Similar Microsatellite Allelic Distribution Between Anopheles darlingi Population Collected by Human Landing Catch or Mosquito Magnet Traps in French Guiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Mosquito Collections
2.2. Extraction of DNA for Genotyping
2.3. Microsatellite Genotyping
2.4. Statistical Analyses
3. Results
3.1. Anopheles darlingi Field Collection
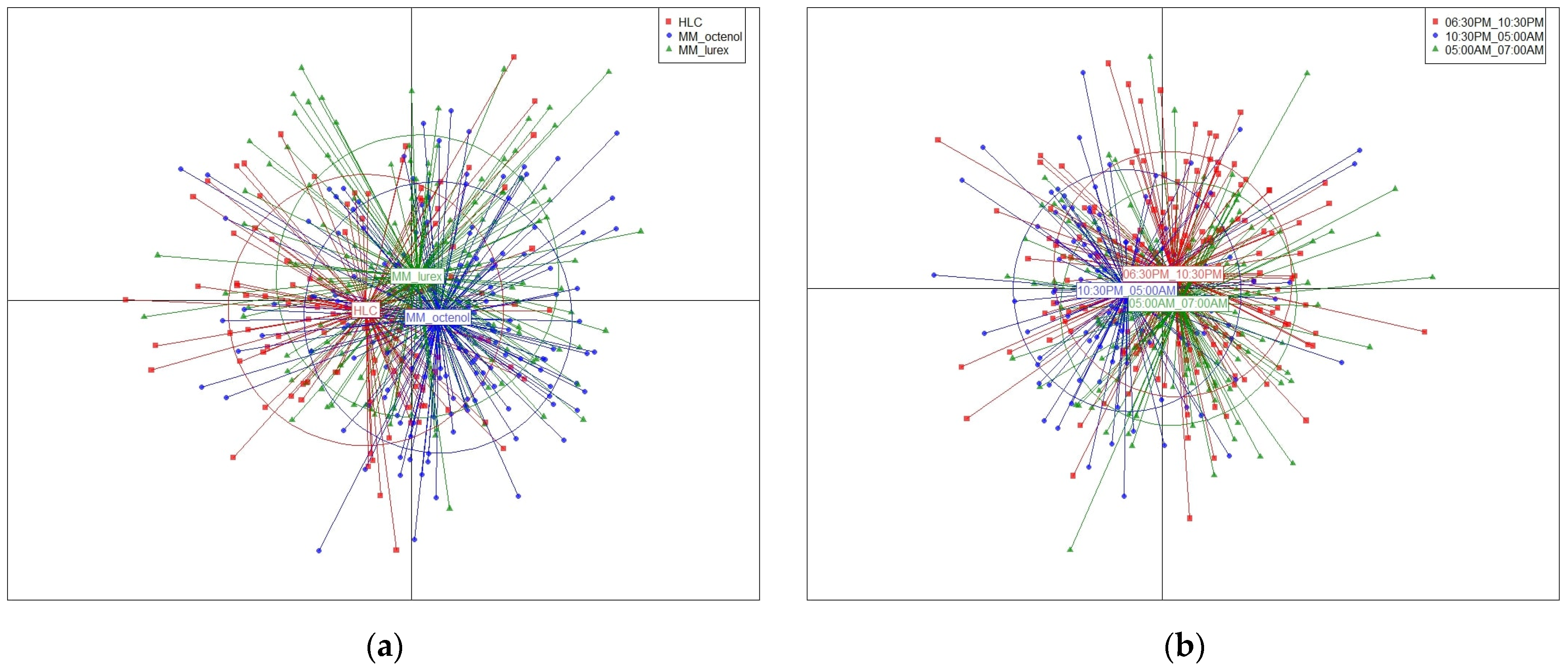
3.2. Anopheles darlingi Populations Collected with Different Methods
3.3. Anopheles darlingi Populations Collected at Different Time Slots
4. Discussion
4.1. Collection Methods and Sampling Bias of Anopheles darlingi
4.2. Anopheles darlingi Populations According to the Time Slots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Center for Disease Control |
| DAPC | Discriminant analysis of principal component |
| HBR | Human biting rate |
| HLC | Human landing catch |
| HWE | Hardy–Weinberg equilibrium |
| MM | Mosquito magnet |
References
- Loaiza, J.R.; Bermingham, E.; Sanjur, O.I.; Scott, M.E.; Bickersmith, S.A.; Conn, J.E. Review of Genetic Diversity in Malaria Vectors (Culicidae: Anophelinae). Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vezenegho, S.; Carinci, R.; Issaly, J.; Nguyen, C.; Gaborit, P.; Ferraro, L.; Lacour, G.; Mosnier, E.; Pommier de Santi, V.; Epelboin, Y.; et al. Variation in Pyrethroid Resistance Phenotypes in Anopheles darlingi in an Area with Residual Malaria Transmission: Warning of Suspected Resistance in French Guiana. Am. J. Trop. Med. Hyg. 2023, 108, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Vezenegho, S.B.; Adde, A.; Gaborit, P.; Carinci, R.; Issaly, J.; Pommier de Santi, V.; Dusfour, I.; Briolant, S.; Girod, R. Mosquito Magnet® Liberty plus Trap Baited with Octenol Confirmed Best Candidate for Anopheles Surveillance and Proved Promising in Predicting Risk of Malaria Transmission in French Guiana. Malar. J. 2014, 13, 384. [Google Scholar] [CrossRef]
- Dusfour, I.; Carinci, R.; Issaly, J.; Gaborit, P.; Girod, R. A Survey of Adult Anophelines in French Guiana: Enhanced Descriptions of Species Distribution and Biting Responses. J. Vector Ecol. J. Soc. Vector Ecol. 2013, 38, 203–209. [Google Scholar] [CrossRef]
- Reis, I.C.; Codeço, C.T.; Câmara, D.C.P.; Carvajal, J.J.; Pereira, G.R.; Keppeler, E.C.; Honório, N.A. Diversity of Anopheles spp. (Diptera: Culicidae) in an Amazonian Urban Area. Neotrop. Entomol. 2018, 47, 412–417. [Google Scholar] [CrossRef]
- Fouque, F.; Gaborit, P.; Carinci, R.; Issaly, J.; Girod, R. Annual Variations in the Number of Malaria Cases Related to Two Different Patterns of Anopheles darlingi Transmission Potential in the Maroni Area of French Guiana. Malar. J. 2010, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Adde, A.; Dusfour, I.; Vezenegho, S.B.; Carinci, R.; Issaly, J.; Gaborit, P.; Nguyen, C.; Ardillon, V.; Girod, R.; Briolant, S. Spatial and Seasonal Dynamics of Anopheles Mosquitoes in Saint-Georges de l’Oyapock, French Guiana: Influence of Environmental Factors. J. Med. Entomol. 2017, 54, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, Y.; Chaney, S.C.; Guidez, A.; Habchi-Hanriot, N.; Talaga, S.; Wang, L.; Dusfour, I. Successes and Failures of Sixty Years of Vector Control in French Guiana: What Is the next Step? Mem. Inst. Oswaldo Cruz 2018, 113, e170398. [Google Scholar] [CrossRef]
- Conn, J.E.; Ribolla, P.E.M. Chapter 5—Ecology of Anopheles darlingi, the Primary Malaria Vector in the Americas and Current Nongenetic Methods of Vector Control. In Genet Control Malar Dengue; Adelman, Z.N., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 81–102. Available online: http://www.Sciencedirect.Com/Science/Article/Pii/B9780128002469000053 (accessed on 5 July 2023).
- Moutinho, P.R.; Gil, L.H.S.; Cruz, R.B.; Ribolla, P.E.M. Population Dynamics, Structure and Behavior of Anopheles darlingi in a Rural Settlement in the Amazon Rainforest of Acre, Brazil. Malar. J. 2011, 10, 174. [Google Scholar] [CrossRef]
- Campos, M.; Conn, J.E.; Alonso, D.P.; Vinetz, J.M.; Emerson, K.J.; Ribolla, P.E.M. Microgeographical Structure in the Major Neotropical Malaria Vector Anopheles darlingi Using Microsatellites and SNP Markers. Parasites Vectors 2017, 10, 76. [Google Scholar] [CrossRef]
- Angêlla, A.F.; Salgueiro, P.; Gil, L.H.S.; Vicente, J.L.; Pinto, J.; Ribolla, P.E.M. Seasonal Genetic Partitioning in the Neotropical Malaria Vector. Anopheles darlingi. Malar. J. 2014, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, S.P.; Costantini, C.; Pennetier, C.; Diabaté, A.; Gibson, G.; Dabiré, R.K. Differences in Timing of Mating Swarms in Sympatric Populations of Anopheles coluzzii and Anopheles gambiae s.s. (Formerly An. gambiae M and S Molecular Forms) in Burkina Faso, West Africa. Parasites Vectors 2013, 6, 275. [Google Scholar] [CrossRef] [PubMed]
- Guelbeogo, W.M.; Sagnon, N.; Liu, F.; Besansky, N.J.; Costantini, C. Behavioural Divergence of Sympatric Anopheles funestus Populations in Burkina Faso. Malar. J. 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Jiménez, J.I.; Gimnig, J.; Rodríguez, M.H.; Washino, R.K. Genetic Differences among Anopheles vestitipennis Subpopulations Collected Using Different Methods in Chiapas State, Southern México. J. Am. Mosq. Control Assoc. 1996, 12, 396–401. [Google Scholar]
- Wondji, C.; Simard, F.; Fontenille, D. Evidence for Genetic Differentiation between the Molecular Forms M and S within the Forest Chromosomal Form of Anopheles gambiae in an Area of Sympatry. Insect Mol. Biol. 2002, 11, 11–19. [Google Scholar] [CrossRef]
- Harischandra, I.N.; Dassanayake, R.S.; De Silva, B.G.D.N.K. Three Sympatric Clusters of the Malaria Vector Anopheles culicifacies E (Diptera: Culicidae) Detected in Sri Lanka. Parasites Vectors 2016, 9, 3. [Google Scholar] [CrossRef]
- Cornel, A.J.; Brisco, K.K.; Tadei, W.P.; Secundino, N.F.; Rafael, M.S.; Galardo, A.K.; Medeiros, J.F.; Pessoa, F.A.; Ríos-Velásquez, C.M.; Lee, Y.; et al. Anopheles darlingi Polytene Chromosomes: Revised Maps Including Newly Described Inversions and Evidence for Population Structure in Manaus. Mem. Inst. Oswaldo Cruz 2016, 111, 335–346. [Google Scholar] [CrossRef]
- WHO. Sampling Malaria Vector. In Malar Entomol Vector Control—Guide Particip—Train Module Malar Control; World Health Organization: Geneva, Switzerland, 2013; p. 31. Available online: https://iris.who.int/bitstream/handle/10665/85890/9789241505819_eng.pdf (accessed on 5 February 2023).
- Lima, J.B.P.; Rosa-Freitas, M.G.; Rodovalho, C.M.; Santos, F.; Lourenço-de-Oliveira, R. Is There an Efficient Trap or Collection Method for Sampling Anopheles darlingi and Other Malaria Vectors That Can Describe the Essential Parameters Affecting Transmission Dynamics as Effectively as Human Landing Catches?—A Review. Mem. Inst. Oswaldo Cruz 2014, 109, 685–705. [Google Scholar] [CrossRef]
- Briët, O.J.T.; Huho, B.J.; Gimnig, J.E.; Bayoh, N.; Seyoum, A.; Sikaala, C.H.; Govella, N.; Diallo, D.A.; Abdullah, S.; Smith, T.A.; et al. Applications and Limitations of Centers for Disease Control and Prevention Miniature Light Traps for Measuring Biting Densities of African Malaria Vector Populations: A Pooled-Analysis of 13 Comparisons with Human Landing Catches. Malar. J. 2015, 14, 247. [Google Scholar] [CrossRef]
- Gama, R.A.; da Silva, I.M.; Geier, M.; Eiras, A.E. Development of the BG-Malaria Trap as an Alternative to Human-Landing Catches for the Capture of Anopheles darlingi. Mem. Inst. Oswaldo Cruz 2013, 108, 763–771. [Google Scholar] [CrossRef]
- Service, M.W. A Critical Review of Procedures for Sampling Populations of Adult Mosquitoes. Bull. Entomol. Res. 1977, 67, 343–382. [Google Scholar] [CrossRef]
- Dusfour, I.; Carinci, R.; Gaborit, P.; Issaly, J.; Girod, R. Evaluation of Four Methods for Collecting Malaria Vectors in French Guiana. J. Econ. Entomol. 2010, 103, 973–976. [Google Scholar] [CrossRef]
- Bidlingmayer, W.L. A Comparison of Trapping Methods for Adult Mosquitoes: Species Response and Environmental Influence. J. Med. Entomol. 1967, 4, 200–220. [Google Scholar] [CrossRef]
- St. Laurent, B.; Sukowati, S.; Burton, T.A.; Bretz, D.; Zio, M.; Firman, S.; Sumardi; Sudibyo, H.; Safitri, A.; Suwito; et al. Comparative Evaluation of Anopheline Sampling Methods in Three Localities in Indonesia. Malar. J. 2018, 17, 13. [Google Scholar] [CrossRef]
- Campos, M.; Alonso, D.P.; Conn, J.E.; Vinetz, J.M.; Emerson, K.J.; Ribolla, P.E.M. Genetic Diversity of Nyssorhynchus (Anopheles) darlingi Related to Biting Behavior in Western Amazon. Parasites Vectors 2019, 12, 242. [Google Scholar] [CrossRef]
- Vezenegho, S.B.; Adde, A.; Pommier de Santi, V.; Issaly, J.; Carinci, R.; Gaborit, P.; Dusfour, I.; Girod, R.; Briolant, S. High Malaria Transmission in a Forested Malaria Focus in French Guiana: How Can Exophagic Anopheles darlingi Thwart Vector Control and Prevention Measures? Mem. Inst. Oswaldo Cruz 2016, 111, 561–569. [Google Scholar] [CrossRef]
- Mosnier, E.; Dusfour, I.; Lacour, G.; Saldanha, R.; Guidez, A.; Gomes, M.S.; Sanna, A.; Epelboin, Y.; Restrepo, J.; Davy, D.; et al. Resurgence Risk for Malaria, and the Characterization of a Recent Outbreak in an Amazonian Border Area between French Guiana and Brazil. BMC Infect. Dis. 2020, 20, 373. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A Global Map of Dominant Malaria Vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Musset, L.; Pelleau, S.; Girod, R.; Ardillon, V.; Carvalho, L.; Dusfour, I.; Gomes, M.S.M.; Djossou, F.; Legrand, E. Malaria on the Guiana Shield: A Review of the Situation in French Guiana. Mem. Inst. Oswaldo Cruz 2014, 109, 525–533. [Google Scholar] [CrossRef]
- Floch, H.; Abonnenc, E. Anophèles de La Guyane Française. Arch. Inst. Pasteur Guyane Territ. Inini. 1951, 236, 1–92. [Google Scholar]
- Faran, M.E.; Linthicum, K.J. A Handbook of the Amazonian Species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 1981, 13, 1–81. [Google Scholar]
- Conn, J.E.; Bollback, J.P.; Onyabe, D.Y.; Robinson, T.N.; Wilkerson, R.C.; Póvoa, M.M. Isolation of Polymorphic Microsatellite Markers from the Malaria Vector Anopheles darlingi. Mol. Ecol. Notes 2001, 1, 223–225. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. Micro-Checker: Software for Identifying and Correcting Genotyping Errors in Microsatellite Data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Nei, M. Estimation of Average Heterozygosity and Genetic Distance from a Small Number of Individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, N.; Bonhomme, F. GENETIX 4.03, Logiciel Sous Windows TM Pour La Génétique Des Populations, Laboratoire Génome, Populations, Interactions CNRS UMR 5000, Université de Montpellier II, Montpellier (France). 2004. Available online: https://kimura.univ-montp2.fr/genetix/ (accessed on 6 August 2019).
- Wright, S. The Interpretation of Population Structure by F-Statistics with Special Regard to Systems of Mating. Evolution 1965, 19, 395. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices, Version 2.9.3. 2001. Available online: http://www2.Unil.Ch/Popgen/Softwares/Fstat.Htm (accessed on 5 May 2019).
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-Project.Org/ (accessed on 5 June 2023).
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New Tools for the Analysis of Genome-Wide SNP Data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Scarpassa, V.M.; Conn, J.E. Population Genetic Structure of the Major Malaria Vector Anopheles darlingi (Diptera: Culicidae) from the Brazilian Amazon, Using Microsatellite Markers. Mem. Inst. Oswaldo Cruz 2007, 102, 319–327. [Google Scholar] [CrossRef]
- Mirabello, L.; Vineis, J.H.; Yanoviak, S.P.; Scarpassa, V.M.; Póvoa, M.M.; Padilla, N.; Achee, N.L.; Conn, J.E. Microsatellite Data Suggest Significant Population Structure and Differentiation Within the Malaria Vector Anopheles darlingi in Central and South America. BMC Ecol. 2008, 8, 3. [Google Scholar] [CrossRef]
- Lainhart, W.; Bickersmith, S.A.; Nadler, K.J.; Moreno, M.; Saavedra, M.P.; Chu, V.M.; Ribolla, P.E.; Vinetz, J.M.; Conn, J.E. Evidence for Temporal Population Replacement and the Signature of Ecological Adaptation in a Major Neotropical Malaria Vector in Amazonian Peru. Malar. J. 2015, 14, 375. [Google Scholar] [CrossRef] [PubMed]
- Rosero, C.Y.; Jaramillo, G.I.; Gonzalez, R.; Cardenas, H. Genetic Differentiation of Colombian Populations of Anopheles darlingi Root (Diptera: Culicidae). Neotrop. Entomol. 2017, 46, 487–498. [Google Scholar] [CrossRef] [PubMed]
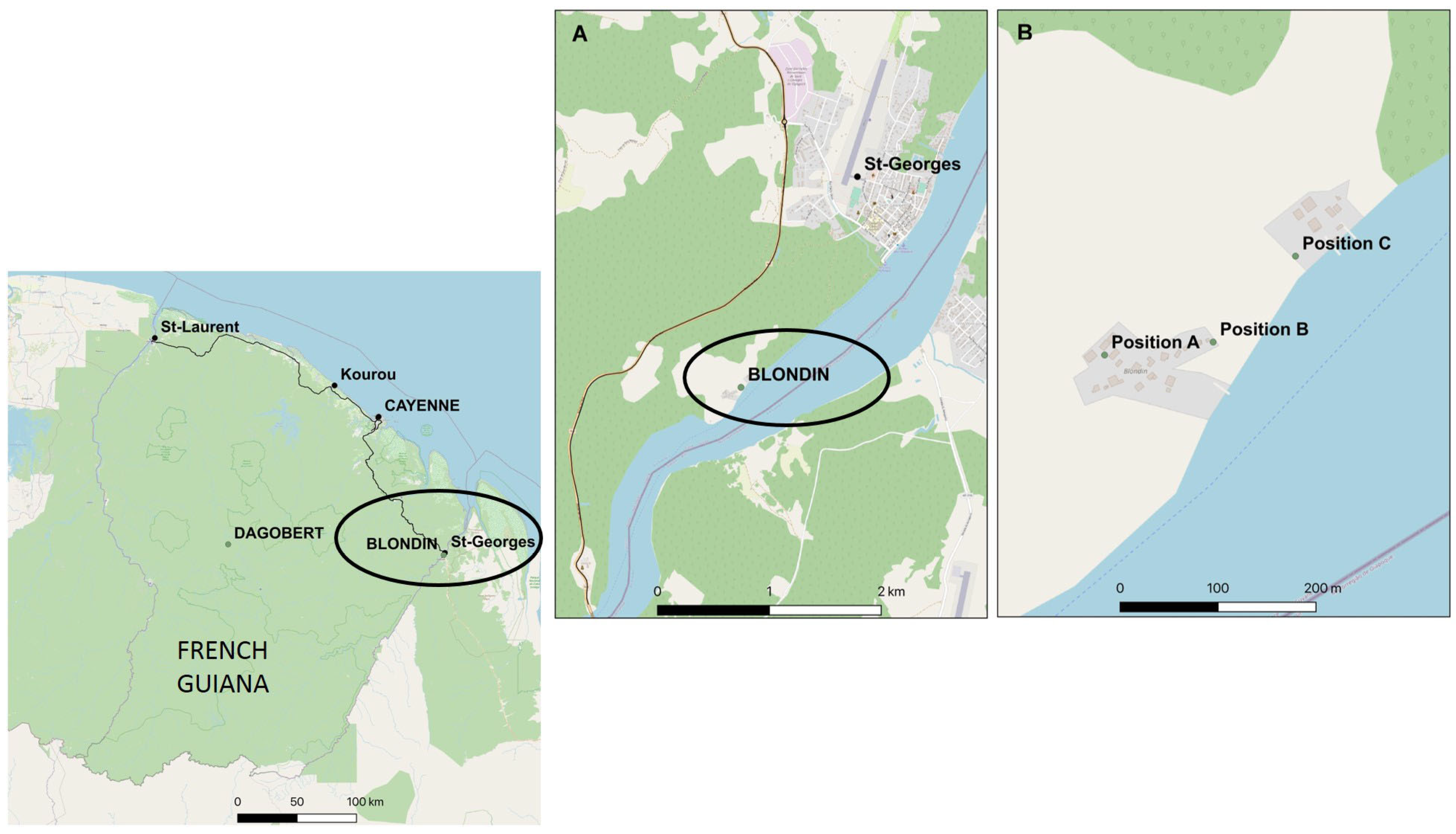

| Capture Session Date | Trap Position | |||
|---|---|---|---|---|
| A | B | C | ||
| 16 and 17 September 2013 | MM octenol | HLC | ||
| 17 and 18 September 2013 | MM lurex | HLC | ||
| 18 and 19 September 2013 | MM octenol | MM lurex | ||
| 19 and 20 September 2013 | HLC | |||
| 20 and 21 September 2013 | MM lurex | MM octenol | ||
| 14 and 15 October 2013 | MM lurex | HLC | ||
| 15 and 16 October 2013 | MM octenol | HLC | MM lurex | |
| 16 and 17 October 2013 | HLC | MM lurex | MM octenol | |
| 11 and 12 November 2013 | MM octenol | HLC | ||
| 12 and 13 November 2013 | HLC | |||
| 13 and 14 November 2013 | MM octenol | |||
| 14 and 15 November 2013 | HLC | MM octenol | ||
| Collection Methods | Trap Position A | Trap Position B | Trap Position C | Total All Trap Positions |
|---|---|---|---|---|
| HLC | 40 | 37 | 38 | 115 (26.7%) |
| MM Octenol | 41 | 23 | 97 | 161 (37.3%) |
| MM Lurex | 22 | 55 | 78 | 155 (36%) |
| Total all collection methods | 103 (23.9%) | 115 (26.7%) | 213 (49.4%) | 431 |
| COLLECTION METHODS (N = 431) | TIME SLOTS (N = 431) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele Size (bp) N = 522 | Allele Number N = 522 | HLC N = 115 | MM Octenol N = 161 | MM Lurex N = 155 | Evening N = 175 | Middle of the Night N = 114 | Early Morning N = 142 | |||||||
| He | FIS | He | FIS | He | FIS | He | FIS | He | FIS | He | FIS | |||
| ADC01 | 153–263 | 38 | 0.933 | 0.113 * | 0.941 | 0.102 * | 0.929 | 0.106 * | 0.935 | 0.085 | 0.928 | 0.152 * | 0.935 | 0.094 * |
| ADC02 | 131–223 | 21 | 0.883 | 0.315 * | 0.862 | 0.374 * | 0.867 | 0.227 * | 0.880 | 0.306 * | 0.855 | 0.324 * | 0.868 | 0.290 * |
| ADC28 | 125–137 | 7 | 0.450 | −0.013 | 0.421 | 0.159 * | 0.441 | 0.107 | 0.438 | 0.122 | 0.430 | 0.123 | 0.436 | 0.031 |
| ADC29 | 246–316 | 19 | 0.829 | 0.036 | 0.838 | 0.023 | 0.844 | 0.008 | 0.835 | 0.009 | 0.849 | 0.083 | 0.830 | −0.016 |
| ADC107 | 125–201 | 14 | 0.668 | 0.199 | 0.714 | 0.086 | 0.697 | 0.102 | 0.712 | 0.096 | 0.694 | 0.077 | 0.677 | 0.189 |
| ADC110 | 160–184 | 12 | 0.819 | 0.074 | 0.803 | 0.056 | 0.779 | 0.100 | 0.797 | 0.034 | 0.794 | 0.239 * | 0.818 | 0.015 |
| ADC137 | 121–149 | 13 | 0.791 | 0.024 | 0.771 | 0.001 | 0.747 | −0.062 | 0.764 | −0.023 | 0.761 | 0.008 | 0.783 | −0.016 |
| ADMP9 | 166–214 | 15 | 0.872 | 0.023 | 0.863 | −0.015 | 0.875 | 0.042 | 0.872 | 0.043 | 0.859 | −0.021 | 0.875 | 0.011 |
| Total | 142 | 0.781 | 0.103 | 0.776 | 0.097 | 0.772 | 0.079 | 0.779 | 0.084 | 0.771 | 0.125 | 0.778 | 0.076 | |
| Compared Populations | HLC | MM Octenol | MM Lurex |
|---|---|---|---|
| HLC | - | 0.00146 | 0.00069 |
| MM Octenol | 0.35000 | - | −0.00039 |
| MM Lurex | 0.25000 | 0.88333 | - |
| Time of the Night | Trap Position A | Trap Position B | Trap Position C | Total All Trap Positions |
|---|---|---|---|---|
| Evening (6:30 p.m.–10:30 p.m.) | 53 | 48 | 74 | 175 (40.6%) |
| Middle of the night (10:30 p.m.–05:00 a.m.) | 18 | 28 | 68 | 114 (26.5%) |
| Early morning (05:00 a.m.–07:00 a.m.) | 32 | 39 | 71 | 142 (32.9%) |
| Total all time slots | 103 (23.9%) | 115 (26.7%) | 213 (49.4%) | 431 |
| Compared Populations | Evening | Middle of the Night | Early Morning |
|---|---|---|---|
| Evening | - | −0.00025 | −0.00132 |
| Middle of the night | 0.70000 | - | 0.00002 |
| Early morning | 0.95000 | 0.76667 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, L.; Briolant, S.; Nacher, M.; Vezenegho, S.; Adde, A.; Nguyen, C.; Gaborit, P.; Issaly, J.; Carinci, R.; Pommier de Santi, V.; et al. Similar Microsatellite Allelic Distribution Between Anopheles darlingi Population Collected by Human Landing Catch or Mosquito Magnet Traps in French Guiana. Trop. Med. Infect. Dis. 2025, 10, 174. https://doi.org/10.3390/tropicalmed10060174
Ferraro L, Briolant S, Nacher M, Vezenegho S, Adde A, Nguyen C, Gaborit P, Issaly J, Carinci R, Pommier de Santi V, et al. Similar Microsatellite Allelic Distribution Between Anopheles darlingi Population Collected by Human Landing Catch or Mosquito Magnet Traps in French Guiana. Tropical Medicine and Infectious Disease. 2025; 10(6):174. https://doi.org/10.3390/tropicalmed10060174
Chicago/Turabian StyleFerraro, Laetitia, Sébastien Briolant, Mathieu Nacher, Samuel Vezenegho, Antoine Adde, Christophe Nguyen, Pascal Gaborit, Jean Issaly, Romuald Carinci, Vincent Pommier de Santi, and et al. 2025. "Similar Microsatellite Allelic Distribution Between Anopheles darlingi Population Collected by Human Landing Catch or Mosquito Magnet Traps in French Guiana" Tropical Medicine and Infectious Disease 10, no. 6: 174. https://doi.org/10.3390/tropicalmed10060174
APA StyleFerraro, L., Briolant, S., Nacher, M., Vezenegho, S., Adde, A., Nguyen, C., Gaborit, P., Issaly, J., Carinci, R., Pommier de Santi, V., Girod, R., Dusfour, I., & Bogreau, H. (2025). Similar Microsatellite Allelic Distribution Between Anopheles darlingi Population Collected by Human Landing Catch or Mosquito Magnet Traps in French Guiana. Tropical Medicine and Infectious Disease, 10(6), 174. https://doi.org/10.3390/tropicalmed10060174








