Effectiveness of Pyrethroid-Piperonyl Butoxide Nets Versus Standard Pyrethroid-Only Nets in Preventing Malaria in Children Under 10 Years Living in Kisantu Health Zone, Democratic Republic of the Congo
Abstract
1. Introduction
2. Materials and Methods
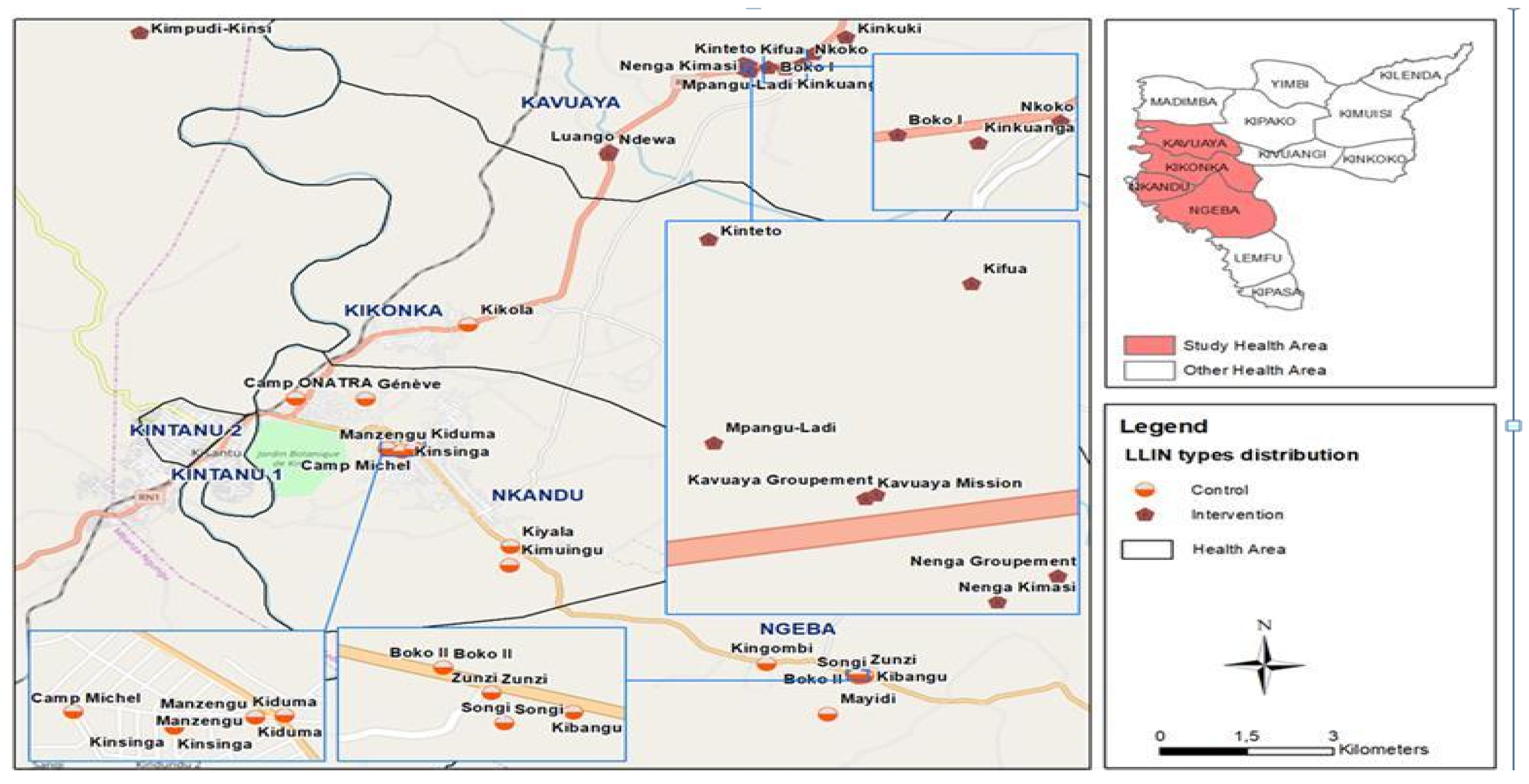
2.1. Study Area
2.2. Design
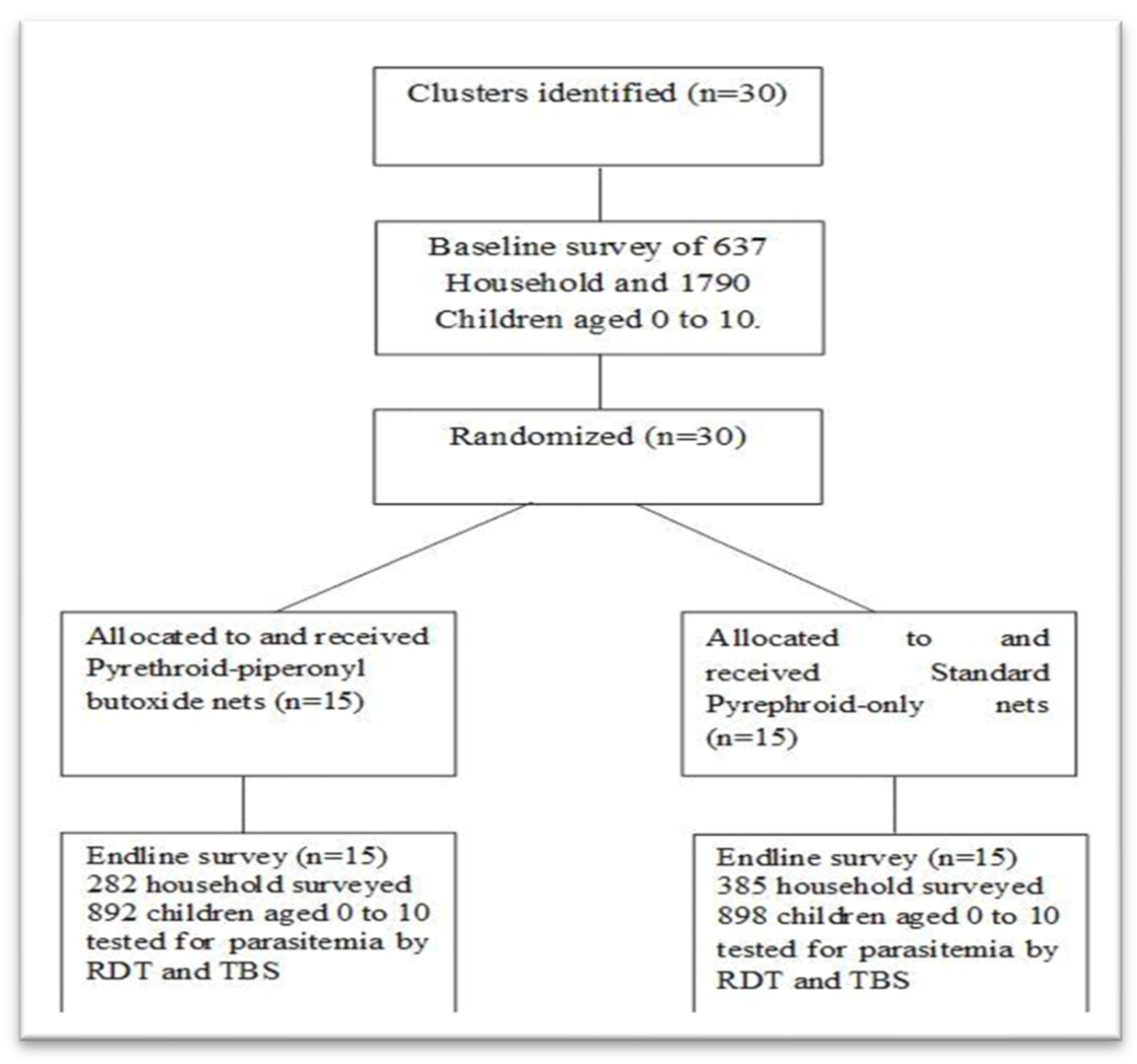
2.3. Study Population and Sample Size
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Data Collection and Study Procedures
2.4.1. Case Surveillance
2.4.2. Mosquito Net Distribution
2.4.3. Mosquito Collection and Transmission Risk Determination
2.4.4. Durability Surveys: Physical Integrity and Bio-Efficacy of LLINs
2.5. Operational Definition of Key Study Variables
- The sporozoite rate = (total CSP ELISA positive/total number tested) × 100;
- Human biting rate (HBR) = total # of each Anopheles species collected by HLCs during a specific period/total number of trap nights;
- Nightly EIR = nightly HBR × sporozoite rate;
- Monthly EIR = nightly mean EIR × number of nights in the month;
- Physical net survival: the proportion of cohort nets received from the LLIN campaign still in serviceable physical condition (WHO 2013 estimating functional of LLIN).
2.5.1. Outcomes
2.5.2. Randomization and Masking
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
3.1. General Characteristics of Inclusion
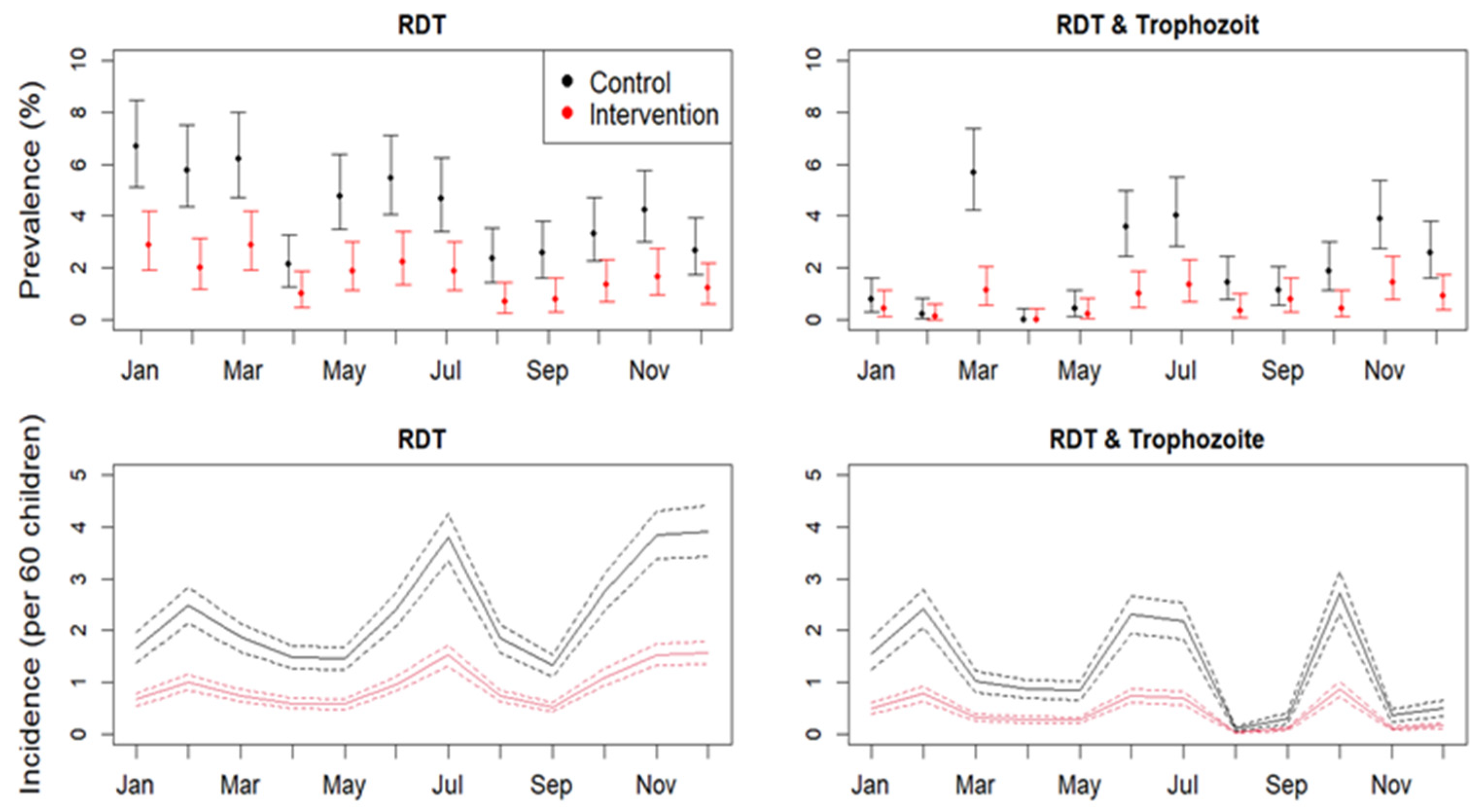
3.2. Incidence of Malaria
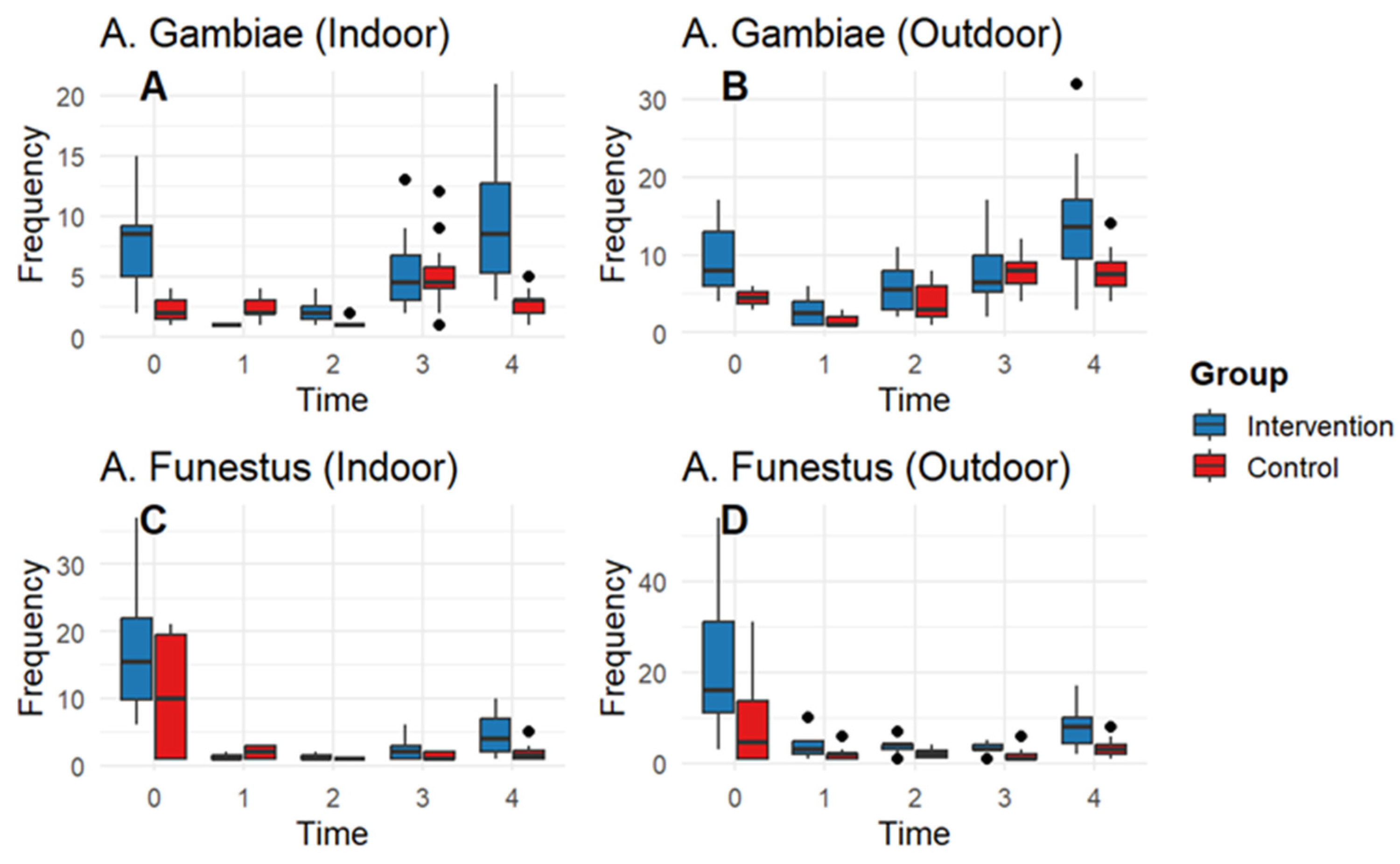
3.3. Entomological Impact
3.4. LLIN Physical Integrity, WHO Cone Bio-Assays and LLIN Chemical Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deutsch-Feldman, M.; Parr, J.B.; Keeler, C.; Brazeau, N.F.; Goel, V.; Emch, M.; Edwards, J.K.; Kashamuka, M.; Tshefu, A.K.; Meshnick, S.R. The Burden of Malaria in the Democratic Republic of the Congo. J. Infect. Dis. 2021, 223, 1948–1952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024; Licence: CC BY-NC-SA 3.0 IGO.
- World Malaria Report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo (accessed on 21 December 2024).
- République Démocratique du Congo MdlSPPNdLClPP (2016) Plan Stratégique National de Communication 2017–2020. Available online: https://pnlprdc.org/psn/ (accessed on 9 February 2017).
- Babalola, S.; Kumoji, K.; Awantang, G.N.; Oyenubi, O.A.; Toso, M.; Tsang, S.; Bleu, T.; Achu, D.; Hedge, J.; Schnabel, D.C.; et al. Ideational factors associated with consistent use of insecticide-treated nets: A multi-country, multilevel analysis. Malar. J. 2022, 21, 374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Philippe, C.M.; Odile, N.N.; Numbi, O.L. The problem of the use of Long-Lasting Insecticide Impregnated Mosquito Nets (LLIN) in children less than five years of age in Democratic Republic of Congo. Pan Afr. Med. J. 2016, 23, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macintyre, K.; Littrell, M.; Keating, J.; Hamainza, B.; Miller, J.; Eisele, T.P. Determinants of hanging and use of ITNs in the context of near universal coverage in Zambia. Health Policy Plan. 2012, 27, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Awolola, T.; Oyewole, I.; Amajoh, C.; Idowu, E.; Ajayi, M.; Oduola, A.; Manafa, O.; Ibrahim, K.; Koekemoer, L.; Coetzee, M. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop. 2005, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Recommended Long-Lasting Insecticidal Mosquito Nets; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kleinschmidt, I.; Bradley, J.; Knox, T.B.; Mnzava, A.P.; Kafy, H.T.; Mbogo, C.; Ismail, B.A.; Bigoga, J.D.; Adechoubou, A.; Raghavendra, K.; et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: A WHO-coordinated, prospective. Lancet Infect. Dis. 2018, 18, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K.; et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wat’senga, F.; Manzambi, E.Z.; Lunkula, A.; Mulumbu, R.; Mampangulu, T.; Lobo, N.; Hendershot, A.; Fornadel, C.; Jacob, D.; Niang, M.; et al. Nationwide insecticide resistance status and biting behaviour of malaria vector species in the Democratic Republic of Congo. Malar. J. 2018, 17, 129. [Google Scholar] [CrossRef]
- President’s Malaria Initiative. The President’s Malaria Initiative (PMI) Project. Indoor Residual Spraying Task Order Six; President’s Malaria Initiative: Kinshasa, Democratic Republic of Congo, 2019. [Google Scholar]
- Churcher, T.S.; Lissenden, N.; Griffin, J.T.; Worrall, E.; Ranson, H.; Kingdom, U. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife 2016, 5, e16090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilombe, G.; Matangila, J.R.; Lulebo, A.; Mutombo, P.; Linsuke, S.; Maketa, V.; Mabanzila, B.; Wat’senga, F.; Van Bortel, W.; Fiacre, A.; et al. Malaria among children under 10 years in 4 endemic health areas in Kisantu Health Zone: Epidemiology and transmission. Malar. J. 2023, 22, 3. [Google Scholar] [CrossRef]
- Djènontin, A.; Moiroux, N.; Bouraïma, A.; Zogo, B.; Sidick, I.; Corbel, V.; Pennetier, C. Field efficacy of a new deltamethrin long lasting insecticidal net (LifeNet©) against wild pyrethroid-resistant Anopheles gambiae in Benin. BMC Public Health 2018, 18, 947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouattara, A.F.; Dagnogo, M.; Olliaro, P.L.; Raso, G.; Tanner, M.; Utzinger, J.; Koudou, B.G. Plasmodium falciparum infection and clinical indicators in relation to net coverage in central Côte d’Ivoire. Parasites Vectors 2014, 7, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pennetier, C.; Bouraima, A.; Chandre, F.; Piameu, M.; Etang, J.; Rossignol, M.; Sidick, I.; Zogo, B.; Lacroix, M.-N.; Yadav, R.; et al. Efficacy of Olyset® Plus, a new long-lasting insecticidal net incorporating permethrin and piperonil-butoxide against multi-resistant malaria vectors. PLoS ONE 2013, 8, e75134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malima, R.; Tungu, P.K.; Mwingira, V.; Maxwell, C.; Magesa, S.M.; Kaur, H.; Kirby, M.J.; Rowland, M. Evaluation of the long-lasting insecticidal net Interceptor LN: Laboratory and experimental hut studies against anopheline and culicine mosquitoes in northeastern Tanzania. Parasites Vectors 2013, 6, 296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngufor, C.; Tungu, P.; Malima, R.; Kirby, M.; Kisinza, W.; Rowland, M. Insecticide-treated net wall hangings for malaria vector control: An experimental hut study in north-eastern Tanzania. Malar. J. 2014, 13, 366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agossa, F.R.; Padonou, G.G.; Gnanguenon, V.; Oké-Agbo, F.; Zola-Sahossi, J.; Dègnonvi, H.; Salako, A.; Sèzonlin, M.; Akogbéto, M.C. Laboratory and field evaluation of the impact of washings on the effectiveness of LifeNet®, Olyset® and PermaNet® 2.0 in two areas, where there is a high level of resistance of Anopheles gambiae to pyrethroids, Benin, West Africa. Malar. J. 2014, 13, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Birhanu, A.; Asale, A.; Yewhalaw, D. Bio-efficacy and physical integrity of piperonylbutoxide coated combination net (PermaNet® 3.0) against pyrethroid resistant population of Anopheles gambiae s.l. and Culex quinquefasciatus mosquitoes in Ethiopia. Malar. J. 2019, 18, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briët, O.J.; Penny, M.A.; Hardy, D.; Awolola, T.S.; Van Bortel, W.; Corbel, V.; Dabiré, R.K.; Etang, J.; Koudou, B.G.; Tungu, P.K.; et al. Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: A modelling study. Malar. J. 2013, 12, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- INS, Enquête par grappes à indicateurs multiples, 2017-2018, Résumés statistiques: Résultats clés de l’enquête. Kinshasa, République Démocratique du Congo. Available online: https://www.unicef.org/drcongo/rapports (accessed on 12 December 2020).
- Groupe de Révision des Preuves de l’OMS—ERG: Conditions de Déploiement des Moustiquaires Traitées avec un Pyréthrinoïde et du Butoxyde de Pipéronyle; Organisation Mondiale de la Santé: Genève, Switzerland, 2017; Volume 3.
- Lignes directrices de l’OMS sur le paludisme, 16 février 2021 [WHO Guidelines for Malaria, 16 February 2021]; Organisation mondiale de la santé: Genève, Switzerland, 2021. (WHO/UCN/GMP/2021.01). Licence: CC BY-NC-SA 3.0 IGO.
- Lynd, A.; Oruni, A.; Van’t Hof, A.E.; Morgan, J.C.; Naego, L.B.; Pipini, D.; O’Kines, K.A.; Bobaga, T.L.; Donnelly, M.J.; Weetman, D. Insecticide resistance in Anopheles gambiae from the northern Democratic Republic of Congo, with externe knockdown resistance (kdr) mutation frequencies revealed by a new diagnostic assay. Malar J. 2008, 17, 412. [Google Scholar] [CrossRef]
- WHO. Guidelines for Monitoring the Durability of Long-Lasting Insecticidal Mosquito Nets Under Operational Conditions. Geneva: World Health Organization; 2011. WHO/HTM/NTD/WHOPES/2011.5. Available online: http://whqlibdoc.who.int/publications/2011/9789241501705_eng.pdf (accessed on 25 October 2019).
- Kelly-Hope, L.A.; McKenzie, F.E. The multiplicity of malaria transmission: A review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar. J. 2009, 8, 19. [Google Scholar] [CrossRef]
- Piorek, S. Field-portable X-ray fluorescence spectrometry: Past, present, and future. Field Anal. Chem. Technol. 1997, 1, 317–329. [Google Scholar] [CrossRef]
- Drakeley, C.; Schellenberg, D.; Kihonda, J.; Sousa, C.A.; Arez, A.P.; Lopes, D.; Lines, J.; Mshinda, H.; Lengeler, C.; Schellenberg, J.A.; et al. An estimation of the entomological inoculation rate for Ifakara: A semi-urban area in a region of intense malaria transmission in Tanzania. Trop. Med. Int. Health 2003, 8, 767–774. [Google Scholar] [CrossRef]
- Mansiangi, P.; Umesumbu, S.; Etewa, I.; Zandibeni, J.; Bafwa, N.; Blaufuss, S.; Olapeju, B.; Ntoya, F.; Sadou, A.; Irish, S.; et al. Comparing the durability of the long—Lasting insecticidal nets D in northwest Democratic Republic of Congo. Malar. J. 2020, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- N’guessan, R.; Odjo, A.; Ngufor, C.; Malone, D.; Rowland, M. A chlorfenapyr mixture net Interceptor® G2 shows high efficacy and wash durability against resistant mosquitoes in west Africa. PLoS ONE 2016, 11, e0165925. [Google Scholar] [CrossRef] [PubMed]
- Tungu, P.K.; Michael, E.; Sudi, W.; Kisinza, W.W.; Rowland, M. Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: Experimental hut trials in north-eastern Tanzania. Malar. J. 2021, 20, 180. [Google Scholar] [CrossRef]
- Gleave, K.; Lissenden, N.; Chaplin, M.; Choi, L.; Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. 2021, 5, CD012776. [Google Scholar] [CrossRef] [PubMed]
- Lukole, E.; Cook, J.; Mosha, J.F.; Messenger, L.A.; Rowland, M.; Kleinschmidt, I.; Charlwood, J.D.; Mosha, F.W.; Manjurano, A.; Wright, A.; et al. Protective efficacy of holed and aging PBO-pyrethroid synergist-treated nets on malaria infection prevalence in north-western Tanzania. PLoS Glob. Public Health 2022, 2, e0000453. [Google Scholar] [CrossRef]
- Gichuki, P.M.; Kamau, L.; Njagi, K.; Karoki, S.; Muigai, N.; Matoke-Muhia, D.; Bayoh, N.; Mathenge, E.; Yadav, R.S. Bioefficacy and durability of Olyset® Plus, a permethrin and piperonyl butoxide-treated insecticidal net in a 3-year long trial in Kenya. Infect. Dis. Poverty 2021, 10, 135. [Google Scholar] [CrossRef]
- Protopopoff, N.; Mosha, J.F.; Lukole, E.; Charlwood, J.D.; Wright, A.; Mwalimu, C.D.; Manjurano, M.A.; Mosha, F.W.; Kisinza, W.; Kleinschmidt, I.; et al. Effectiveness of a long lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 2018, 391, 1577–1588. [Google Scholar]
- Wiseman, V.; Hawley, W.A.; TER Kuile, F.O.; Phillips-Howard, P.A.; Vulule, J.M.; Nahlen, B.L.; Mills, J. The cost-effectiveness of permethrin-treated bed nets in an area of intense malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 2003, 68 (Suppl. S4), 161–167. [Google Scholar] [CrossRef] [PubMed]
| Variables | All | Control | Intervention |
|---|---|---|---|
| Sex | |||
| Female | 870 (48.6) | 437 (48.7) | 433 (48.5) |
| Male | 920 (51.4) | 461 (51.3) | 459 (51.4) |
| Age | |||
| <5 years | 925 (51.9) | 440 (49.0) | 485 (54.8) |
| ≥5 years | 865 (48.3) | 458 (51.0) | 407 (45.6) |
| Type of roof | |||
| Thatch/wood | 258 (14.4) | 12 (1.3) | 246 (27.6) |
| Metal sheet | 791 (44.2) | 570 (63.5) | 221 (24.8) |
| Straw | 741 (41.4) | 316 (35.2) | 425 (47.7) |
| Type of wall | |||
| Clay with pillar | 350 (19.9) | 31 (3.5) | 319 (35.8) |
| Cement brick/baked | 1393 (77.8) | 865 (96.3) | 528 (58.2) |
| Straw | 47 (2.6) | 2 (0.2) | 45 (5.0) |
| Type of floor | |||
| Cement/tiled | 687 (38.4) | 577 (64.3) | 110 (12.3) |
| Ground/bamboo | 1103 (61.6) | 321 (35.8) | 782 (87.8) |
| Wasting | |||
| Moderate/severe | 1211 (67.7) | 631 (70.3) | 580 (65.0) |
| Normal/mild | 268 (14.9) | 134 (14.9) | 134 (15.0) |
| Over | 311 (17.4) | 133 (14.8) | 178 (19.9) |
| Stunting | |||
| Moderate/severe | 608 (33.9) | 312 (34.7) | 296 (33.2) |
| Normal/mild | 967 (54.0) | 492 (54.8) | 475 (53.3) |
| Over | 215 (12.0) | 94 (10.5) | 121 (13.6) |
| Underweight | |||
| Moderate/severe | 461 (25.8) | 223 (24.8) | 238 (26.7) |
| Normal/mild | 1050 (58.7) | 539 (60.0) | 511 (57.3) |
| Over | 279 (15.6) | 136 (15.1) | 143 (16.0) |
| Thick Blood Smear | |||
| No | 1524 (85.1) | 851 (94.77) | 673 (75.5) |
| Yes | 266 (14.8) | 47 (5.3) | 219 (24.5) |
| System | Time | Intervention |
|---|---|---|
| An. gambiae_out | edf = 1.98, chisq = 132 p < 0.001 | est = −0.46; std error =0.007; z value = −613; p < 0.0001 |
| An. gambiae_in | edf = 1.97, chisq = 52.5, p < 0.0001 | est = −0.69; std error= 0.09; z value = −71; p < 0.0001 |
| An. funestus_in | edf = 1.97, chisq = 264.1, p < 0.0001 | est = −0.629; std error = 0.1135; z value = −5.5; p < 0.0001 |
| An. funestus_out | edf = 1.97, chisq = 334; p < 0.0001 | est = −0.7392; std error =0.09774; z value = −7.5; p < 0.0001 |
| Arms | Trimester | Total An Collected HLC | HLC Trap-Nights | HBR/Night | Number of An Tested | Pf Sporozoite Rate | EIR/ Night | EIR/ Month |
|---|---|---|---|---|---|---|---|---|
| Anopheles gambiae sl. | ||||||||
| Control | baseline | 16 | 180 | 0.08 | 16 | 1 | 0.08 | 2.4 |
| T1 | 38 | 180 | 0.21 | 44 | 0.05 | 0.01 | 0.3 | |
| T2 | 41 | 180 | 0.23 | 50 | 0 | 0 | 0.0 | |
| T3 | 181 | 180 | 1.00 | 97 | 0.03 | 0.03 | 0.9 | |
| T4 | 144 | 180 | 0.80 | 99 | 0.01 | 0.00 | 0.0 | |
| Intervention | baseline | 222 | 180 | 1.23 | 50 | 4 | 4.92 | 147.6 |
| T1 | 37 | 180 | 0.20 | 33 | 0.06 | 0.01 | 0.3 | |
| T2 | 85 | 180 | 0.47 | 85 | 0.40 | 0.18 | 5.4 | |
| T3 | 184 | 180 | 1.02 | 93 | 0.03 | 0.03 | 0.9 | |
| T4 | 330 | 180 | 1.83 | 100 | 0.03 | 0.05 | 1.5 | |
| Anopheles funestus sl. | ||||||||
| Control | baseline | 83 | 180 | 0.46 | 39 | 2 | 0.92 | 27.6 |
| T1 | 39 | 180 | 0.22 | 42 | 0.02 | 0.00 | 0.0 | |
| T2 | 15 | 180 | 0.08 | 17 | 0 | 0.00 | 0.0 | |
| T3 | 32 | 180 | 0.17 | 1 | 0 | 0.00 | 0.0 | |
| T4 | 65 | 180 | 0.36 | 1 | 0 | 0.00 | 0.0 | |
| Intervention | baseline | 489 | 180 | 2.71 | 50 | 0 | 0.00 | 0.0 |
| T1 | 54 | 180 | 0.30 | 55 | 0 | 0.00 | 0.0 | |
| T2 | 40 | 180 | 0.22 | 40 | 0 | 0.00 | 0.0 | |
| T3 | 69 | 180 | 0.38 | 1 | 0 | 0.00 | 0.0 | |
| T4 | 171 | 180 | 0.95 | 1 | 0 | 0.00 | 0.0 | |
| Variable | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Control | Intervention | p | Control | Intervention | p | |
| 1. Physical condition (pHI) | N = 364 | N = 369 | N = 364 | N = 368 | ||
| Good (0–64) | 359 | 369 | 0.009 | 359 | 368 | 0.009 |
| Damaged (65–642) | 5 | 0 | 5 | 0 | ||
| Torn (>642) | ||||||
| Serviceable (0–642) | ||||||
| Median pHI if any hole IQR | 2 (0) | 2 (0) | 2 (0) | 2 (0) | ||
| 2. bio-assays (WHO) | N = 30 | N = 30 | N = 30 | N = 30 | ||
| Knockdown 60 min | 0.001 | |||||
| Mean (95% CI) | 46.81 (44.69–49.00) | 53.74 (51.97–55.45) | ||||
| Median (IQR) | 48.0 (41–51) | 53.00 (52–57) | ||||
| Mortality 24 h | 0.001 | |||||
| Mean (95% CI) | 22.47 (20.19–24.69) | 46.74 (44.36–48.99) | ||||
| Median (IQR) | 23 (18–26) | 47 (45–51) | ||||
| Optimal effectiveness | 38 | 60 | ||||
| Minimal effectiveness | 8 | 26 | ||||
| 3. chemistry | N = 30 | N = 30 | N = 30 | N = 30 | ||
| Mean (95% CI) | 29.32 (24.01–35.06) | 69.14 (64–42-73.10) | 0.000 | 37.39 (32.93–42.20) | 52.78 (44.43–60.01) | 0.001 |
| Median (IQR) | 31.35 (18.15–43.10) | 72.25 (61.15–77.45) | 37.95 (30.50–43.60) | 59.60 (41.50–67.45) | ||
| SD | 16.75 (13.29–19.24) | 12.18 (7.87–16.07) | 12.20 (9.46–14.44) | 19.21 (12.30–23.15) | ||
| Roof PermaNet 3.0 (mean) | 121.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilombe, G.; Likwela, J.L.; Lukanu, P.; Lulebo, A.; Muela, N.; Mariën, J.; Mbanzulu, K.M.; Mabanzila, B.; Matangila, J.R.; Agossa, F.; et al. Effectiveness of Pyrethroid-Piperonyl Butoxide Nets Versus Standard Pyrethroid-Only Nets in Preventing Malaria in Children Under 10 Years Living in Kisantu Health Zone, Democratic Republic of the Congo. Trop. Med. Infect. Dis. 2025, 10, 172. https://doi.org/10.3390/tropicalmed10060172
Ilombe G, Likwela JL, Lukanu P, Lulebo A, Muela N, Mariën J, Mbanzulu KM, Mabanzila B, Matangila JR, Agossa F, et al. Effectiveness of Pyrethroid-Piperonyl Butoxide Nets Versus Standard Pyrethroid-Only Nets in Preventing Malaria in Children Under 10 Years Living in Kisantu Health Zone, Democratic Republic of the Congo. Tropical Medicine and Infectious Disease. 2025; 10(6):172. https://doi.org/10.3390/tropicalmed10060172
Chicago/Turabian StyleIlombe, Gillon, Joris Losimba Likwela, Philippe Lukanu, Aimée Lulebo, Nicole Muela, Joachim Mariën, Kennedy Makola Mbanzulu, Baby Mabanzila, Junior Rika Matangila, Fiacre Agossa, and et al. 2025. "Effectiveness of Pyrethroid-Piperonyl Butoxide Nets Versus Standard Pyrethroid-Only Nets in Preventing Malaria in Children Under 10 Years Living in Kisantu Health Zone, Democratic Republic of the Congo" Tropical Medicine and Infectious Disease 10, no. 6: 172. https://doi.org/10.3390/tropicalmed10060172
APA StyleIlombe, G., Likwela, J. L., Lukanu, P., Lulebo, A., Muela, N., Mariën, J., Mbanzulu, K. M., Mabanzila, B., Matangila, J. R., Agossa, F., Mukomena, E., Linsuke, S., Kalonji, A., Lutumba, P., Geertruyden, J.-P. V., & Irish, S. R. (2025). Effectiveness of Pyrethroid-Piperonyl Butoxide Nets Versus Standard Pyrethroid-Only Nets in Preventing Malaria in Children Under 10 Years Living in Kisantu Health Zone, Democratic Republic of the Congo. Tropical Medicine and Infectious Disease, 10(6), 172. https://doi.org/10.3390/tropicalmed10060172






