Epidemiological Trends and Age–Period–Cohort Effects on Dengue Incidence Across High-Risk Regions from 1992 to 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Collection and Processing
2.3. Descriptive Analysis
2.4. Age–Period–Cohort Model of Dengue Incidence
3. Results
3.1. Global Distribution of Dengue Incidence, 1992–2021
3.2. Trends in Dengue Incidence in High-Risk Areas, 1992–2021
3.3. Temporal Changes in Age-Specific Dengue Incidence in High-Risk Areas, 1992–2021
3.4. Age, Period, and Cohort Effects on Dengue Incidence Across High-Risk Regions, 1992–2021
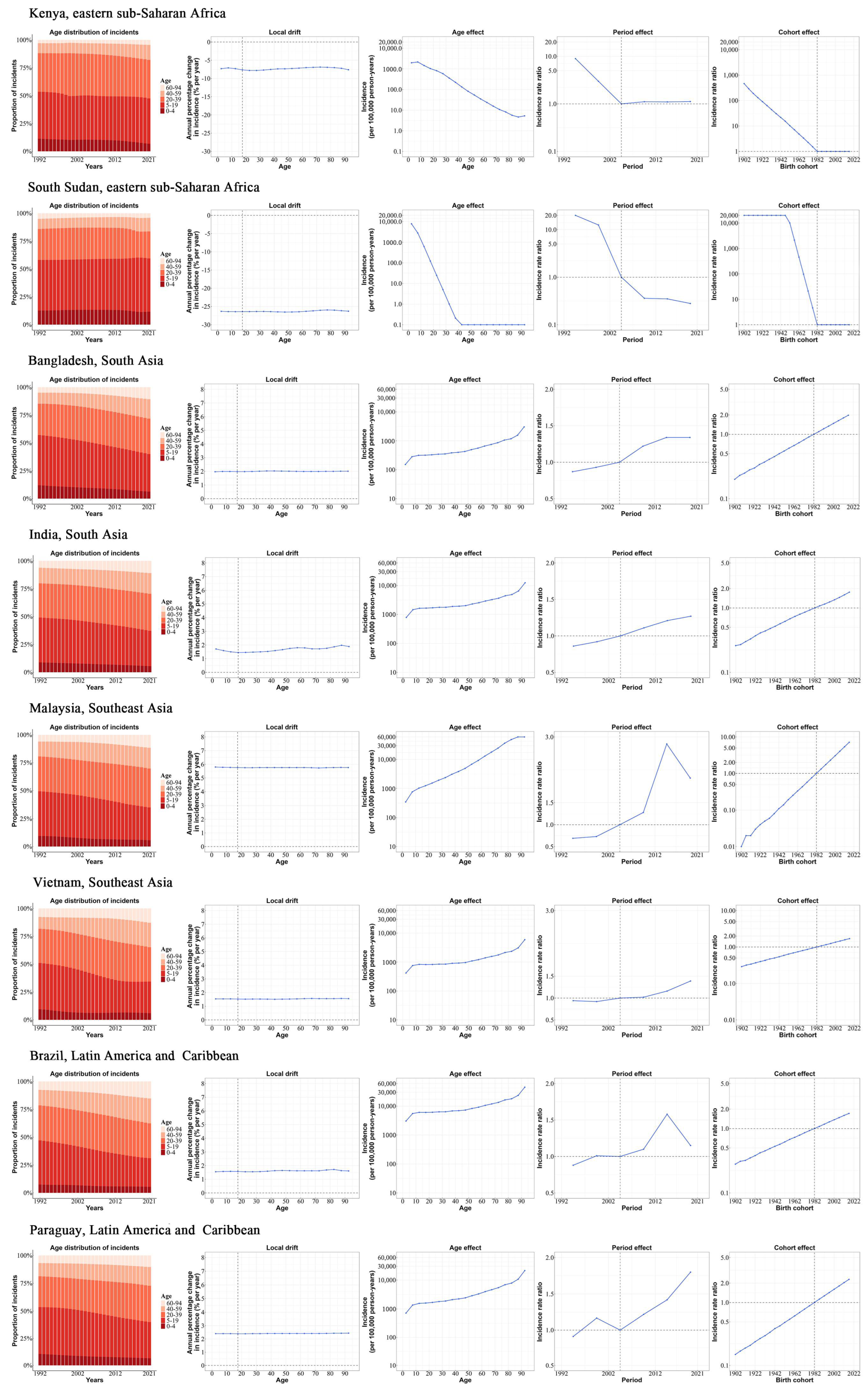
3.5. Age, Period, and Cohort Effects in Exemplary Countries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Age–period–cohort |
| ASIRs | Age-standardised incidence rates |
| CI | Confidence interval |
| DALY | Disability-adjusted life year |
| GBD | Global Burden of Disease Study |
| ICD | International Classification of Diseases |
| RR | Relative risk |
| UI | Uncertainty interval |
| WHO | World Health Organization |
References
- World Health Organization. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 23 April 2024).
- Paz-Bailey, G.; Adams, L.E.; Deen, J.; Anderson, K.B.; Katzelnick, L.C. Dengue. Lancet 2024, 403, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ilic, M. Global Patterns of Trends in Incidence and Mortality of Dengue, 1990–2019: An Analysis Based on the Global Burden of Disease Study. Medicina 2024, 60, 425. [Google Scholar] [CrossRef] [PubMed]
- Cattarino, L.; Rodriguez-Barraquer, I.; Imai, N.; Cummings, D.A.T.; Ferguson, N.M. Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 2020, 12, eaax4144. [Google Scholar] [CrossRef]
- World Bank Data Help Desk. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519/ (accessed on 4 February 2025).
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.; Wint, G.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Lu, B.-Z.; Liang, W.-J.; Shu, Y.-C.; Chuang, K.-T.; Chen, P.-L.; Ko, W.C.; Ko, N.Y. Trajectories of hepatic and coagulation dysfunctions related to a rapidly fatal outcome among hospitalized patients with dengue fever in Tainan, 2015. PLoS Neglected Trop. Dis. 2019, 13, e0007817. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Oliveira, L.; Itria, A.; Lima, E.C. Cost of illness and program of dengue: A systematic review. PLoS ONE 2019, 14, e0211401. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A. Economic and Disease Burden of Dengue in Southeast Asia. PLoS Neglected Trop. Dis. 2013, 7, e2055. [Google Scholar] [CrossRef]
- van den Berg, H.; Velayudhan, R.; Yadav, R.S. Management of insecticides for use in disease vector control: Lessons from six countries in Asia and the Middle East. PLoS Neglected Trop. Dis. 2021, 15, e0009358. [Google Scholar] [CrossRef]
- Scott, L.J. Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease. Drugs 2016, 76, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Malisheni, M.; Khaiboullina, S.F.; Rizvanov, A.A.; Takah, N.; Murewanhema, G.; Bates, M. Clinical Efficacy, Safety, and Immunogenicity of a Live Attenuated Tetravalent Dengue Vaccine (CYD-TDV) in Children: A Systematic Review with Meta-analysis. Front. Immunol. 2017, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Reiner, R.C., Jr.; Achee, N.; Barrera, R.; Burkot, T.R.; Chadee, D.D.; Devine, G.J.; Endy, T.; Gubler, D.; Hombach, J.; Kleinschmidt, I.; et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Neglected Trop. Dis. 2016, 10, e0004588. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Li, Y.; Zhou, Y.; Qiang, N.; Li, T.; Zhang, Y.; Chong, M.K.; Zhao, S.; Deng, X.; Zhang, X.; et al. Global burden associated with rare infectious diseases of poverty in 2021: Findings from the Global Burden of Disease Study 2021. Infect. Dis. Poverty 2024, 13, 85. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Sun, Z.S.; Zheng, J.X.; Zhang, S.X.; Yin, J.X.; Zhao, H.Q.; Shen, H.M.; Baneth, G.; Chen, J.H.; Kassegne, K.; et al. Prevalence and attributable health burdens of vector-borne parasitic infectious diseases of poverty, 1990–2021: Findings from the Global Burden of Disease Study 2021. Infect. Dis. Poverty 2024, 13, 96. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar]
- Bell, A. Age period cohort analysis: A review of what we should and shouldn’t do. Ann. Hum. Biol. 2020, 47, 208–217. [Google Scholar] [CrossRef]
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A Web Tool for Age-Period-Cohort Analysis of Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomarks Prev. 2014, 23, 2296–2302. [Google Scholar] [CrossRef]
- Yang, X.; Quam, M.B.M.; Zhang, T.; Sang, S. Global burden for dengue and the evolving pattern in the past 30 years. J. Travel. Med. 2021, 28, taab146. [Google Scholar] [CrossRef]
- Su, Z.; Zou, Z.; Hay, S.I.; Liu, Y.; Li, S.; Chen, H.; Naghavi, M.; Zimmerman, M.S.; Martin, G.R.; Wilner, L.B.; et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. eClinicalMedicine 2022, 43, 101249. [Google Scholar] [CrossRef]
- Carstensen, B. Age-period-cohort models for the Lexis diagram. Stat. Med. 2007, 26, 3018–3045. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Rahman, M.; Uddin, M.; Ashrafi, S.A.A.; Rahman, K.M.; Paul, K.K.; Sarker, M.F.R.; Haque, F.; Sharma, A.; Papakonstantinou, D.; et al. The 2023 fatal dengue outbreak in Bangladesh highlights a paradigm shift of geographical distribution of cases. Epidemiol. Infect. 2025, 153, e3. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, J.; Wang, L. Long-term effects of climate factors on dengue fever over a 40-year period. BMC Public Health 2024, 24, 1451. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue—Global situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 21 December 2023).
- Chen, Y.; Li, N.; Lourenço, J.; Wang, L.; Cazelles, B.; Dong, L.; Li, B.; Liu, Y.; Jit, M.; Bosse, N.I.; et al. Measuring the effects of COVID-19-related disruption on dengue transmission in southeast Asia and Latin America: A statistical modelling study. Lancet Infect. Dis. 2022, 22, 657–667. [Google Scholar] [CrossRef]
- Butala, C.B.; Cave, R.N.R.; Fyfe, J.; Coleman, P.G.; Yang, G.J.; Welburn, S.C. Impact of COVID-19 on the neglected tropical diseases: A scoping review. Infect. Dis. Poverty 2024, 13, 55. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Li, L.; He, J.; Guo, J.; Wang, Z.; Huang, Y.; Xi, Z.; Yuan, F.; Li, Y.; et al. The effect of temperature on dengue virus transmission by Aedes mosquitoes. Front. Cell. Infect. Microbiol. 2023, 13, 1242173. [Google Scholar] [CrossRef]
- Bhatia, S.; Bansal, D.; Patil, S.; Pandya, S.; Ilyas, Q.M.; Imran, S. A Retrospective Study of Climate Change Affecting Dengue: Evidences, Challenges and Future Directions. Front. Public Health 2022, 10, 884645. [Google Scholar] [CrossRef]
- Andhikaputra, G.; Lin, Y.H.; Wang, Y.C. Effects of temperature, rainfall, and El Niño Southern Oscillations on dengue-like-illness incidence in Solomon Islands. BMC Infect. Dis. 2023, 23, 206. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109 (Suppl. S1), 141–161. [Google Scholar]
- Reiter, P. Global-warming and vector-borne disease in temperate regions and at high altitude. Lancet 1998, 351, 839–840. [Google Scholar] [CrossRef]
- TheGlobalEconomy. Demographics. Available online: https://www.theglobaleconomy.com/rankings/population_density/ (accessed on 26 May 2025).
- Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Colón-González, F.J.; Gibb, R.; Khan, K.; Watts, A.; Lowe, R.; Brady, O.J. Projecting the future incidence and burden of dengue in Southeast Asia. Nat. Commun. 2023, 14, 5439. [Google Scholar] [CrossRef] [PubMed]
- Tsheten, T.; Gray, D.J.; Clements, A.C.A.; Wangdi, K. Epidemiology and challenges of dengue surveillance in the WHO South-East Asia Region. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Roche, J.; Redondo-Bravo, L.; Ruiz-Huerta, C.; Gomez-Barroso, D.; Benito, A.; Herrador, Z. The impact of climate change on mosquito-borne diseases in Africa. Pathog. Glob. Health 2020, 114, 287–301. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, H.; Wang, Y.; Liu, Q.; Du, M.; Yan, W.; Qin, C.; Zhang, S.; Chen, W.; Zhou, L.; et al. Global, regional, and national burden of dengue infection in children and adolescents: An analysis of the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 78, 102943. [Google Scholar] [CrossRef]
- Nicholson, S.E. Climate and climatic variability of rainfall over eastern Africa. Rev. Geophys. 2017, 55, 590–635. [Google Scholar] [CrossRef]
- Forget, Y.; Shimoni, M.; Gilbert, M.; Linard, C. Mapping 20 Years of Urban Expansion in 45 Urban Areas of Sub-Saharan Africa. Remote Sens. 2021, 13, 525. [Google Scholar] [CrossRef]
- Gainor, E.M.; Harris, E.; LaBeaud, A.D. Uncovering the Burden of Dengue in Africa: Considerations on Magnitude, Misdiagnosis, and Ancestry. Viruses 2022, 14, 233. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Zhang, Y.; Wu, X.; Li, J.; Chen, H.; Gao, W. Time trends in malaria incidence from 1992 to 2021 in high-risk regions: An age-period-cohort analysis based on the Global Burden of Disease study 2021. Int. J. Infect. Dis. 2025, 153, 107770. [Google Scholar] [CrossRef]
- Lim, J.K.; Carabali, M.; Lee, J.S.; Lee, K.S.; Namkung, S.; Lim, S.K.; Ridde, V.; Fernandes, J.; Lell, B.; Matendechero, S.H.; et al. Evaluating dengue burden in Africa in passive fever surveillance and seroprevalence studies: Protocol of field studies of the Dengue Vaccine Initiative. BMJ Open 2018, 8, e017673. [Google Scholar] [CrossRef]
- Lopes, R.; Basagaña, X.; Bastos, L.S.L.; Bozza, F.A.; Ranzani, O.T. Ambient temperature and dengue hospitalization in Brazil: A 10-year period case time series analysis. Environ. Epidemiol. 2025, 9, e360. [Google Scholar] [CrossRef] [PubMed]
- Phanhkongsy, S.; Suwannatrai, A.; Thinkhamrop, K.; Somlor, S.; Sorsavanh, T.; Tavinyan, V.; Sentian, V.; Khamphilavong, S.; Samountry, B.; Phanthanawiboon, S. Spatial analysis of dengue fever incidence and serotype distribution in Vientiane Capital, Laos: A multi-year study. Acta Trop. 2024, 256, 107229. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.Z.M.; Ruzlin, A.N.M.; Mohamad, M.; Zainuddin, M.A. Factors Associated with Dengue Outbreaks in Pulau Pinang from the eDengue Database 2023: A Cross-Sectional Study. J. Vector Borne Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Kongsomboon, K.; Singhasivanon, P.; Kaewkungwal, J.; Nimmannitya, S.; Mammen, M.P., Jr.; Nisalak, A.; Sawanpanyalert, P. Temporal trends of dengue fever/dengue hemorrhagic fever in Bangkok, Thailand from 1981 to 2000: An age-period-cohort analysis. Southeast Asian J. Trop. Med. Public Health 2004, 35, 913–917. [Google Scholar]
- Rodriguez, D.M.; Madewell, Z.J.; Torres, J.M.; Rivera, A.; Wong, J.M.; Santiago, G.A.; Rivera-Amill, V.; Paz-Bailey, G.; Marzan-Rodriguez, M.; Adams, L.E. Epidemiology of Dengue—Puerto Rico, 2010–2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 1112–1117. [Google Scholar] [CrossRef]
- Lee, V.J.; Lye, D.C.; Sun, Y.; Fernandez, G.; Ong, A.; Leo, Y.S. Predictive value of simple clinical and laboratory variables for dengue hemorrhagic fever in adults. J. Clin. Virol. 2008, 42, 34–39. [Google Scholar] [CrossRef]
- Lien, C.E.; Chou, Y.J.; Shen, Y.J.; Tsai, T.; Huang, N. A Population-Based Cohort Study on Chronic Comorbidity Risk Factors for Adverse Dengue Outcomes. Am. J. Trop. Med. Hyg. 2021, 105, 1544–1551. [Google Scholar] [CrossRef]
- Macias, A.E.; Werneck, G.L.; Castro, R.; Mascareñas, C.; Coudeville, L.; Morley, D.; Recamier, V.; Guergova-Kuras, M.; Etcheto, A.; Puentes-Rosas, E.; et al. Mortality among Hospitalized Dengue Patients with Comorbidities in Mexico, Brazil, and Colombia. Am. J. Trop. Med. Hyg. 2021, 105, 102–109. [Google Scholar] [CrossRef]
- Lee, I.K.; Liu, J.W.; Yang, K.D. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 2008, 79, 149–153. [Google Scholar] [CrossRef]
- Lye, D.C.; Lee, V.J.; Sun, Y.; Leo, Y.S. The benign nature of acute dengue infection in hospitalized older adults in Singapore. Int. J. Infect. Dis. 2010, 14, e410–e413. [Google Scholar] [CrossRef]
- Htun, N.S.; Odermatt, P.; Eze, I.C.; Boillat-Blanco, N.; D’Acremont, V.; Probst-Hensch, N. Is diabetes a risk factor for a severe clinical presentation of dengue?–review and meta-analysis. PLoS Neglected Trop. Dis. 2015, 9, e0003741. [Google Scholar] [CrossRef]
- Lin, R.J.; Lee, T.H.; Leo, Y.S. Dengue in the elderly: A review. Expert. Rev. Anti Infect. Ther. 2017, 15, 729–735. [Google Scholar] [CrossRef]



| Global | Eastern Sub-Saharan Africa | South Asia | Southeast Asia | Latin America and Caribbean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1992 | 2021 | 1992 | 2021 | 1992 | 2021 | 1992 | 2021 | 1992 | 2021 | |
| Population | ||||||||||
| Number, n × 1,000,000 | 5497 (5379 to 5624) | 7891 (7667 to 8131) | 202 (196 to 208) | 426 (406 to 447) | 1136 (1069 to 1205) | 1847 (1668 to 2039) | 482 (463 to 501) | 698 (670 to 728) | 405 (391 to 419) | 594 (560 to 626) |
| Percentage of global population, % | 100.0 | 100.0 | 3.67 | 5.40 | 20.67 | 23.41 | 8.1 | 8.85 | 7.37 | 7.53 |
| Incident cases | ||||||||||
| Number, n × 100,000 | 288.47 (62.07 to 530.97) | 589.64 (154.73 to 1068.85) | 23.07 (1.76 to 57.35) | 3.88 (0.07 to 14.62) | 134.08 (2.08 to 278.03) | 318.12 (18.72 to 670.71) | 28.64 (7.35 to 62.97) | 67.28 (47.88 to 104.31) | 90.6 (12.17 to 196.14) | 165.49 (65.78 to 305.44) |
| Percentage of global cases, % | 100.0 | 100.0 | 8.0 | 0.7 | 46.48 | 53.95 | 9.93 | 11.41 | 31.41 | 28.07 |
| Percent change for 1992–2021, % | 104.40 | −83.20 | 137.26 | 134.90 | 82.65 | |||||
| Percent change for 2019–2021, % | 3.81 | 3.88 | 3.22 | 6.46 | 3.04 | |||||
| All-age incidence rate | ||||||||||
| Rate per 100,000 | 524.76 (112.91 to 965.89) | 747.2 (196.08 to 1354.46) | 1143.51 (87.08 to 2842.94) | 90.97 (1.54 to 343.2) | 1180.36 (18.33 to 2447.58) | 1722.77 (101.4 to 3632.17) | 594.63 (152.64 to 1307.19) | 963.54 (685.65 to 1493.81) | 2236.28 (300.3 to 4841.16) | 2785.41 (1107.14 to 5141.04) |
| Percent change for 1992–2021, % | 42.39 | −92.04 | 45.95 | 62.04 | 24.56 | |||||
| Percent change for 2019–2021, % | 1.89 | −0.80 | 1.03 | 4.39 | 1.53 | |||||
| ASIRs | ||||||||||
| Rate per 100,000 | 510.67 (108.43 to 941.74) | 752.04 (196.33 to 1363.35) | 1142.19 (87.44 to 2843.11) | 94.37 (1.55 to 364.39) | 1182.78 (18.6 to 2452.17) | 1726.94 (102.48 to 3635.94) | 587.51 (150.84 to 1281.39) | 971.89 (691.33 to 1500.41) | 2235.8 (298.96 to 4852.39) | 2781.19 (1107.73 to 5129.38) |
| Percent change for 1992–2021, % | 47.26 | −91.74 | 46.01 | 65.43 | 24.39 | |||||
| Percent change for 2019–2021, % | 1.85 | −0.33 | 1.05 | 4.41 | 1.46 | |||||
| APC model estimates | ||||||||||
| Net drift of ASIR for 1992–2021, % per year | 2.14 (2.1 to 2.19) | −9.02 (−9.07 to −8.98) | 1.57 (1.56 to 1.58) | 2.47 (2.44 to 2.51) | 1.78 (1.76 to 1.8) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Chen, H.; Wu, H.; Wu, B.; Wang, L.; Liu, X.; Yang, Y.; Tan, H.; Gao, W. Epidemiological Trends and Age–Period–Cohort Effects on Dengue Incidence Across High-Risk Regions from 1992 to 2021. Trop. Med. Infect. Dis. 2025, 10, 173. https://doi.org/10.3390/tropicalmed10060173
Cao Y, Chen H, Wu H, Wu B, Wang L, Liu X, Yang Y, Tan H, Gao W. Epidemiological Trends and Age–Period–Cohort Effects on Dengue Incidence Across High-Risk Regions from 1992 to 2021. Tropical Medicine and Infectious Disease. 2025; 10(6):173. https://doi.org/10.3390/tropicalmed10060173
Chicago/Turabian StyleCao, Yu, Hanwu Chen, Hao Wu, Bin Wu, Lu Wang, Xin Liu, Yuyue Yang, Hui Tan, and Wei Gao. 2025. "Epidemiological Trends and Age–Period–Cohort Effects on Dengue Incidence Across High-Risk Regions from 1992 to 2021" Tropical Medicine and Infectious Disease 10, no. 6: 173. https://doi.org/10.3390/tropicalmed10060173
APA StyleCao, Y., Chen, H., Wu, H., Wu, B., Wang, L., Liu, X., Yang, Y., Tan, H., & Gao, W. (2025). Epidemiological Trends and Age–Period–Cohort Effects on Dengue Incidence Across High-Risk Regions from 1992 to 2021. Tropical Medicine and Infectious Disease, 10(6), 173. https://doi.org/10.3390/tropicalmed10060173






