from Water Samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Eichmiller, J.J.; Best, S.E.; Sorensen, P.W. Effects of Temperature and Trophic State on Degradation of Environmental DNA in Lake Water. Environ. Sci. Technol. 2016, 50, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Taguchi, J.; Yamagishi, S.; Doi, H.; Ogata, S.; Yamanaka, H.; Minamoto, T. Suppression of Environmental DNA Degradation in Water Samples Associated with Different Storage Temperature and Period Using Benzalkonium Chloride. Limnol. Ocean. Methods 2020, 18, 437–445. [Google Scholar] [CrossRef]
- Martínez-Ortí, A.; Vilavella, D.; Bargues, M.D.; Mas–Coma, S. Risk Map of Transmission of Urogenital Schistosomiasis by Bulinus truncatus (Audouin, 1827) (Mollusca Gastropoda, Bulinidae) in Spain and Portugal. Anim. Biodiv. Conserv. 2019, 42, 257–266. [Google Scholar] [CrossRef]
- Guido, G.; Frallonardo, L.; Cotugno, S.; De Vita, E.; Patti, G.; De Santis, L.; Segala, F.V.; Nicastri, E.; Gobbi, F.; Morea, A.; et al. Prevalence of Neglected Tropical Diseases among Migrants Living in Europe: A Systematic Review and Meta-Analysis. Travel Med. Infect. Dis. 2025, 64, 102823. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Bocanegra, C.; Treviño, B.; Sulleiro, E.; Sánchez-Montalvá, A.; Serre-Delcor, N.; Bosch-Nicolau, P.; Aznar, M.L.; Goterris, L.; Pou, D.; et al. Imported Schistosomiasis in Travelers: Experience from a Referral Tropical Medicine Unit in Barcelona, Spain. Travel Med. Infect. Dis. 2024, 60, 102742. [Google Scholar] [CrossRef] [PubMed]
- Depaquit, J.; Akhoundi, M.; Haouchine, D.; Mantelet, S.; Izri, A. No Limit in Interspecific Hybridization in Schistosomes: Observation from a Case Report. Parasite 2019, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Le Govic, Y.; Kincaid-Smith, J.; Allienne, J.-F.; Rey, O.; de Gentile, L.; Boissier, J. Schistosoma haematobium–Schistosoma mansoni Hybrid Parasite in Migrant Boy, France, 2017. Emerg. Infect. Dis. 2019, 25, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronas, J.; Cabezas-Fernández, M.T.; Lozano-Serrano, A.B.; Soriano-Pérez, M.J.; Vázquez-Villegas, J.; Cuenca-Gómez, J.Á. Newly Arrived African Migrants to Spain: Epidemiology and Burden of Disease. Am. J. Trop. Med. Hyg. 2018, 98, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronas, J.; Vázquez-Villegas, J.; Lozano-Serrano, A.B.; Soriano-Pérez, M.J.; Cabeza-Barrera, I.; Cabezas-Fernández, M.T.; Villarejo-Ordóñez, A.; Sánchez-Sánchez, J.C.; Abad Vivas-Pérez, J.I.; Vázquez-Blanc, S.; et al. Severe Complications of Imported Schistosomiasis, Spain: A Retrospective Observational Study. Travel. Med. Infect. Dis. 2020, 35, 101508. [Google Scholar] [CrossRef] [PubMed]
- Soentjens, P.; Cnops, L.; Huyse, T.; Yansouni, C.; De Vos, D.; Bottieau, E.; Clerinx, J.; Van Esbroeck, M. Diagnosis and Clinical Management of Schistosoma haematobium—Schistosoma bovis Hybrid Infection in a Cluster of Travelers Returning from Mali. Clin. Infect. Dis. 2016, 63, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Cnops, L.; Huyse, T.; Maniewski, U.; Soentjens, P.; Bottieau, E.; Van Esbroeck, M.; Clerinx, J. Acute Schistosomiasis with a Schistosoma mattheei × Schistosoma haematobium Hybrid Species in a Cluster of 34 Travelers Infected in South Africa. Clin. Infect. Dis. 2021, 72, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, P.F.; Artigas, P.; Lorenzo-Morales, J.; Bargues, M.D.; Mas-Coma, S. Ecological Niche Modelling Approaches: Challenges and Applications in Vector-Borne Diseases. Trop. Med. Infect. Dis. 2023, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, P.F.; Bargues, M.D.; Artigas, P.; Buchon, P.; Angles, R.; Mas-Coma, S. Heterogeneous Zonal Impacts of Climate Change on a Wide Hyperendemic Area of Human and Animal Fascioliasis Assessed within a One Health Action for Prevention and Control. PLoS Negl. Trop. Dis. 2025, 19, e0012820. [Google Scholar] [CrossRef] [PubMed]
| Final Model: eDNA ng/µl 0.25 ~ Snail Density × Time2 + BAC × Time2 + (1|Aquaria ID) + (1|Sample/Section) | |||
|---|---|---|---|
| Predictors | Estimates | CI | p-Value |
| Intercept | 0.09 | 0.04–0.14 | 0.001 |
| BAC [YES] | 0.36 | 0.33–0.39 | <0.001 |
| Time [1st degree] | −1.13 | −2.10–−0.16 | 0.022 |
| Time [2nd degree] | 0.78 | −0.13–1.69 | 0.094 |
| SnailDens [1] | 0.08 | 0.02–0.14 | 0.006 |
| SnailDens [10] | 0.11 | 0.05–0.17 | <0.001 |
| SnailDens [30] | 0.32 | 0.26–0.37 | <0.001 |
| BAC [YES] × Time [1st degree] | 0.37 | −0.24–0.98 | 0.232 |
| BAC [YES] × Time [2nd degree] | −0.90 | −1.42–−0.38 | 0.001 |
| SnailDens [1] × Time [1st degree] | −1.03 | −2.03–−0.04 | 0.042 |
| SnailDens [1] × Time [2nd degree] | 0.03 | −0.94–0.99 | 0.958 |
| SnailDens [10] × Time [1st degree] | −1.32 | −2.33–−0.30 | 0.011 |
| SnailDens [10] × Time [2nd degree] | 0.25 | −0.76–1.25 | 0.627 |
| SnailDens [30] × Time [1st degree] | −2.10 | −3.10–−1.10 | <0.001 |
| SnailDens [30] × Time [2nd degree] | 0.17 | −0.80–1.13 | 0.730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Marqués, R.; Cuervo, P.F.; De Elías-Escribano, A.; Martínez-Ortí, A.; Artigas, P.; Fantozzi, M.C.; Mas-Coma, S.; Bargues, M.D. Benzalkonium Chloride Significantly Improves Environmental DNA Detection from Schistosomiasis Snail Vectors in Freshwater Samples. Trop. Med. Infect. Dis. 2025, 10, 201. https://doi.org/10.3390/tropicalmed10080201
Sánchez-Marqués R, Cuervo PF, De Elías-Escribano A, Martínez-Ortí A, Artigas P, Fantozzi MC, Mas-Coma S, Bargues MD. Benzalkonium Chloride Significantly Improves Environmental DNA Detection from Schistosomiasis Snail Vectors in Freshwater Samples. Tropical Medicine and Infectious Disease. 2025; 10(8):201. https://doi.org/10.3390/tropicalmed10080201
Chicago/Turabian StyleSánchez-Marqués, Raquel, Pablo Fernando Cuervo, Alejandra De Elías-Escribano, Alberto Martínez-Ortí, Patricio Artigas, Maria Cecilia Fantozzi, Santiago Mas-Coma, and Maria Dolores Bargues. 2025. "Benzalkonium Chloride Significantly Improves Environmental DNA Detection from Schistosomiasis Snail Vectors in Freshwater Samples" Tropical Medicine and Infectious Disease 10, no. 8: 201. https://doi.org/10.3390/tropicalmed10080201
APA StyleSánchez-Marqués, R., Cuervo, P. F., De Elías-Escribano, A., Martínez-Ortí, A., Artigas, P., Fantozzi, M. C., Mas-Coma, S., & Bargues, M. D. (2025). Benzalkonium Chloride Significantly Improves Environmental DNA Detection from Schistosomiasis Snail Vectors in Freshwater Samples. Tropical Medicine and Infectious Disease, 10(8), 201. https://doi.org/10.3390/tropicalmed10080201










