Systematic Review of the Ovitrap Surveillance of Aedes Mosquitoes in Brazil (2012–2022)
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
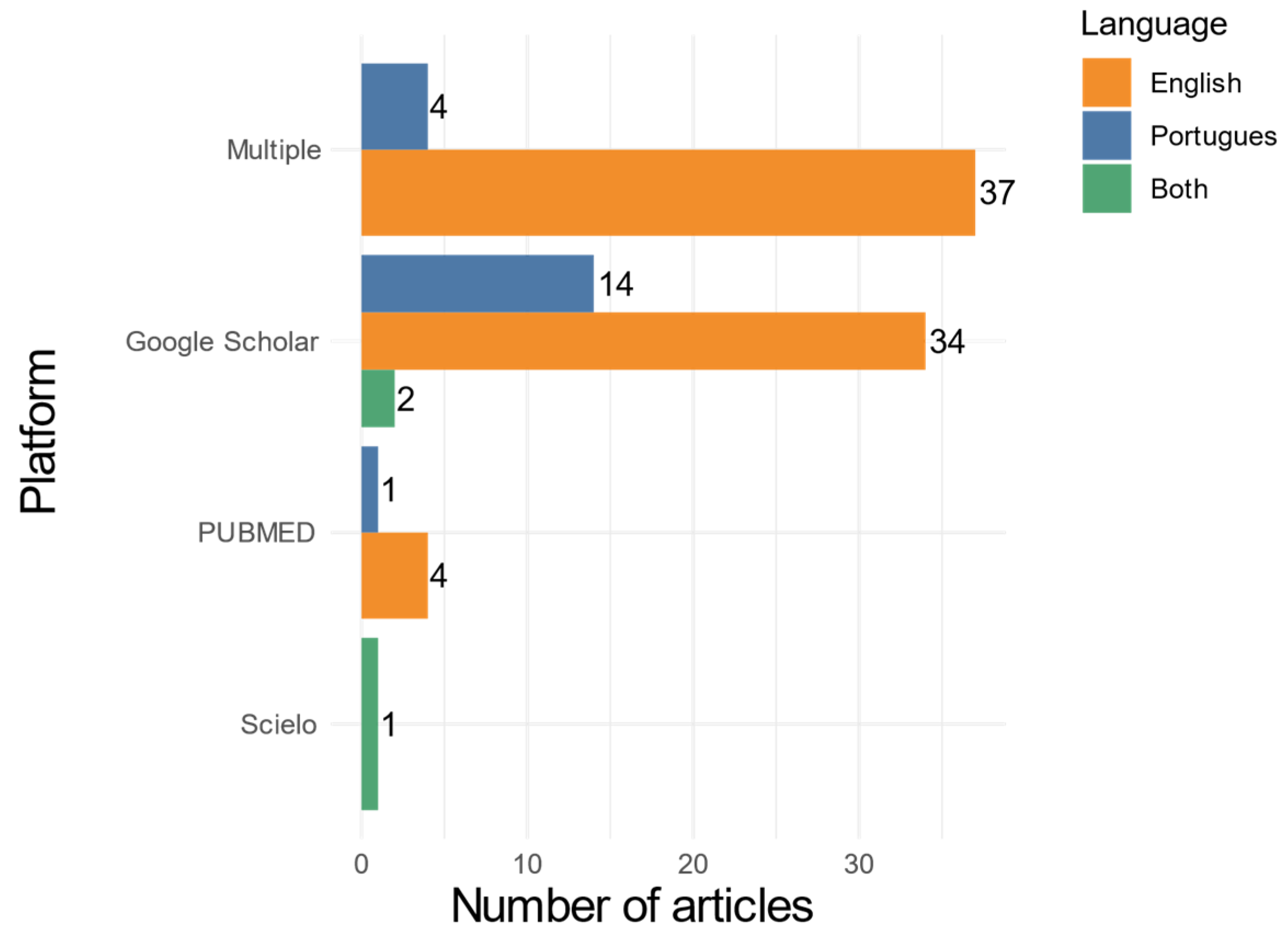
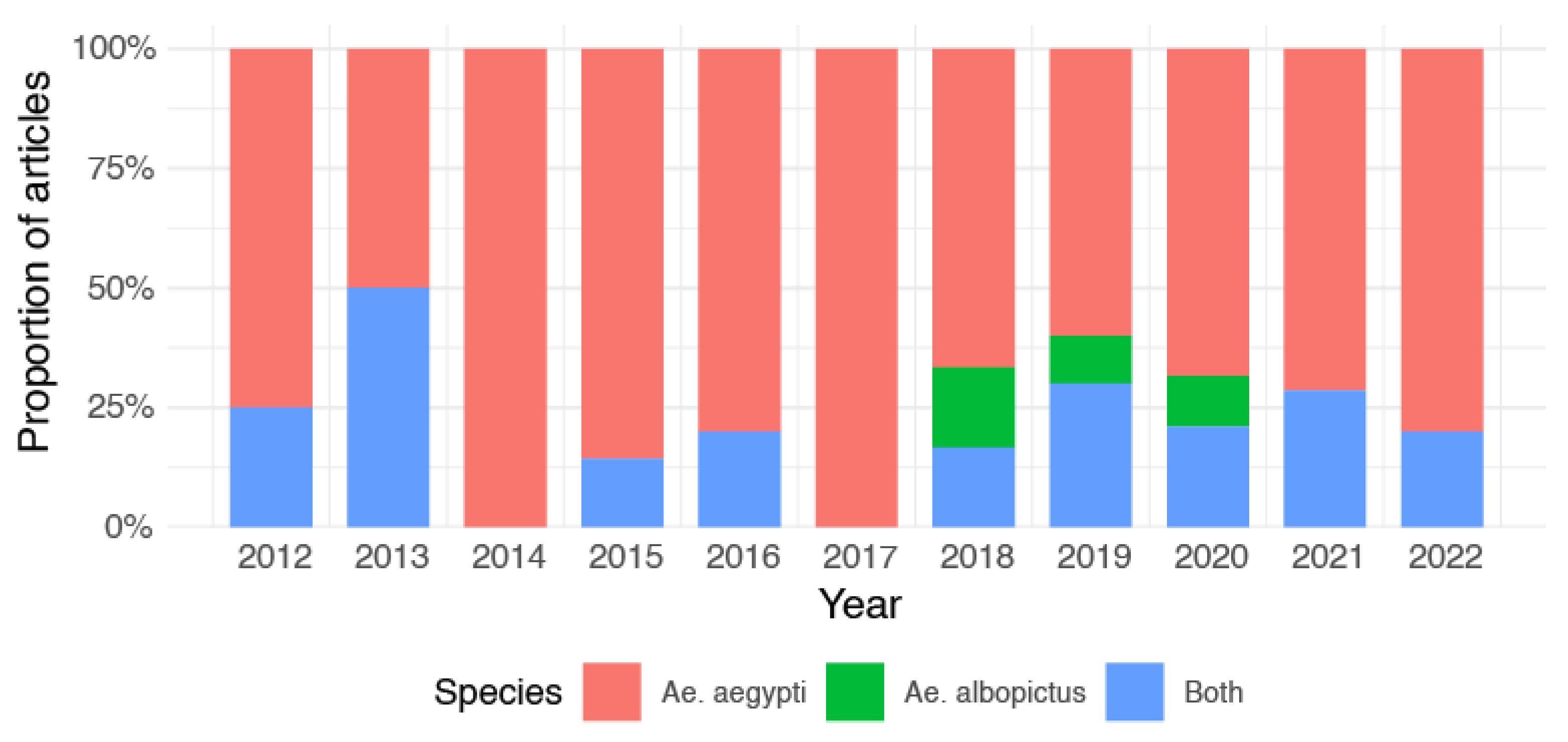
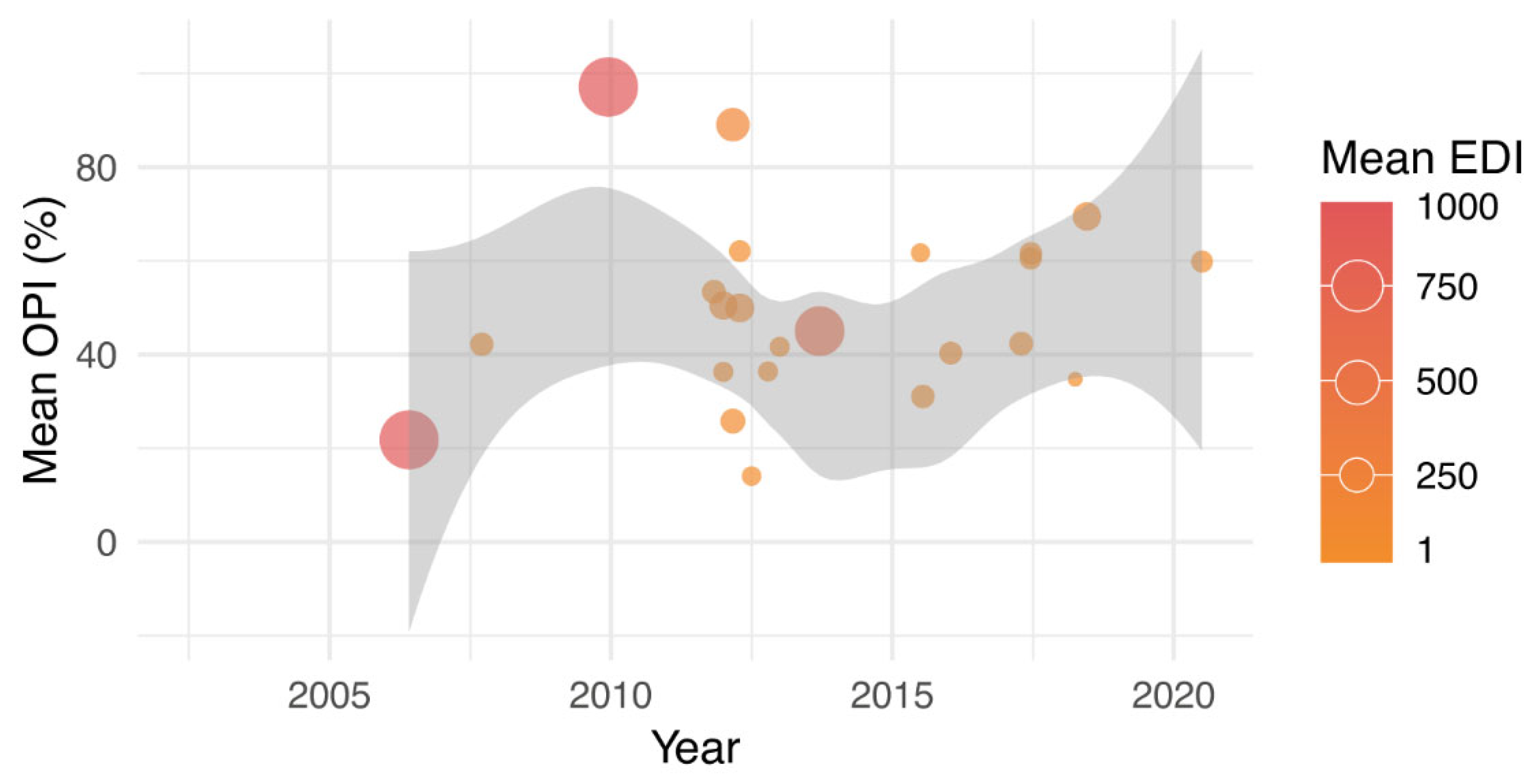
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima-Camara, T.N. Arboviroses emergentes e novos desafios para a saúde pública no Brasil. Rev. Saúde Pública 2016, 50, 36. [Google Scholar]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.P.; Pissinatti, A.; Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 10, e180278. [Google Scholar] [CrossRef]
- Resck, M.E.B.; Câmara, D.C.P.; Dos Santos, F.B.; Dos Santos, J.P.C.; Alto, B.W.; Honório, N.A. Spatial-temporal distribution of chikungunya virus in Brazil: A review on the circulating viral genotypes and Aedes (Stegomyia) albopictus as a potential vector. Front. Public Health 2024, 11, 12. [Google Scholar] [CrossRef]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil, 1st ed.; Editora Fiocruz: Rio de Janeiro, Brazil, 1994; 228p. [Google Scholar]
- Clements, A.N. The Biology of Mosquitoes, 3rd ed.; Neville, A., Ed.; Clements Hardcover: London, UK, 2012; 584p. [Google Scholar]
- Nadim, S.S.; Ghosh, I.; Martcheva, M.; Chattopadhyay, J. Impact of venereal transmission on the dynamics of vertically transmitted viral diseases among mosquitoes. Math. Biosci. 2020, 325, 108366. [Google Scholar]
- Yang, H.M. The transovarial transmission in the dynamics of dengue infection: Epidemiological implications and thresholds. Math. Biosci. 2017, 286, 1–15. [Google Scholar] [CrossRef]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 5, e1005548. [Google Scholar] [CrossRef] [PubMed]
- Janjoter, S.; Kataria, D.; Yadav, M.; Dahiya, N.; Sehrawat, N. Transovarial transmission of mosquito-borne viruses: A systematic review. Front. Cell. Infect. Microbiol 2024, 13, 1304938. [Google Scholar] [CrossRef] [PubMed]
- Beaty, J.B.; William, C.M. The Biology of Disease Vectors, 1st ed.; University Press of Colorado: Denver, CO, USA, 1996; 632p. [Google Scholar]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasites Vectors 2018, 1, 77. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes aegypti (L.), the Yellow Fever Mosquito—Its Life History, Bionomics, and Structure; Cambridge University Press: London, UK, 1960; 739p. [Google Scholar]
- Souza, L.R.S. Viabilidade Econômica da TIE (Técnica do Inseto Estéril) no Controle Epidemiológico do Mosquito Aedes aegypti. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Arduino, M.B.; de-Ávila, G.O. Aspectos físico-químicos da água de criadouros de Aedes aegypti em ambiente urbano e as implicações para o controle da dengue. Rev. Patolog. Trop. 2015, 44, 89–100. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef]
- Honório, N.A.; Silva, W.C.; Leite, P.J.; Gonçalves, J.M.; Lounibos, L.P.; Lourenço-de-Oliveira, R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2003, 2, 191–198. [Google Scholar] [CrossRef]
- Gomes, A.C.; Souza, J.M.P.; Bergamaschia, D.P.; dos-Santos, J.L.F.; Andrade, V.R.; Leite, O.F.; Rangel, O.; Souza, S.S.L.; Guimarães, N.S.N.; Lima, V.L.C. Atividade antropofílica de Aedes aegypti e Aedes albopictus em área sob controle e vigilância. Rev. Saúde Pública 2005, 39, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Câmara, D.C.P.; Codeço, C.T.; Ayllón, T.; Nobre, A.A.; Azevedo, R.C.; Ferreira, D.F.; da Silva Pinel, C.; Rocha, G.P.; Honório, N.A. Entomological surveillance of Aedes mosquitoes: Comparison of different collection methods in an endemic area in Rio de Janeiro, Brazil. Trop. Med. Infect. Dis. 2022, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Soares-Pinheiro, V.C.; Dasso-Pinheiro, W.; Trindade-Bezerra, J.M.; Tadei, W.P. Eggs viability of Aedes aegypti Linnaeus (Diptera, Culicidae) under different environmental and storage conditions in Manaus, Amazonas, Brazil. Braz. J. Biol. 2017, 2, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, J.P.; Wilke, A.B.B.; Ostrum, E.; Vasquez, C.; Cardenas, G.; Carvajal, A.; Moreno, M.; Petrie, W.D.; Rodruiguez, A.; Presas, H.; et al. Diel activity patterns of two distinct populations of Aedes aegypti in Miami, FL and Brownsville, TX. Sci. Rep. 2022, 12, 5315. [Google Scholar] [CrossRef]
- Reiter, P.; Amador, M.A.; Colon, N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J. Am. Mos. Control Assoc. 1991, 7, 52–55. [Google Scholar]
- Forattini, O.P. Culicidologia Médica, 2nd ed.; EdUSP: São Paulo, Brazil, 2002; 860p. [Google Scholar]
- Carvalho, R.G.; Lourenço-de-Oliveira, R.; Braga, I.A. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Mem. Inst. Oswaldo Cruz 2014, 109, 787–796. [Google Scholar] [CrossRef]
- Pancetti, F.G.M.; Honório, N.A.; Urbinatti, P.R.; Lima-Camara, T.N. Twenty-eight years of Aedes albopictus in Brazil: A rationale to maintain active entomological and epidemiological surveillance. Short Communications. Rev. Soc. Bras. Med. Trop. 2015, 48, 87–89. [Google Scholar] [CrossRef]
- Castro, M.G.; Nogueira, R.M.R.; Schatzmayr, H.G.; Miagostovich, M.P.; Lourenço-de Oliveira, R. Dengue virus detection by using reverse transcription polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Mem. Inst. Oswaldo Cruz 2004, 99, 809–814. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya Virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef]
- Honório, N.A.; Wiggins, K.; Câmara, D.C.P.; Eastmond, B.; Alto, B.W. Chikungunya virus vector competency of Brazilian and Florida mosquito vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006521. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C. Vigilância entomológica. Inf. Epidemiol. Sus 2002, 11, 79–90. [Google Scholar] [CrossRef]
- Braga, I.A.; Gomes, A.C.; Nelson, M.; Mello, R.C.; Bergamaschi, D.P.; Souza, J.M.P. Comparação entre pesquisa larvária e armadilha de oviposição, para detecção de Aedes aegypti. Rev. Soc. Bras. Med. 2000, 33, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.S.; Trindade, R.B.R.; Souto, R.N.P. Avaliação da atratividade de ovitrampas a Aedes (Stegomyia) aegypti Linnaeus (Diptera: Culicidae) no bairro Hospitalidade, Santana, Amapá. Biota Amazon. 2011, 1, 26–31. [Google Scholar] [CrossRef]
- Codeço, C.T.; Lima, A.W.; Araújo, S.C.; Lima, J.B.; Maciel-de-Freitas, R.; Honório, N.A.; Galardo, A.K.; Braga, I.A.; Coelho, G.E.; Valle, D. Surveillance of Aedes aegypti: Comparison of house index with four alternative traps. PLoS Negl. Trop. Dis. 2015, 9, e0003475. [Google Scholar] [CrossRef]
- Fay, R.W.; Perry, A.S. Laboratory studies of ovipositional preferences of Aedes aegypti. Mosq. News 1965, 25, 276–281. [Google Scholar]
- Fay, R.W.; Eliason, D.A. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- Focks, D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Gainsville, FL, USA, 2003; 40p. [Google Scholar]
- Zequi, J.A.C.; Oliveira, A.A.; Santos, F.P.; Lopes, J. Monitoramento e controle de Aedes aegypti (Linnaeus, 1762) e Aedes albopictus (Skuse, 1984) com uso de ovitrampas. Semin. Cienc. Biol. Saude 2018, 39, 93–102. [Google Scholar] [CrossRef]
- Câmara, D.C.P.; Codeço, C.T.; Juliano, S.A.; Lounibos, L.P.; Riback, T.I.S.; Pereira, G.R.; Honório, N.A. Seasonal differences in density but similar competitive Impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLoS ONE 2016, 11, e0157120. [Google Scholar] [CrossRef]
- Acero-Sandoval, M.A.; Palacio-Cortés, A.M.; Navarro-Silva, M.A. Surveillance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) as a method for prevention of Arbovirus transmission in urban and seaport areas of the Southern Coast of Brazil. J. Med. Entomol. 2023, 1, 173–184. [Google Scholar] [CrossRef]
- Braga, I.A.; Valle, D. Aedes aegypti: Histórico do controle no Brasil. Epidemiol. Serv. Saúde 2007, 16, 113–118. [Google Scholar]
- Siqueira, A.S.P.; Praça, H.L.F.; Santos, J.P.C.; Albuquerque, H.G.; Pereira, L.H.V.; César, T.S.; Gusmão, E.V.V.; Pereira, A.A.T.; Pimenta Júnior, F.G.; Nobre, A.A.; et al. ARBOALVO: Método de estratificação da receptividade territorial às arboviroses urbanas. Rev. Saude Publica 2022, 56, 39. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.B.; Erbisti, R.S.; Nobre, A.A.; Simões, T.C.; Tavares, A.M.; Melo, M.C.; Pedreira, R.M.; de Araújo, J.P.M.; Carvalho, M.S.; Honório, N.A. ARBOALVO: A Bayesian spatiotemporal learning and predictive model for dengue cases in the endemic Northeast city of Natal, Rio Grande do Norte, Brazil. PLoS Negl. Trop. Dis. 2025, 19, e0012984. [Google Scholar] [CrossRef] [PubMed]
- Technical Note, No. 3/2014/IOC-FIOCRUZ/DIRETORIA. Available online: https://fiocruz.br/ioc/media/nota_tecnica_ioc_3.pdf (accessed on 8 July 2025).
- Technical Note, No. 33/2022/CGARB/DEIDT/SVS/MS. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/estudos-e-notas-informativas/2023/nota-informativa-no-37-2023-cgarb-dedt-svsa-ms/@@download/file/Nota%2037_2023%20novas%20tecnologias.pdf (accessed on 8 July 2025).
- BRASIL; Ministry of Health; Brasília. Diretrizes do MS (Diretrizes Nacionais para Prevenção e Controle das Arboviroses Urbanas: Vigilância Entomológica e Controle Vetorial [recurso eletrônico]/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Doenças Transmissíveis. Coordenação-Geral de Vigilância de Arboviroses 2025, 190 p. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/dengue/diretrizes-nacionais-para-prevencao-e-controle-das-arboviroses-urbanas-vigilancia-entomologica-e-controle-vetorial.pdf/view (accessed on 8 July 2025).
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Site DeCS. Available online: http://decs.bvsalud.org/ (accessed on 8 July 2025).
- Site R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 16 July 2025).
- Cleveland, W.S.; Grosse, E.; Shyu, W.M. Local Regression Models. Chapter 8 of Statistical Models in S; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; 68p. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Pereira, R.H.M.; Gonçalves, C.N. geobr: Loads Shapefiles of Official Spatial Data Sets of Brazil. GitHub Repository. 2019. Available online: https://github.com/ipeaGIT/geobr (accessed on 8 July 2025).
- Ministério da Saúde. Painel de Monitoramento de Arboviroses. 2025. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses (accessed on 16 July 2025).
- Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Relatório da Reunião internacional para implementação de alternativas para o controle do Aedes aegypti no Brasil. Bol. Epidemiol. 2016, 47, 1–9. [Google Scholar]
- Di Bitetti, M.S.; Ferraras, J.A. Publish (in English) or perish: The effect on citation rate of using languages other than English in scientific publications. Ambio 2017, 46, 121–127. [Google Scholar] [CrossRef]
- Day, J.F. Mosquito oviposition behavior and vector control. Insects 2016, 4, 65. [Google Scholar] [CrossRef]
- da Costa, C.F.; da Silva, A.V.; do Nascimento, V.A.; de Souza, V.C.; Monteiro, D.C.D.S.; Terrazas, W.C.M.; Dos Passos, R.A.; Nascimento, S.; Lima, J.B.P.; Naveca, F.G. Evidence of vertical transmission of Zika virus in field-collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2018, 12, e0006594. [Google Scholar] [CrossRef]
- Chadee, D.D.; Lakhan, A.; Ramdath, W.R.; Persad, R.C. Oviposition response of Aedes aegypti mosquitoes to different concentrations of hay infusion in Trinidad, West Indies. J. Am. Mosq. Control Assoc. 1993, 3, 346–349. [Google Scholar]
- De Melo, D.P.; Scherrer, L.R.; Eiras, Á.E. Dengue fever occurrence and vector detection by larval survey, ovitrap and MosquiTRAP: A space-time clusters analysis. PLoS ONE 2012, 7, e42125. [Google Scholar] [CrossRef]
- Resende, M.C.; Silva, I.V.; Ellis, B.R.; Eira, A.E. A comparison of larval, ovitrap and MosquiTRAP surveillance for Aedes (Stegomyia) aegypti. Mem. Inst. Oswaldo Cruz 2013, 8, 1024–1030. [Google Scholar] [CrossRef]
- Silva, C.E.; Limongi, J.E. Avaliação comparativa da eficiência de armadilhas para a captura e coleta de Aedes aegypti em condições de campo. Cad. Saúde Coletiva 2018, 26, 241–248. [Google Scholar] [CrossRef]
- Monteiro, R.M.M.; Oliveira, C.A.; Sousa, M.H.; Santos, A.O.; Soares, T.M.S.; Santos, E.P.; Amorim, L.L.B.; Amorim, L.B. Estudo comparativo entre ovitrampa e o método LIRAa para avaliação da presença de Aedes aegypti (Diptera:Culicidae) em Pedro II, Piauí, Brasil. Bras. J. Dev. 2020, 6, 38890–38912. [Google Scholar] [CrossRef]
- Jesus, C.P.; Dias, F.B.S.; Villela, D.M.A.; Freitas, R.F.M. Ovitraps provide a reliable estimate of Wolbachia frequency during WMelBr Strain deployment in a geographically isolated Aedes aegypti population. Insects 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.A.; Silva, E.M.; Silva, I.M.; Resende, M.C.; Eiras, A.E. Evaluation of the sticky MosquiTRAP for detecting Aedes (Stegomyia) aegypti (L.) (Diptera: Culicidae) during the dry season in Belo Horizonte, Minas Gerais, Brazil. Neotrop. Entomol. 2007, 36, 294–302. [Google Scholar] [CrossRef]
- Morato, V.C.G.; Teixeira, M.G.; Gomes, A.C.; Bergamaschi, D.P.; Barreto, M.L. Infestation of Aedes aegypti estimated by oviposition traps in Brazil. Rev. Saúde Pública 2005, 4, 553–558. [Google Scholar] [CrossRef]
- Honório, N.A.; Castro, M.G.; Barros, F.S.; Magalhães Mde, A.; Sabroza, P.C. The spatial distribution of Aedes aegypti and Aedes albopictus in a transition zone, Rio de Janeiro, Brazil. Cad. Saude Publica 2009, 25, 1203–1214. [Google Scholar] [CrossRef]
- Rawlins, S.C.; Martinez, R.; Wiltshire, S.; Legall, G. A comparison of surveillance systems for the dengue vector Aedes aegypti in Port of Spain, Trinidad. J. Am. Mosq. Control Assoc. 1998, 2, 131–136. [Google Scholar]
- Romero-Vivas, C.M.; Falconar, A.K. Investigation of relationships between Aedes aegypti egg, larvae, pupae, and adult density indices where their main breeding sites were located indoors. J. Am. Mosq. Control Assoc. 2005, 1, 15–21. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Tavares, A.M.; Torres, U.P.S.; Nascimento, C.A.; Moura, M.C.B.M.; Vieira, V.B.; Araújo, J.M.G.; Gama, R.A. Identification of surveillance and control priority for dengue and other arboviruses transmitted by areas Aedes aegypti in Natal-RN, Brazil: Experience report. Epidemiol. Serv. Saude 2017, 26, 629–638. [Google Scholar] [CrossRef]
- Heinisch, M.R.S.; Diaz-Quijano, F.A.; Chiaravalloti-Neto, F.; Pancetti, F.G.M.; Coelho, R.R.; Andrade, P.S.; Urbinatti, P.R.; Almeida, R.M.M.S.; Lima-Camara, T.N. Seasonal and spatial distribution of Aedes aegypti and Aedes albopictus in a municipal urban park in São Paulo, SP, Brazil. Acta Trop. 2019, 189, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mafra, N.S.C.; Everton, G.O.; Ferreira, A.M.; Sales, E.H.; Júnior, P.S.S.; Filho, V.E.M. Potenciais biológicos do óleo essencial de Ocimum basilicum Linn coletada na região Pré-Amazônica do Maranhão. Res. Soc. Dev. 2020, 9, e203985596. [Google Scholar] [CrossRef]
- Martins, T.G.T.; Everton, G.O.; Rosa, P.V.S.; Arruda, M.O.; Souto, L.A.S.; Fonseca, D. Atividade larvicida do óleo essencial de Pimenta dioica Lindl. frente as larvas do mosquito Aedes aegypti. Res. Soc. Dev. 2020, 9, e151985518. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Gonzaga, E.A.R.; Souza, G.S.G.; Filho, J.F. As contribuições de ovitrampas no monitoramento de vetores: Possibilidades e desafios. Rev. Multi. Saúde 2023, 4, 42–58. [Google Scholar]
- Oliveira, J.C.; Lima, S.C. Mobilização comunitária e vigilância em saúde no controle dos Aedes e prevenção do dengue no distrito de Martinésia, Uberlândia (MG). Bolet. Campineiro Geogr. 2012, 2, 121–136. [Google Scholar] [CrossRef]
- Silva, M.G.N.M.; Rodrigues, M.A.B.; Araujo, R.E. Sistema de aquisição e processamento de imagens de ovitrampas para o combate à dengue. Rev. Bras. Eng. Biomed. 2012, 28, 364–374. [Google Scholar] [CrossRef]
- Oliveira, T.E.S.; Musis, C.R. Análise da flutuação das populações de Aedes aegypti e Aedes albopictus em uma escola de Cuiabá-MT. Rev. Eletrônica Gest. Educ. Tecnol. Ambient. 2014, 18, 178–186. [Google Scholar] [CrossRef]
- Serpa, L.L.N.; Barbosa, G.L.; Arduino, M.B.; Andrade, V.R.; Voltolini, J.C.; Lima, V.L.C.; Marques, G.R.A.M. Segregação espacial de Aedes aegypti e Aedes albopictus, estado de São Paulo, Brasil. Bolet. Epidemiol. Paul. 2013, 18, 39–56. [Google Scholar] [CrossRef]
- Bellinato, D.F.; Viana-Medeiros, P.F.; Araújo, S.C.; Martins, A.J.; Lima, J.B.P.; Valle, D. Resistance status to the insecticides temephos, deltamethrin, and diflubenzuron in Brazilian Aedes aegypti populations. Biomed. Res. Int. 2016, 2016, 8603263. [Google Scholar] [CrossRef]
- Costa, I.M.P.; Calado, D.C. Incidência dos casos de dengue (2007) e distribuição sazonal de culicídeos (2012–2013) em Barreiras, Bahia. Epidemiol. Serv. Saude 2016, 25, 735–744. [Google Scholar] [CrossRef]
- Depoli, P.A.C.; Zequi, J.A.C.; Nascimento, K.L.C.; Lopes, J. Eficácia de ovitrampas com diferentes atrativos na vigilância e controle de Aedes. EntomoBrasilis 2016, 9, 51–55. [Google Scholar] [CrossRef]
- Alves Virgulino, A.C.L.; Silva Costa, T.I.; Azevedo Feitosa, F.R.; Lima Rocha, E.P.; Azevedo, R. Attractive activity of plant extracts for the oviposition of Aedes aegypti L. (Diptera: Culicidae). Idesia 2018, 36, 225–231. [Google Scholar]
- Barbosa, G.L.; Lage, M.O.; Andrade, V.R.; Gomes, A.H.A.; Quintanilha, J.A.; Chiaravalloti-Neto, F. Influence of strategic points in the dispersion of Aedes aegypti in infested areas. Rev. Saude Publica 2019, 53, 29. [Google Scholar] [CrossRef] [PubMed]
- Custódio, J.M.O.; Nogueira, L.M.S.; Souza, D.A.; Fernandes, M.F.; Oshiro, E.T.; Oliveira, E.F.; Piranda, E.M.; Oliveira, A.G. Abiotic factors and population dynamic of Aedes aegypti and Aedes albopictus in an endemic area of dengue, Brazil. Rev. Inst. Med. Trop. 2019, 61, e18. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.O.; Macoris, M.L.G.; Santos, R.F.; Morato, D.G.; Isabel, M.D.S.S.; Cerqueira, N.A.; Monte-Alegre, A.F. Estudo experimental sobre a ação de larvicidas em pop de Aedes aegypti do município de Itabuna, Bahia. Epidemiol. Serv. Saude 2019, 28, e2017316. [Google Scholar] [PubMed]
- Gonçalves e Sá, Á.K.; Matias Gomes, E.J.; Beltrão Rameh Barbosa, I.M.; Medeiros de Araújo Frutuoso, M.N.; Costa Castro Lyra, M.R.; Mansur Custódio Nogueira, R.J.; Ferreira Brandão Rodrigues, S.S. Monitoramento de Aedes aegypti por ovitrampas e pelo método LIRAa em Salgueiro, Pernambuco, Brasil. Hygeia—Rev. Bras. Geogr. Méd. Saúde 2019, 15, 134–148. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Filho, V.E.M.; Mafra, N.S.C.; Sales, E.H.; Júnior, P.S.S.; Everton, G.O. Constituintes químicos, toxicidade, potencial antioxidante e atividade larvicida frente a larvas de Aedes aegypti do óleo essencial de Aniba rosaeodora Ducke. Res. Soc. Dev. 2020, 9, e520985663. [Google Scholar] [CrossRef]
- Moraes, L.D.; Cerqueira-Silva, T.; Nobrega, V.; Akrami, K.; Santos, L.A.; Orge, C.; Casais, P.; Cambui, L.; Rampazzo, R.D.; Trinta, K.S.; et al. A clinical scoring system to predict long-term arthralgia in Chikungunya disease: A cohort study. PLOS Neglected Trop. Dis. 2020, 14, e0008467. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Gil-Santana, H.R.; Valka Alves, R.J.; Alencar, J. Aedes aegypti invades Trindade Island, 1,140 km from the Brazilian coast, in the South Atlantic. J. Am. Mosq. Control Assoc. 2020, 36, 112–114. [Google Scholar] [CrossRef]
- Andrade, J.N.; Neto, E.M.C.; Brandão, H.N.; Lucchese, A.M.; Neto, E.B.N.; Peixoto, T.M. Avaliação de extratos de Phyllanthus acuminatus Vahl (Phyllantaceae) na mortalidade de larvas de Aedes aegypti Linnaeus, 1762 (Culicidae). Brazil. J. Dev. 2021, 7, 5278–5295. [Google Scholar] [CrossRef]
- Júnior, P.P.G.; Bezerra, A.C.; Ferraz, E.X.L. Análise espacial de casos de dengue em município no semiárido pernambucano. Res. Soc. Dev. 2021, 10, e8510615473. [Google Scholar] [CrossRef]
- Campos, M.; Spenassatto, C.; Macoris, M.L.G.; Paduan, K.S.; Pinto, J.; Ribolla, P.E.M. Seasonal population dynamics and the genetic structure of the mosquito vector Aedes aegypti in São Paulo, Brazil. Ecol. Evol. 2012, 2, 2794–2802. [Google Scholar] [CrossRef]
- Gambarra, W.P.T.; Martins, W.F.S.; Lucena Filho, M.L.; Albuquerque, I.M.C.; Apolinário, O.K.S.; Beserra, E.B. Spatial distribution and esterase activity in populations of Aedes (Stegomyia) aegypti (Linnaeus) resistant to temephos. Rev. Soc. Bras. Med. Trop. 2013, 46, 178–184. [Google Scholar] [CrossRef]
- Padilla-Torres, S.D.; Ferraz, G.; Luz, S.L.B.; Zamora-Perea, E.; Abad-Franch, F. Modeling dengue vector dynamics under imperfect detection: Three years of site-occupancy by Aedes aegypti and Aedes albopictus in urban Amazonia. PLoS ONE 2013, 8, e58420. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.C.; Rego, R.; Maciel-de-Freitas, R. The use of the Premise Condition Index (PCI) to provide guidelines for Aedes aegypti surveys. J. Vector Ecol. 2013, 38, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.; Rosa, S.L.; Rocha, M.L.; Azevedo, T.S.; Von Zuben, C.J. Entomological surveillance, spatial distribution, and diversity of Culicidae (Diptera) immatures in a rural area of the Atlantic Forest biome, State of São Paulo, Brazil. J. Vector Ecol. 2013, 38, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Regis, L.N.; Acioli, R.V.; Silveira, J.C., Jr.; Melo-Santos, M.A.V.; Souza, W.V.; Ribeiro, C.M.; da Silva, J.C.; Monteiro, A.M.; Oliveira, C.M.; Barbosa, R.M.; et al. Sustained reduction of the dengue vector population resulting from an integrated control strategy applied in two Brazilian cities. PLoS ONE 2013, 8, e67682. [Google Scholar] [CrossRef]
- Lana, R.M.; Carneiro, T.G.S.; Honório, N.A.; Codeco, C.T. Seasonal and nonseasonal dynamics of Aedes aegypti in Rio de Janeiro, Brazil: Fitting mathematical models to trap data. Acta Trop. 2014, 129, 25–32. [Google Scholar] [CrossRef]
- Leal-Santos, F.A.; Santana, M.B.A.; Figueiredo, D.A.; Oliveira, M.M.; Acel, A.M.; Ribeiro, A.L.; Rodrigues, J.S.; Carvalho-Leandro, D.; Miyazaki, R.D.; Leite-Jr, D.P. Effective surveillance of vector dynamics of Aedes aegypti in a hospital setting in Cuiabá, Mato Grosso, Brazil. J. Infect. Dev. Ctries 2014, 8, 1356–1360. [Google Scholar] [CrossRef]
- Linss, J.G.B.; Brito, L.P.; Garcia, G.A.; Araki, A.S.; Bruno, R.V.; Lima, J.B.P.; Valle, D.; Martins, A.J. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites Vectors 2014, 7, 25. [Google Scholar] [CrossRef]
- Monteiro, F.J.C.; Carvalho, J.C.T.; Souto, R.N.P. Distribuição da oviposição e dinâmica temporal do Aedes aegypti (Linnaeus) por meio de ovitrampas. EntomoBrasilis 2014, 7, 188–192. [Google Scholar] [CrossRef]
- Pessanha, J.E.M.; Brandão, S.T.; Almeida, M.C.M.; Cunha, M.C.M.; Sonoda, I.V.; Bessa, A.M.; Nascimento, J.C. Ovitrap surveillance as dengue epidemic predictor in Belo Horizonte City, Brazil. J. Health Biol. Sci. 2014, 2, 51–56. [Google Scholar] [CrossRef]
- Regis, L.N.; Acioli, R.V.; Silveira, J.C., Jr.; Melo-Santos, M.A.V.; da Cunha, M.C.; Souza, F.; Batista, C.A.; Barbosa, R.M.; de Oliveira, C.M.; Ayres, C.F.; et al. Characterization of the spatial and temporal dynamics of the dengue vector population established in urban areas of Fernando de Noronha, a Brazilian oceanic island. Acta Trop. 2014, 137, 80–87. [Google Scholar] [CrossRef]
- Santos, N.D.L.; Napoleão, T.H.; Coelho, L.C.B.B.; Paiva, P.M.G. Evaluation of Moringa oleifera seed lectin in traps for the capture of Aedes aegypti eggs and adults under semi-field conditions. Parasitol. Res. 2014, 113, 1837–1842. [Google Scholar] [CrossRef]
- Abreu, F.V.S.; Morais, M.M.; Ribeiro, S.P.; Eiras, Á.E. Influence of breeding site availability on the oviposition behaviour of Aedes aegypti. Mem. Inst. Oswaldo Cruz 2015, 110, 669–676. [Google Scholar] [CrossRef]
- Brasil, L.M.; Gomes, M.M.F.; Miosso, C.J.; Silva, M.M.; Amvame-Nze, G.D. Web platform using digital image processing and GIS tools: A Brazilian case study on dengue. Biomed. Eng. Online 2015, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef] [PubMed]
- Cecílio, S.G.; Silva Júnior, W.F.; Tótola, A.H.; Magalhães, C.L.B.; Ferreira, J.M.S.; Magalhães, J.C. Dengue virus detection in Aedes aegypti larvae from Southeastern Brazil. J. Vector Ecol. 2015, 40, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Chapadense, F.G.G.; Fernandes, E.K.K.; Lima, J.B.P.; Martins, A.J.; Silva, L.C.; Rocha, W.T.; Santos, A.H.; Cravo, P. Phenotypic and genotypic profile of pyrethroid resistance in populations of the mosquito Aedes aegypti from Goiânia, Central West Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 607–609. [Google Scholar] [CrossRef]
- Cruz, L.C.T.A.; Serra, O.P.; Leal-Santos, F.A.; Ribeiro, A.L.M.; Slhessarenko, R.D.; Santos, M.A. Natural transovarial transmission of dengue virus 4 in Aedes aegypti from Cuiabá, State of Mato Grosso, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 18–25. [Google Scholar] [CrossRef]
- Dias, C.N.; Alves, L.P.L.; Rodrigues, K.A.F.; Brito, M.C.A.; Rosa, C.S.; Amaral, F.M.; Monteiro, O.D.; Andrade, E.H.; Maia, J.G.; Moraes, D.F. Chemical composition and larvicidal activity of essential oils extracted from Brazilian Legal Amazon plants against Aedes aegypti L. (Diptera: Culicidae). Evid.-Based Complement. Altern. Med. 2015, 2015, 490765. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.T.; Trindade, F.T.T.; Stabeli, R.G.; Silva, A.A.E.; Militão, J.S.L.T.; Facundo, V.A. Essential oils of leaves of Piper species display larvicidal activity against the dengue vector, Aedes aegypti (Diptera: Culicidae). Rev. Bras. Plantas Med. 2015, 17, 105–111. [Google Scholar] [CrossRef]
- Schultes, O.L.; Morais, M.H.F.; Cunha, M.C.M.; Sobral, A.; Caiaffa, W.T. Spatial analysis of dengue incidence and Aedes aegypti ovitrap surveillance in Belo Horizonte, Brazil. Trop. Med. Int. Health 2021, 26, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.A.; Silva, J.C.; Oliveira, J.B.B.S.; Abreu, F.V.S. Study of oviposition behavior of Aedes aegypti in two neighborhoods under the influence of semi-arid climate in the municipality of Salinas, State of Minas Gerais, Brazil. Rev. Patol. Trop. 2015, 44, 77–88. [Google Scholar]
- Taranto, M.F.R.; Pessanha, J.E.M.; Santos, M.; Andrade, A.C.S.P.; Camargos, V.N.; Alves, S.N.; Oliveira, C.D.L.; Taranto, A.G.; Santos, L.L.; Magalhães, J.C.; et al. Dengue outbreaks in Divinópolis, south-eastern Brazil and the geographic and climatic distribution of Aedes albopictus and Aedes aegypti in 2011–2012. Trop. Med. Int. Health 2015, 20, 77–88. [Google Scholar] [CrossRef]
- Wermelinger, E.D.; Ferreira, A.P.; Carvalho, R.W.; Silva, A.A.; Benigno, C.V. Aedes aegypti eggs oviposited on water surface collected from field ovitraps in Nova Iguaçu City, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 770–772. [Google Scholar] [CrossRef]
- Aguirre-Obando, O.A.; Pietrobon, A.J.; Dalla Bona, A.C.; Navarro-Silva, M.A. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in Aedes aegypti populations from Jacarezinho (Brazil) after a dengue outbreak. Rev. Bras. Entomol. 2016, 60, 94–100. [Google Scholar] [CrossRef]
- Chediak, M.; Pimenta, F.G., Jr.; Coelho, G.E.; Braga, I.A.; Lima, J.B.P.; Cavalcante, K.R.L.J.; Sousa, L.C.; Melo-Santos, M.A.V.; Macoris, M.L.G.; Araújo, A.P.; et al. Spatial and temporal country-wide survey of temephos resistance in Brazilian populations of Aedes aegypti. Mem. Inst. Oswaldo Cruz 2016, 111, 311–321. [Google Scholar] [CrossRef]
- Costa, C.F.; Passos, R.A.; Lima, J.B.P.; Roque, R.A.; Sampaio, V.S.; Campolina, T.B.; Secundino, N.F.C.; Pimenta, P.F.P. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: A local model of xenomonitoring. Parasites Vectors 2017, 10, 249. [Google Scholar] [CrossRef]
- Dias, L.S.; Macoris, M.L.G.; Andrighetti, M.T.M.; Otrera, V.C.G.; Dias, A.S.; Bauzer, L.G.S.R.; Rodovalho, C.D.; Martins, A.J.; Lima, J.B. Toxicity of spinosad to temephos-resistant Aedes aegypti populations in Brazil. PLoS ONE 2017, 12, e0173689. [Google Scholar]
- Fontoura, N.G.; Bellinato, D.F.; Valle, D.; Lima, J.B.P. The efficacy of a chitin synthesis inhibitor against field populations of organophosphate-resistant Aedes aegypti in Brazil. Mem. Inst. Oswaldo Cruz 2012, 107, 387–395. [Google Scholar] [CrossRef]
- Hendy, A.; Valério, D.; Fé, N.F.; Hernandez-Acosta, E.; Mendonça, C.; Andrade, E.; Pedrosa, I.; Costa, E.R.; Andes Júnior, J.T.; Assunção, F.P.; et al. Microclimate and the vertical stratification of potential bridge vectors of mosquito-borne viruses captured by nets and ovitraps in a central Amazonian forest bordering Manaus, Brazil. Sci. Rep. 2021, 11, 21129. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.P.; Maciel, J.S.; Leite, G.R.; Souza, M.A.A. Influence of shading and pedestrian traffic on the preference of Aedes (Stegomyia) aegypti (Diptera: Culicidae) for oviposition microenvironments. J. Vector Ecol. 2017, 42, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.C.; Pinto, R.C.; Sadahiro, M.; Sampaio, V.S.; Castro, D.B.; Terrazas, W.C.M.; Mustafa, L.M.; Costa, C.F.; Passos, R.A.; Lima, J.B.P.; et al. Relationship between local presence and density of Aedes aegypti eggs with dengue cases: A spatial analysis approach. Trop. Med. Int. Health 2018, 23, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, T.; Câmara, D.C.P.; Morone, F.C.; Gonçalves, L.S.; Barros, F.S.M.; Brasil, P.; Carvalho, M.S.; Honorio, N.A. Dispersion and oviposition of Aedes albopictus in a Brazilian slum: Initial evidence of Asian tiger mosquito domiciliation in urban environments. PLoS ONE 2018, 13, e0195014. [Google Scholar] [CrossRef]
- Garcia, G.A.; David, M.R.; Martins, A.J.; Maciel-de-Freitas, R.; Linss, J.G.B.; Araújo, S.C.; Lima, J.B.; Valle, D. The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLoS Negl. Trop. Dis. 2018, 12, e0006227. [Google Scholar] [CrossRef]
- Garziera, L.; Pedrosa, M.C.; Souza, F.A.; Gomez, M.; Moreira, M.B.; Virginio, J.F.; Capurro, M.L.; Carvalho, D.O. Effect of interruption of over-flooding releases of transgenic mosquitoes over wild population of Aedes aegypti: Two case studies in Brazil. Entomol. Exp. Appl. 2017, 164, 327–339. [Google Scholar] [CrossRef]
- La Corte, R.; Melo, V.A.D.; Dolabella, S.S.; Marteis, L.S. Variation in temephos resistance in field populations of Aedes aegypti (Diptera: Culicidae) in the State of Sergipe, Northeast Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 284–290. [Google Scholar] [CrossRef]
- Dos Santos, T.P.; Roiz, D.; Abreu, F.V.S.; Luz, S.L.B.; Santalucia, M.; Jiolle, D.; Santos Neves, M.S.; Simard, F.; Lourenço-de-Oliveira, R.; Paupy, C. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Sacramento, R.H.M.; Araújo, F.M.C.; Lima, D.M.; Alencar, C.H.; Martins, V.E.P.; Araújo, L.V.; Oliveira, T.C.; Cavalcanti, L.P.G. Dengue fever and Aedes aegypti in indigenous Brazilians: Seroprevalence, risk factors, knowledge and practices. Trop. Med. Int. Health 2018, 23, 596–604. [Google Scholar] [CrossRef]
- Silva, W.R.; Soares-da-Silva, J.; Ferreira, F.A.S.; Rodrigues, I.B.; Tadei, W.P.; Zequi, J.A.C. Oviposition of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) under laboratory and field conditions using ovitraps associated to different control agents, Manaus, Amazonas, Brazil. Rev. Bras. Entomol. 2018, 62, 304–310. [Google Scholar] [CrossRef]
- Noleto, J.V.O.; Moraes, H.L.M.N.; Lima, T.M.; Rodrigues, J.G.M.; Cardoso, D.T.; Lima, K.C.; Melo, R.S.S.; Miranda, G.S. Use of ovitraps for the seasonal and spatial monitoring of Aedes spp. in an area endemic for arboviruses in Northeast Brazil. J. Infect. Dev. Ctries 2020, 14, 387–393. [Google Scholar] [CrossRef]
- Piovezan, R.; Visockas, A.; Azevedo, T.S.; Von Zuben, C.J.; Sallum, M.A.M. Spatial–temporal distribution of Aedes (Stegomyia) aegypti and locations of recycling units in southeastern Brazil. Parasites Vectors 2019, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Sá, E.L.R.; Rodovalho, C.M.; Sousa, N.P.R.; Sá, I.L.R.; Bellinato, D.F.; Dias, L.S.; Silva, L.C.; Martins, A.J.; Lima, J.B.P. Evaluation of insecticide resistance in Aedes aegypti populations connected by roads and rivers: The case of Tocantins state in Brazil. Mem. Inst. Oswaldo Cruz 2019, 114, e180318. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.F.; Maitra, A.; Galardo, A.K.R.; Scarpassa, V.M. First record of Aedes (Stegomyia) albopictus in the state of Amapá, northern Brazil. Acta Amaz. 2019, 49, 36–40. [Google Scholar] [CrossRef]
- Soares, E.N.L.; Santos, M.A.B.; Macedo, L.O.; Santos, C.V.B.; Agra, M.C.R.; Alves, L.C.; Ramos, R.A.N.; Carvalho, G.A.C. Spatial distribution of Aedes aegypti (Diptera: Culicidae) in vulnerable areas for the transmission of arboviruses. J. Bras. Soc. Trop. Med. 2019, 52, e20180341. [Google Scholar] [CrossRef]
- Alencar, J.; Mello, C.F.; Guimarães, A.É.; Maia, D.A.; Balbino, V.Q.; Freitas, M.T.S.; Marcondes, C.B. The first detection of a population of Aedes aegypti in the Atlantic Forest in the state of Rio de Janeiro, Brazil. Trop. Zool. 2020, 33, 77–82. [Google Scholar] [CrossRef]
- Barbosa, R.M.R.; Melo-Santos, M.A.V.; Silveira, J.C., Jr.; Silva-Filha, M.H.N.L.; Souza, W.V.; Oliveira, C.M.F.; Ayres, C.F.J.; Xavier, M.N.; Rodrigues, M.P.; Santos, S.A.; et al. Infestation of an endemic arbovirus area by sympatric populations of Aedes aegypti and Aedes albopictus in Brazil. Mem. Inst. Oswaldo Cruz 2020, 115, e190437. [Google Scholar] [CrossRef]
- Carvalho, B.L.; Germano, R.N.L.; Braga, K.M.L.; Araújo, E.R.F.; Rocha, D.A.; Obara, M.T. Susceptibility of Aedes aegypti populations to Pyriproxyfen in the Federal District of Brazil. J. Bras. Soc. Trop. Med. 2020, 53, e20190489. [Google Scholar]
- Leandro, A.S.; Lopes, R.D.; Martins, C.A.; Rivas, A.V.; da Silva, I.; Galvão, S.R.; Maciel-de-Freitas, R. The adoption of the One Health approach to improve surveillance of venomous animal injury, vector-borne and zoonotic diseases in Foz do Iguaçu, Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0009109. [Google Scholar] [CrossRef]
- MacCormack-Gelles, B.; Lima Neto, A.S.; Sousa, G.S.; do Nascimento, O.J.; Castro, M.C. Evaluation of the usefulness of Aedes aegypti rapid larval surveys to anticipate seasonal dengue transmission between 2012–2015 in Fortaleza, Brazil. Acta Trop. 2020, 205, 105391. [Google Scholar] [CrossRef]
- Maia, D.A.; Bastos, A.Q.; Leite, P.J.; Gil-Santana, H.R.; Silva, J.S.; Alencar, J. Comparative analysis between sampling methods for immature mosquitoes in an Atlantic Forest fragment in Brazil. J. Am. Mosq. Control Assoc. 2020, 36, 245–248. [Google Scholar] [CrossRef]
- Moura, M.C.B.M.; Oliveira, J.V.; Pedreira, R.M.; Tavares, A.M.; Souza, T.A.; Lima, K.C.; Barbosa, I.R. Spatio-temporal dynamics of Aedes aegypti and Aedes albopictus oviposition in an urban area of northeastern Brazil. Trop. Med. Int. Health 2020, 25, 1510–1521. [Google Scholar] [CrossRef]
- Nascimento, K.L.C.; Silva, J.F.M.; Zequi, J.A.C.; Lopes, J. Comparison between larval survey index and positive ovitrap index in the evaluation of populations of Aedes (Stegomyia) aegypti (Linnaeus, 1762) north of Paraná, Brazil. Environ. Health Insights 2020, 14, 1178630219886570. [Google Scholar] [CrossRef]
- Pedrosa, M.C.; Borges, M.A.Z.; Eiras, Á.E.; Caldas, S.; Cecílio, A.B.; Brito, M.F.; Ribeiro, S.P. Invasion of tropical montane cities by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) depends on continuous warm winters and suitable urban biotopes. J. Med. Entomol. 2021, 58, 333–342. [Google Scholar] [CrossRef]
- Sá, G.; Gómez-Hernández, C.; Rezende-Oliveira, K. Ovitrap to monitor the incidence of Aedes aegypti. Int. J. Mosq. Res. 2020, 6, 40–44. [Google Scholar]
- Santos, V.S.V.; Limongi, J.E.; Pereira, B.B. Association of low concentrations of pyriproxyfen and spinosad as an environment-friendly strategy to rationalize Aedes aegypti control programs. Chemosphere 2020, 247, 125795. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.M.; Rosário, I.N.G.; Silva, I.M. Distribution and preference for oviposition sites of Aedes albopictus (Skuse) in the metropolitan area of Belém, in the Brazilian Amazon. J. Vector Ecol. 2020, 45, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, S.F.; Silva, K.A.; Vasconcelos, R.B.; Sousa, I.V.; Mesquita, L.P.S.; Barakat, R.D.M.; Fernandes, H.M.C.; Queiroz, A.C.M.; Santos, G.P.G.; Filho, V.C.B.; et al. Scaling up of eco-bio-social strategy to control Aedes aegypti in highly vulnerable areas in Fortaleza, Brazil: A cluster, non-randomized controlled trial protocol. Int. J. Environ. Res. Public Health 2021, 18, 1278. [Google Scholar] [CrossRef]
- Martinez, J.M.; Rodrigues, J.; Marreto, R.N.; Mascarin, G.M.; Fernandes, É.K.K.; Humber, R.A.; Luz, C. Efficacy of focal applications of a mycoinsecticide to control Aedes aegypti in Central Brazil. Appl. Microbiol. Biotechnol. 2021, 105, 8703–8714. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Silva, J.S.; Viana, J.L.; Tadei, W.P.; Pinheiro, V.C.S. Efficiency of oviposition traps with biolarvicides for monitoring Aedes aegypti and Aedes albopictus (Diptera, Culicidae), northeast Brazil. Acta Sci. Biol. Sci. 2022, 44, e59231. [Google Scholar] [CrossRef]
- da Silva, K.R.; da Silva, W.R.; Silva, B.P.; Arcos, A.N.; da Silva Ferreira, F.A.; Soares-da-Silva, J.; Pontes, G.O.; Roque, R.A.; Tadei, W.P.; Navarro-Silva, M.A.; et al. New traps for the capture of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse) (Diptera: Culicidae) eggs and adults. PLoS Negl. Trop. Dis. 2021, 15, e0008813. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; de Brito, B.B.; Correia, T.M.L.; Viana, A.I.S.; Carvalho, J.C.; da Silva, F.A.F.; Santos, M.L.; da Silveira, E.A.; Neto, H.P.; da Silva, N.M.; et al. Simultaneous circulation of Zika, Dengue, and Chikungunya viruses and their vertical co-transmission among Aedes aegypti. Acta Trop. 2021, 215, 105819. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gendriz, I.; de Souza, G.F.; de Andrade, I.G.M.; Doria Neto, A.D.; Tavares, A.M.; Barros, D.M.S.; de Morais, A.H.; Galvão-Lima, L.J.; de Medeiros Valentim, R.A. Data-driven computational intelligence applied to dengue outbreak forecasting: A case study at the scale of the city of Natal, RN-Brazil. Sci. Rep. 2022, 12, 6550. [Google Scholar] [CrossRef]
- do Nascimento, J.F.; Palioto-Pescim, G.F.; Pescim, R.R.; Suganuma, M.S.; Zequi, J.A.C.; Golias, H.C. Influence of abiotic factors on the oviposition of Aedes (Stegomyia) aegypti (Diptera: Culicidae) in Northern Paraná, Brazil. Int. J. Trop. Insect Sci. 2022, 42, 2215–2220. [Google Scholar] [CrossRef]
- Pereira, L.P.; Teixeira, C.W.; Rodrigues, M.F.R.; Marteleto, N.C.; da Silva, S.I.A.; Pujoni, D.G.F.; Casteluber, M.C.F. Contribution of ovitraps in the control of the Aedes aegypti vector and reduction of dengue cases in the municipality of Ibirité in Minas Gerais. Uningá Rev. 2022, 37, eURJ4418. [Google Scholar] [CrossRef]
- Piovezan, R.; de Azevedo, T.S.; Faria, E.; Veroneze, R.; Von Zuben, C.J.; Von Zuben, F.J.; Sallum, M.A.M. Assessing the effect of Aedes (Stegomyia) aegypti (Linnaeus, 1762) control based on machine learning for predicting the spatiotemporal distribution of eggs in ovitraps. Dialogues Health 2022, 1, 100003. [Google Scholar] [CrossRef]
- de Souza, S.J.P.; de Camargo Guaraldo, A.; Honório, N.A.; Câmara, D.C.P.; Sukow, N.M.; Machado, S.T.; dos Santos, C.N.D.; da Costa-Ribeiro, M.C.V. Spatial and temporal distribution of Aedes aegypti and Aedes albopictus oviposition on the coast of Paraná, Brazil, a recent area of dengue virus transmission. Trop. Med. Infect. Dis. 2022, 7, 246. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes Silva Chagas do Nascimento, R.; da Silva Xavier, A.; Ayllón Santiago, T.; Câmara, D.C.P.; dos Reis, I.C.; Delatorre, E.; de Sequeira, P.C.; Ferreira-de-Lima, V.H.; Lima-Camara, T.N.; Honório, N.A. Systematic Review of the Ovitrap Surveillance of Aedes Mosquitoes in Brazil (2012–2022). Trop. Med. Infect. Dis. 2025, 10, 212. https://doi.org/10.3390/tropicalmed10080212
Fernandes Silva Chagas do Nascimento R, da Silva Xavier A, Ayllón Santiago T, Câmara DCP, dos Reis IC, Delatorre E, de Sequeira PC, Ferreira-de-Lima VH, Lima-Camara TN, Honório NA. Systematic Review of the Ovitrap Surveillance of Aedes Mosquitoes in Brazil (2012–2022). Tropical Medicine and Infectious Disease. 2025; 10(8):212. https://doi.org/10.3390/tropicalmed10080212
Chicago/Turabian StyleFernandes Silva Chagas do Nascimento, Raquel, Alexandre da Silva Xavier, Tania Ayllón Santiago, Daniel Cardoso Portela Câmara, Izabel Cristina dos Reis, Edson Delatorre, Patrícia Carvalho de Sequeira, Vitor Henrique Ferreira-de-Lima, Tamara Nunes Lima-Camara, and Nildimar Alves Honório. 2025. "Systematic Review of the Ovitrap Surveillance of Aedes Mosquitoes in Brazil (2012–2022)" Tropical Medicine and Infectious Disease 10, no. 8: 212. https://doi.org/10.3390/tropicalmed10080212
APA StyleFernandes Silva Chagas do Nascimento, R., da Silva Xavier, A., Ayllón Santiago, T., Câmara, D. C. P., dos Reis, I. C., Delatorre, E., de Sequeira, P. C., Ferreira-de-Lima, V. H., Lima-Camara, T. N., & Honório, N. A. (2025). Systematic Review of the Ovitrap Surveillance of Aedes Mosquitoes in Brazil (2012–2022). Tropical Medicine and Infectious Disease, 10(8), 212. https://doi.org/10.3390/tropicalmed10080212






