Abstract
Metal(loid)s and nonmetals in the soil of urban parks can pose risks to human health. Thus, we aimed to study the soil elemental content, soil types and their pH, contamination factor, geo-accumulation index, pollution load index, total carcinogenic risk, and hazard quotient for children and adults by ingestion, inhalation, and dermal routes contact in ecological parks (EP) in Central-West Brazil. In Lago do Amor EP, high concentrations of Mg and Mn and lower pH values predominate, while in the Águas do Prosa EP, there is a greater influence of Zn. Except for the range of average concentrations of Al, Fe, P, Mg, and Mn in all EP soils, the range of the average concentrations of As, Cd, Co, Pb, Cr, Cu, Mo, Se, and Zn were generally higher than those permissible limits. There is moderate contamination by Mo, Ni, Cd, and mainly Se in Lago do Amor, Anhanduí, and Sóter EPs. The geo-accumulation index revealed that Lago do Amor EP is moderately polluted by Cd. Oral ingestion was evidenced as the main route of possible contamination by heavy metals, especially for children, who presented a carcinogenic risk greater than 10−1 for As, Cr, and Ni.
Keywords:
assessment; biomonitoring; carcinogenic risk; ecotourism; hazard index; leisure; metals; pollution; savanna; urban areas 1. Introduction
Despite reductions in the deaths attributable to the types of pollution associated with lower-income groups, who tend to be more exposed and vulnerable, these reductions in deaths are offset by increases in the deaths attributable to air pollution, chemical pollution, and environmental contamination (soil and water mainly) generated by chemicals [1]. Indeed, in the current era of industrialization, pollution deteriorates the quality and diversity of life, with contamination by heavy metals and nonmetals being one of the main causes of environmental deterioration. The basic reasons are natural and anthropogenic [2]. Human exposure to heavy metals is possible through several pathways, including inhalation of dust in the air [3], ingestion of contaminated water or soil, or through the food chain, river sediments, sewage sludge, fertilizers from urban waste, agricultural chemicals such as fertilizers and pesticides, and industrial waste, such as factories that release chemicals, including mainly contaminants such as cadmium (Cd), copper (Cu), zinc (Zn), nickel (Ni), copper (Co), lead (Pb), mercury (Hg), and arsenic (As). Due to the prolonged use of phosphate fertilizers, foundry dust, and industrial waste, agricultural soils are generally contaminated by Cd, Cu, Zn, Ni, Co, Pb, and Hg [4]. In addition, heavy metals emitted into the atmosphere in large quantities during the burning process of burning domestic solid waste with wood, dirt, and dust produced by transportation, energy, metallurgy, and during building mainly cause lung diseases [5].
Street dust may have become one of the main air quality problems in the atmospheric environment [6,7]. Different studies have proved that air pollution through soil and dust not only affects people regularly exposed to it but also affects climate, agriculture, and natural environments [8,9]. Urban parks are an important part of the urban ecosystem, providing semi-natural habitats for many plant and animal species, and are also used by residents for recreation. They improve air quality; however, the environmental quality of soils can be altered due to contamination by heavy metals (Cu, Zn, Pb, Cd, As, and Hg), which influence human health after prolonged exposure [9]. According to Huang et al. (2021) [10], elements such as Hg, Cd, and Pb were the main pollutants in urban parks in Shanghai, China. They represented greater ecological risks than other metal(loid)s.
Studies carried out in China [9,11,12,13], Spain [14], India [8], and Africa [15,16] have evaluated heavy metals soil contamination and its role in population health, demonstrating that information, prevention, and control of heavy metal pollution in urban parks are necessary. According to the results of the authors, concentrations of metal(loid)s were associated with human influences, including urbanization duration, park age, agriculture, mining industry, and coal consumption [9,17]. Therefore, human health risk assessments in urban environments are very important. Some elements such as Fe, Zn, Se, P, Al, Mn, and Cr(III) are present in foods such as saffron [18], fruits [19], caviar [20], or canned fish [21]. However, Al, Se, Co, Zn, and Ni in high content cause adverse health effects. On the other hand, chromium compounds Cr(VI), As, Pb, and Cd are toxic and known human carcinogens [20,21,22].
In Brazilian cities like Campo Grande, in the State of Mato Grosso do Sul (MS), there are several urban ecological parks (EPs), places frequented daily by many people for the practice of sport and leisure. However, we support the hypothesis that the soil of these places can be contaminated and cause damage to the health of animals and especially to human health due to dermal contact, inhalation, and even direct or indirect ingestion of the soil. According to previous studies, to assess the state of pollution of metal(loid)s in the soil, several indices can be used, such as the contamination factor (CF), geo-accumulation index (Igeo), and pollutant load index (PLI) [17,18]. Under other conditions, to assess the total carcinogenic risk (TCR) or hazard quotient (HQ) by ingestion, inhalation, and dermal routes, equations established by the US Environmental Protection Agency (USEPA) are used [23]. However, there has been very little data about urban park pollution in Brazil and other countries. The limited data on pollution from urban parks in Brazil and other countries highlight a significant gap in environmental research. While urban parks are generally perceived as beneficial for health and pollution mitigation, studies indicate that they can also be sites of considerable pollution exposure, particularly from traffic-related sources [8,9,11,12,13,15,16].
As investigations to identify the sources of heavy metals in urban surface soils of ecological parks and to assess the associated risks to human health had not been previously conducted in the City of Campo Grande, we aimed to (i) determine the types of soil and its pH; (ii) quantify chemical elements such as As, Pb, Co, Cr, Al Cd, Ni, Co, Fe, Zn, Mn, Mg, Se, P, and Mo in the soils of four ecological parks located close to urban areas in the city of Campo Grande (MS), Brazil; (iii) and perform calculations of pollution rates and risk calculations to human health due to dermal, inhalation and oral contact with heavy metals present in the soil.
2. Materials and Methods
2.1. Sample Collection and Study Area Background
Soil samples were collected in the following ecological parks (EPs) located in the City of Campo Grande/MS, Brazil: Anhanduí EP, Sóter EP, Águas do Prosa EP, and Lago do Amor EP. Campo Grande is the capital and largest city of the Brazilian state of Mato Grosso do Sul in the Center-West region of the country. The location of ecological parks is presented in Figure 1. The population of the City of Campo Grande (MS) reached 898,100 people in 2022. In the surroundings of the city, there are large industries, including the shipping of cattle, meatpacking, and processing of beef, hides, skins, mate (tea), and agricultural products, particularly the extraction of soybean oil. Before collecting soil samples, the soil surface was cleaned to remove plant debris and organic residues. The collection of each sample was carried out using a shovel with a clean tip; only the core part of the soil samples was collected with a stainless steel spoon. The collections were carried out at a depth of 10 to 20 cm, making a zigzag circuit that covered the entire area. Regarding the number of samples, in each selected area, we collected 20 samples, which we mixed well and removed an amount of 1/2 kg. In addition, all soil samples were air-dried to a constant weight, then ground and sieved through a 2 mm sieve as described by Miclean et al. (2019) [24] and Rosa et al. (2022) [25]. Sampling was conducted during June and July 2022.
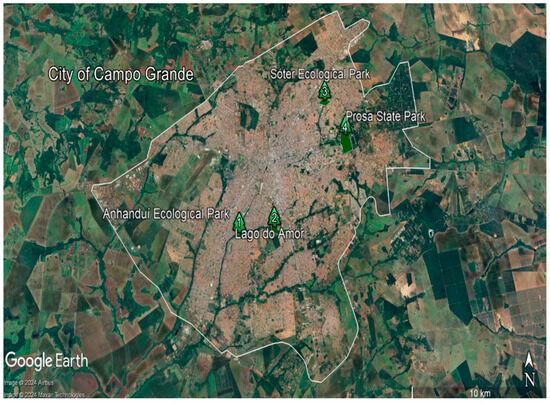
Figure 1.
Location of ecological parks: 1—Anhanduí EP (20°30′22.9″ S 54°38′34.5″ W), 2—Lago do Amor EP (20°30′07.5″ S, 54°37′07.3″ W), 3—Sóter EP (20°25′42.2″ S, 54°34′36.6″ W), and 4—Águas do Prosa EP (20°26′53.3″ S, 54°33′37.8″ W). (Satellite image from Google Earth).
2.2. Determination of Physical-Chemical Characterization of the Soil
For pH determination, it was adjusted to 7.3 with 4.0 mol/L of HCl solution. In addition, the classification of soil types was carried out according to the Brazilian Embrapa soil manual [26], that is:
Soil type 1: Soils with a sandy texture, with a minimum clay content of 10% and less than 15% or with a clay content equal to or greater than 15%, in which the difference between the percentage of sand and the percentage of clay is greater than or equal to 50%.
Soil type 2: Soils with a medium texture, with a minimum clay content of 15% and less than 35%, in which the difference between the percentage of sand and the percentage of clay is less than 50%.
Soil type 3: Soils with a clay texture, with a clay content greater than or equal to 35%.
2.3. Sample Preparation and Digestion
After collection, the soil samples were dried at room temperature until they reached a constant weight and then sieved through a 2 mm polyethylene sieve (Verder Scientific, São Bernardo do Campo, Brazil). An amount of 0.5 g of each soil sample was weighed into the digestion Teflon DAP60® vessels (Berghof Products + Instruments GmbH, Eningen, Germany). The digestion was performed by adding 9.0 mL of HCl (35%; Merck, Darmstadt, Germany) and 3.0 mL of H2O2 (65%; Merck, Darmstadt, Germany) to the sample. The digestion of soil using hydrochloric acid (HCl) and hydrogen peroxide (H2O2) serves multiple analytical purposes, primarily aimed at preparing soil samples for the detection of various elements, including heavy metals. This method enhances the oxidizing capacity of the digestion process, leading to more accurate and reliable results in subsequent analyses. All vessels with the samples of soils were placed in the microwave digestion system (Speedwave 4-Microwave Digestion System; BERGHOF Products + Instruments GmbH) according to the digestion program instruction depicted in the USEPA method 3051A guidelines [27]. After cooling, the samples were filtered, transferred into 25 mL volumetric flasks, and made up to the mark with ultrapure water.
2.4. Metal Quantification Using ICP-OES
Soil samples were quantified using inductively coupled plasma–optical emission spectrometry (ICP-OES) (iCAP 6300 Duo, Thermo Fisher Scientific, Bremen, Germany). For the quantification of the elements, an axial view was used, operating power of 1250 W; sample flow = 0.35 L/min; plasma gas flow = 12 L/min; integration time = 5 s; stabilization time = 20 s; and fogging pressure = 20 psi. In addition, the following emission wavelengths (nm) were configured and used by ICP-OES for analysis of each of the elements: Al 309.271 nm, As 189.042 nm, Cd 228.000 nm, Co 228.616 nm, Cr 267.716 nm, Cu 324.754 nm, Fe 259.940 nm, Mg 279.553 nm, Mn 257.610 nm, Mo 202.030 nm, Ni 221.647 nm, P 214.914 nm, Se 196.00 nm, Pb 220.353 nm, and Zn 213.856 nm.
2.5. Calibration Procedure
The calibration curves and stock solution to mineral detection were set up with high-purity water and nitric acid. The concentrations of the different elements in these samples were measured using the corresponding multi-element standard solution (100 mg/L; Specsol, São Paulo, Brazil) containing Co, Cu, Fe, Na, and Zn and monoelement standard solution (100 mg/L, Specsol, São Paulo, Brazil) containing Ca, Cr, and Pb.
Standard solutions were prepared by diluting a multi-element standard stock solution (100 mg/L; Specsol, São Paulo, Brazil) containing 100 mg/L of each element (Al, As, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, P, S, V, Se, and Zn) and 1000 mg/L of Mg. For the quantitative analyses of the macro- and microelements in the soil, external calibration curves were generated using the following concentrations: 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.0, and 4.0 ppm of the element standards. Optimization conditions were evaluated in terms of precision (by recovery test) and the detection limit. The spike solution was made from a 1000 ppm multi-element stock solution. In addition, a recovery test was performed, and the solutions were enriched with 1 ppm. The method had a recovery range of 87–110%. The limits of detection (LOD) were calculated as 3 times the standard deviation of the mean of the sing blank (SB) determinations divided by the slope of the calibration curve (Sp); that is, 3 × SB/Sp [28]. On the other hand, the limit of quantification (LOQ) was calculated as follows: LOQ = 10 × SB/Sp. The range of all LOD elements was 0.02–0.3 µg/L, and the range of all LOQ elements was 0.06 to 10 µg/L. The correlation coefficient range (R2) was 0.9992–0.9995.
2.6. Contamination Factor
The contamination factor (CF) is used to evaluate the contamination status of pollutants in the soil (Ci) based on their concentrations in the sample and their reference concentration (Cb) [29]. It is an effective tool for monitoring pollution over some time and is calculated according to Equation (1).
The contamination classes are defined as follows: CF < 1 (low contamination), 1 < CF < 3 (moderate contamination), 3 < CF < 6 (considerable contamination), and CF > 6 (very high contamination) [30,31,32]. The results measured in the soil were used to portray the classification of contamination by metals such as As, Cd, Co, Cr, Mo, Ni, Se, and Pb. In our study, the reference values for the concentration of metals in surface soil were considered those established by Resolution No. 420 of 28 December 2009 of the National Council for the Environment (Conama) [33], which provides for quality criteria and guiding values of the soil for the presence of chemical substances and establishes guidelines for the environmental management of areas contaminated by these substances as a result of anthropogenic activities that established the values standard of soils for Brazil, that is, As = 15 mg/kg, Cd = 1.3 mg/kg, Co = 25 mg/kg, Cr = 75 mg/kg, Mo = 30 mg/kg, Ni = 30 mg/kg, Se = 5 mg/kg, and Pb = 72 mg/kg.
2.7. Pollution Load Index
For the entire sampling site, the quality of soil was evaluated via the pollution load index [34]. It was determined by calculating the product of Equation (1) according to Equation (2) below:
The classification of the contamination level by the pollution load index (PLI) follows the following values: PLI < 0.7 safety domain; 0.7 ≤ PLI < 1.0 caution region; 1.0 < PLI < 2.0 lightly polluted region; 2.0 ≤ PLI < 3.0 moderately polluted region; and PLI > 3.0 severely polluted region [34].
2.8. Calculation of Geo-Accumulation Index (Igeo)
The degree of metal contamination can be assessed by determining the geo-accumulation index (Igeo). The Igeo was developed to assess sediment contamination but has been widely applied in studies to assess surface soils [9,17,18]. To characterize the level of soil pollution, the Igeo values can be calculated using Equation (3).
where Cn is the metal (n) concentration recorded in the sediments of the study area (mg/kg), and Bn is the background value of the corresponding metal (n), here, is the geochemical background concentration of the average established by Conama, Brazil [33], while 1.5 is the factor background matrix correction due to lithogenic effects. The geo-accumulation index consists of seven classes. Class 0 (uncontaminated): Igeo ≤ 0; Class 1 (uncontaminated to moderately contaminated): 0 < Igeo < 1; Class 2 (moderately contaminated): 1 < Igeo < 2; Class 3 (moderately to highly contaminated): 2 < Igeo < 3; Class 4 (highly contaminated): 3 < Igeo < 4; Class 5 (highly to very highly contaminated): 4 < Igeo < 5; and Class 6 (very highly contaminated): 5 > Igeo [35,36].
2.9. Estimated Daily Dose Exposure Risk (ADD)
The average daily dose (ADD) of heavy metals in soil was calculated for exposure via oral, dermal, and inhalation ingestion for children and adults, according to Equation (4), Equation (5), and Equation (6) [37], respectively.
The following parameters were used to calculate the estimated daily intake dose (ADD) of heavy metals present in soil for children and adults:
- -
- Concentration of heavy metals in soils Cs (mg/kg) [23].
- -
- Ingestion rate of soil (IngR): 1000 mg/day for children and 100 for adults [38].
- -
- Conversion factor (CF): 10−6 kg/mg for children and adults [39].
- -
- Exposure frequency (EF): 104 days/year for children and 350 days/year for adults [39].
- -
- Exposure duration (ED): 6 for children per year and 24 for adults [38,39].
- -
- Body weight (BW): 15 kg for children and 70 kg for adults [23,40].
- -
- Average time for non-carcinogenic effects (AT): 2190 days for children and 8760 days for adults [40].
- -
- Average time for carcinogenic effects (AT): there is no value for children—25.550 days for adults [41].
- -
- Exposure time (ET): 1 h/day for children and adults [42].
- -
- Particles suspended in the air (PM): 1.36 × 10−9 kg⋅m−3 for children and adults [38].
- -
- Surface area of the skin that contacts the soil (SA): 2800 cm2 for children and 5700 cm2 for adults [38].
- -
- Skin adherence factor for soil (AF): 0.20 mg⋅cm2 for children and 0.07 mg⋅cm2 for adults [43].
- -
- Dermal absorption factor (ABS): 10−3 for children and adults for all elements [44].
- -
- Inhalation rate of soil (InhR): 7.6 m3/day for children and 20 m3/day for adults [45].
2.10. Hazard Quotient (HQ) and Hazard Index (HI)
The hazard quotient (HQ) is defined as the ratio of heavy metal exposure to the non-carcinogenic reference dose of the metal (RfD). Thus, the hazard quotients (HQ) of each heavy metal in each exposure route (Equations (4)–(6)) were obtained through Equations (7)–(9) provided by the USEPA [23,38]. The hazard index (HI) was calculated as the sum of the HQs (oral, dermal, and inhalation) according to Equation (10). The reference values of the toxicity dose used are listed in Table 1.

Table 1.
Toxicity reference values (RfD) for oral, dermal, and inhalation routes of exposure.
2.11. Carcinogenic Risk (CR)
The carcinogenic risk was estimated by Equations (11)–(13). The cancer slope factor (SF) values in different pathways are presented in Table 2. Elements such as Al, Co, Cu, Fe, Mg, Mn, Mo, P, Se, and Zn do not have a defined SF value. According to the USEPA (1989) [23], the acceptable carcinogenic risk value ranges from 10−6 to 10−4, considering the number of different exposure routes. However, the sum of values (total carcinogenic risk, TCR) with concentrations greater than 10−4 is considered unacceptable, as it indicates a significant risk of developing cancer, and below 10−6 represents an insignificant health risk. The risk range from >1 10−6 to <1 10−4 presents no concern for an increased cancer risk. Total carcinogenic risk (TCR) was calculated by (Equation (14)) summing the risk from each exposure pathway (ingestion + dermal + inhalation).

Table 2.
Cancer slope factor CSF (mg/kg/day) values in different pathways.
2.12. Statistical Analysis
Data were reported using the Origin 9.0 software (OriginLab Corporation, Northampton, MA, USA). The concentrations were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) and principal component analysis (PCA) were used to test for the differences in element levels in soil samples collected at different collection sites.
3. Results and Discussion
3.1. Physical-Chemical Characterization of the Soil
According to the parameters related to soil acidity, pH 5.8 in EP Lago do Amor presented low acidity and was classified as soil type 3; that is, clayey soils with a clay content greater than or equal to 35%. On the other hand, Anhanduí EP presented an average acidity of 5.1, being classified as soil type 2; that is, medium-textured soils with a minimum clay content of 15% and less than 35%. High acidity was obtained for the Sóter EP soil (pH = 4.6), which was classified as soil type 1, soils with a sandy texture, with a minimum clay content of 10% and less than 15%, or with a clay content equal to or greater than 15%. The pH of Águas do Prosa EP was 3.9, being a type 3 soil, and as such, a clayey soil with a clay content greater than or equal to 35% [59].
The pH is a parameter that can be used to estimate the mobility of chemical elements in soil and measure the toxicity levels and soil contamination [60]. The higher the pH, the lower the mobility of Cd, Zn, Ni, Cu, and Pb. At pH 7, Zn and Cd ions begin to break free of their compounds; meanwhile, Cd, Co, and Cr, as less mobile metals, will begin to dissolve at pH < 5 [60]. In addition, a low pH means that the mobility of metals in the soil is higher, which creates a lower availability of metals in the soil [61]. Soil pH influences precipitation and the release of various chemical elements. Toxicity levels rise as the pH drops below 3.0, affecting Cu, Zn, Cd, and Pb contamination, with Cd exceeding the critical value of 25 mg/kg [62].
The pH of urban soil influences heavy metal accumulation, which is related to toxicity levels. According to a study published by [63], specific relationships exist between the soil pH, copper, and overall contamination index and anthropogenic impact. Different soil types and pH levels affect metal concentrations in soil; therefore, the relationship between the soil pH, soil types, and metal concentrations is complex for understanding metal mobility and toxicity in various environments. Therefore, understanding the relationship between the soil pH, soil type, and metal(loid)s is crucial for assessing soil health and remediation strategies. Thus, the discussion of the values of concentrations of metal(loid)s in the different types of soils, as well as the different pH values analyzed in each soil from the ecological parks, are presented in the next subsection.
3.2. Metal Quantification Using ICP-OES and Principal Component Analysis (PCA)
The elements Al, As, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, P, Se, Zn, and Pb quantified in soil samples from EPs are presented in Table 3. In addition, the concentration values of various elements present in the soils studied in Table 3 were compared with those published by Conama (2009) [33], which is a Brazilian agency that establishes the values of concentrations of heavy metals in soils, and with the values obtained from Concentrations in Soils by State (Arizona) by the USEPA (2003) [64] and Canadian soil quality guidelines [65]. By comparing the regulatory limits on soil pollution across countries, it is also important to recognize that local environmental conditions and regulatory frameworks can significantly influence soil management practices and the interpretation of results. Thus, while global comparisons are valuable, they must be contextualized within local realities to be truly effective.

Table 3.
Concentrations of elements in soil (median and standard deviation) compared to Conama/Brazil (Conama, 2009) [33], soil from Canadian guidelines [65] and USEPA (2003) [64].
The metal(loid)s accumulated in urban park soils, as well as the pH and soil types, can be arranged in descending order of concentration values as follows:
- -
- Anhanduí park (pH 5.1, soil type 2): Mn > Mg > Zn > Fe > Cu > Mo > Cr > Pb > Se > Co > Ni > P > As > Al > Cd.
- -
- Águas do Prosa park (pH 3.8, soil type 3): Zn > Mg > Cu > Fe > Mn > Cr > Al > Ni > Mo > P > Se > As > Pb > Co > Cd.
- -
- Sóter park (pH 4.6, soil type 1): Zn > Mn > Mg > Cu > Fe > Mo > Cr > Ni > Pb > Se > P > Co > Al > As > Cd.
- -
- Lago do Amor park (pH 5.8, soil type 3): Mg > Cu > Mn > Fe > Zn > Cr > Ni > Mo > Pb > Se > As >Al > Co > P > Cd.
According to studies [55,59,66,67], the contamination of several soil types is associated with the pH in them [59]. That is, most metal(loid)s become more available at a lower pH (higher acidity) [66]. According to Pandey et al. (2015) [67], the pH influences the levels of trace elements in the soil, causing toxicity, contamination, and impacting soil health. Although the ecological parks Águas do Prosa and Lago do Amor both have the same soil type, the pH values and element concentrations are different. The distance of each urban park from the city center or close to highways can influence the presented results on the concentration of metal(loid)s and nonmetals. Depending on the location, soil can also contain heavy metals and metalloids originating from industrial emissions, waste disposal sites, agriculture, urban centers, or emissions generated by motor vehicles [25].
In addition to the type of soil and pH being important factors for soil quality, the age of the park and the type of local vegetation are factors that can significantly influence the concentration of heavy metals. According to Setälä et al. (2017) [68], lawns in parks of ca. 50 years old had the highest contents of Cr, Cu, Fe, Mn, Ni, and Zn, and in these and older parks (>100 years old), the contents of most metals were lowest under evergreen trees. Indeed, our results of metal concentrations in park soils corroborate the hypotheses of Setälä et al. (2017) [68], where at approximately 52 years of age, Lago do Amor Park has significant concentrations of elements such as Cr, Cu, Fe, Mn, Ni, and Zn, when compared to other parks (Table 3) that are approximately 20 years old.
Soil pH plays a crucial role in the mobility and bioavailability of heavy metals and metalloids in soils. The relationship between the soil pH and heavy metal mobility is complex and is influenced by various chemical and physical processes that occur in the soil environment. A decrease in soil pH typically enhances the solubility of heavy metals, thereby increasing their mobility. For instance, lower pH levels change the stable binding states of heavy metals, promoting their solubility and increasing the bioavailable fraction in the soil [63,68]. This is particularly significant for metals such as cadmium (Cd), chromium (Cr), and zinc (Zn), which exhibit increased mobility under acidic conditions. In our study, higher concentrations of Zn were quantified in Águas do Prosa park, which has a pH of 3.8 and soil with a clay content greater than or equal to 35%.
Conversely, higher soil pH values can lead to the precipitation of heavy metals as hydroxides or carbonates, thereby reducing their mobility [60,63]. Studies emphasized that moderate pH levels correlate with moderate metal content, suggesting that pH is a key factor influencing the bioavailability and mobility of heavy metals in soils [63,68]. Moreover, the interaction between the soil pH and organic matter content is critical. Organic matter can form stable complexes with heavy metals, influencing their availability. For instance, the presence of organic urban soil can alter the soil pH and enhance the retention of metal(loid)s, thereby mitigating their mobility [63]. This interplay between pH, organic matter, and heavy metal mobility is essential for effective soil management practices aimed at reducing contamination risks. However, the ecological parks presented in Table 3 are located in different locations in the City of Campo Grande/MS, Brazil, and may contain different concentrations of organic matter. Lower pH levels generally enhance metal solubility and mobility, while higher pH levels promote precipitation and immobilization. Understanding these dynamics is crucial for developing effective soil remediation strategies and managing heavy metal contamination in agricultural and urban soils.
In the present study (Table 3), the values of Al concentrations in the soils of the Anhanduí EP, Águas do Prosa EP, and Lago do Amor EP are below those values obtained from Concentrations in Soils by State (Arizona) by the USEPA (2003) [64]. There are no values of concentration of Al in soil set by Conama/Brazil (Conama, 2009) [33] and Canadian soil quality guidelines [65]. However, the concentration of Al in Table 3 is below the Al in the soil sample in the state park Cantareira, Brazil (71,799 mg/kg) [69]. Furthermore, the values for Al in the soils of all parks in Table 3 are above those obtained for playground soils (1.8 mg/kg) in countries such as Sweden [70]. Aluminum concentrations in urban park soils in Brazilian cities, particularly in our study, São Paulo states in Brazil and mainly other countries have great variations. This variation is influenced by anthropogenic activities and the physical properties of the soil, climate, and local environmental management practices [33,69,70]. On average, soils in urban areas in Brazil have aluminum concentrations that can reach relatively high levels, especially in regions with acidic soils, common in tropical and subtropical areas [33,64,69]. Our results for Al (Table 3) are in agreement with those published in previous research [64], in which there are variations in aluminum concentration within several Brazilian states and between different countries. These variations indicate the importance of managing soil acidity and controlling pollution sources in urban areas to mitigate the presence of bioavailable aluminum.
The concentration of As in all ecological parks in Table 3 is below those values obtained by Conama/Brazil [33]. In comparison, the concentration of this element in soil from Anhanduí EP, Águas do Prosa EP, and Sóter EP are below those values obtained by Canadian soil quality guidelines [65]. The content of As found in the soil of the Anhanduí EP and Lago do Amor EP were also higher than the values obtained by the USEPA (2003) [64], while the concentration of As in Águas do Prosa EP and Sóter EP are below of the values obtained by the USEPA [64]. Except for the As concentration value in Águas do Prosa EP, the concentrations of these elements in the other parks are below those values obtained in soils around an industrial zone in Neyshabur, Iran, which was 8.84 mg/kg [17]. However, values of As concentrations in all the soil of parks (Table 3) are lower than those obtained in studies (28 mg/kg) carried out in the topsoil of urban forest parks (Southern Poland) [71]. According to Mohammadi et al. (2020) [17], the high concentrations of metals in their study are due to long-term industrial activities in the area. Indeed, urban parks, particularly those near industrial lands, change the properties of the soil and can exhibit elevated arsenic levels due to anthropogenic sources. However, while the studies highlight significant arsenic concentrations in urban parks, it is essential to consider that not all urban areas exhibit the same levels of contamination. Variability in soil composition and proximity to pollution sources can lead to differing arsenic levels across different parks. Higher levels of arsenic in soils in Águas do Prosa EP indicate significant contamination.
In Table 3, the results showed that Cd concentrations in all soil samples from ecological parks are lower than those obtained by Canadian soil quality guidelines [65] but higher than those obtained by the USEPA (2003) [64]. Only the concentrations of Cd in Sóter EP and Lago do Amor EP are higher than the values established by Conama/Brazil [33]. In addition, Cd concentrations in the soil from Anhanduí EP and Águas do Prosa EP in Table 3 are below the soil concentration values (1.9 mg/kg) around an industrial zone in Neyshabur, Iran [17]. Furthermore, comparative analyses showed that Cd concentrations in all soil samples from ecological parks (Table 3) are higher than those obtained in other urban parks, such as Southern Poland (0.8 mg/kg) [72] and China (0.49 mg/kg) [9]. The assessment of Cd pollution in the topsoil of historical urban parks, such as Planty Park in Krakow (Poland), reveals significant contamination levels influenced by urbanization and industrial activities. However, according to Liu et al. (2020) [9], in addition to industrial activities, vehicle emissions can also contribute to the accumulation of Cd in the soil. It is clear that in all studies [9,33,72], including ours (Sóter EP and Lago do Amor EP), Cd concentrations were higher than the background value established by the regulatory agencies in each country. The study by Liu et al. (2020) [9] investigated the concentrations of Cd in the topsoil of urban parks in Beijing, revealing significant environmental concerns regarding the presence of Cd in the soil.
According to Table 3, the Co concentration values in the soils of Águas do Prosa EP, Sóter EP, and Lago do amor EP were lower than those obtained by Conama/Brazil [33] and the USEPA [64], but the concentration of Co in the soil from Anhanduí EP is higher than those presented by Conama/Brazil [33] and the USEPA [64]. There are no values defined by the Canadian soil quality guidelines for Co in soils. However, the Co concentration values in the soils of parks in Table 3 are below those obtained in the soil samples from selected playgrounds in the City of Sarajevo, Bosnia and Herzegovina (10.6 ± 1.0 mg/kg) [73], and topsoil of urban forest parks in Southern Poland (17.9 mg/kg) [71]. The study by Šapčanin et al. (2017) [73] investigates soil pollution in children’s playgrounds in Sarajevo, highlighting significant contamination from organic pollutants and pesticides. On the other hand, the Anhanduí, Águas do Prosa, Sóter, and Lago do Amor ecological parks have suffered due to the existence of fires close to their areas, which can probably contribute to the accumulation of various metals, including Co, which comes from the combustion of wood [71]. In fact, for authors such as Rahmonov et al. (2024) [71], among the sources of pollution in the urban forest are low emissions from coal combustion.
The values of Cr concentration in the soils of the parks were lower than those reported by the Canadian soil quality guidelines (Canadian Environmental Quality Guidelines, 2007) [65] and the USEPA (2003) [64], except Lago do Amor EP, which was higher than that reported by the USEPA (2003) [63]. There are no Cr values for the soils set by Conama/Brazil [33]. The value of Cr concentration in the soils of the Lago do Amor EP is greater than those obtained for the soils close to industrial zones (37.66 mg/kg), studied in Iran [17]. On the other hand, all Cr concentration values in Table 3 are below the chromium concentrations that varied among European urban parks, with notably higher levels in Turin (188 ± 41 mg/kg) [74]. According to the authors [74], there is insufficient information to claim the high levels of these elements present in their study, although the presence of rocks in the soil is one of the main causes of the increase in Cr in the soil. However, Lago do Amor has a large water reserve that is supplied by two streams that may contribute to the accumulation of Cr in their soil. Soils and streams are vulnerable to degradation processes caused by human activities [17].
The concentration of Cu in the soil of the Lago do Amor EP is higher than the values of the soils from Conama/Brazil (Conama, 2009) [33], the Canadian soil quality guidelines (Canadian Environmental Quality Guidelines, 2007) [49] and the USEPA (2003) [64], while the concentration of this element in the soils of Anhanduí EP, Águas do Prosa EP, and Sóter EP are higher when in comparison with the soils from the Canadian soil quality guidelines [65] and USEPA (2003) [64]. In addition, the Cu concentration values in park soils in Table 3 are above those obtained in playground soils from other countries, such as Bosnia (31.9 ± 4.5 mg/kg) [73] and Spain (0.19 mg/kg) [49], and the topsoil of urban forest parks (70.2 mg/kg) in Southern Poland [71]. Copper concentration in soils is influenced by various factors, including the soil type, which varies significantly across different countries and regions [49,65,71]. Research indicates that the availability of copper in soils is not uniform and is affected by local geochemical conditions, land use, and environmental factors [71]. Other factors, such as agricultural practices and environmental regulations, also play a significant role in the copper concentration variability across regions from different parks. Understanding these dynamics is essential for effective soil management and environmental protection.
There are no Fe, Mg, and P concentration values established or obtained by Conama/Brazil [33] as well as the Canadian soil quality guidelines [65] and the USEPA (2003) [64] (Table 3). However, concentrations of Fe, Mg, and P in the soil of Lago do Amor EP (Table 3) are higher than those obtained in soils with agricultural activities in Brazil, which were 188 mg/kg for Fe, 230.01 mg/kg for Mg, and 20.57 mg/kg for P [25]. There are few studies on the concentration of elements such as Fe, Mg, and P in urban park soils. On the other hand, the concentration of Mg in urban forest topsoil from China (1600–12,800 mg/kg) [75], as well as the concentration of P in soils from urban areas parks of the City of Sulaimani, Kurdistan, Iraq (677.9 mg/kg) [76] are higher than those obtained in our study (Table 3). In a study carried out on soils of children’s urban environments in the small northern European City of Uppsala, the concentration of Fe was quantified at 2.5 mg/kg [70]. Soil contamination by elements such as Fe, Mg, and P is significantly influenced by climate and various geochemical factors. Research indicates that climatic conditions, including temperature and precipitation, affect the chemical forms and mobility of these elements in soils. The study by Khorshid et al. (2019) [70] investigates phosphorus (P) fractions and speciation in rural and urban calcareous soils in the City of Sulaimani, Kurdistan, Iraq. Understanding P dynamics is crucial for agricultural productivity and environmental protection, particularly in semiarid regions. There is no local research on elements such as Fe, Mg, and P in the city park of Campo Grande, Brazil; however, the flow of vehicles, industrial sewage, and even accumulated garbage can contribute to the accumulation of these metals in the soils of the studied parks [25].
The Mn concentrations in the soils of Anhanduí EP, Águas do Prosa EP, Sóter EP, and Lago do Amor EP are below the values obtained by the USEPA (2003) (Table 3). In addition, the values of Mn in Table 3, when compared with other studies, show that they are below those obtained in playground soils in Spain (285 mg/kg) [49] and Hong Kong (518 mg/kg) [77]. Based on the manuscripts published in Refs. [49,77], it is observed that manganese (Mn) concentrations in soils vary significantly based on environmental conditions, anthropogenic influences, and soil properties. In other words, it indicates that Mn exists in different fractions, affecting its availability to plants and its ecological implications.
As shown in Table 3, Mo concentrations in the soils of Anhanduí EP, Águas do Prosa EP, Sóter EP, and Lago do amor EP are higher than the values obtained by Canadian soil quality guidelines [65]. On the other hand, Mo concentrations in the soils of Anhanduí EP and Lago do Amor EP are higher than those established by Conama/Brazil [33]. Furthermore, research indicates that urban environments, including parks, can exhibit significant levels of Mo, particularly in surface dust, soils, and sediments, with the highest concentrations found in the dust (11.9 mg/kg) compared to soil (5.84 mg/kg) and sediment (4.87 mg/kg) [78]; however, all of these values are below those in Table 3. The concentration of molybdenum (Mo) in soils presented in Table 3 and other studies can vary significantly based on geological, environmental, and anthropogenic factors. Understanding these concentrations is crucial for assessing ecological risks. However, the concentrations of Mo in our soils are not fully understood, making it challenging to assess their ecological risk from different sources of contamination.
According to Table 3, the Ni content in the soil samples from Anhanduí EP, Águas do Prosa EP, Sóter EP, and Lago do Amor EP are below the value obtained by Canadian soil quality guidelines [65]. Except for the Ni concentration in the soil of the Lago do Amor EP, in the soils of Anhanduí EP, Águas do Prosa EP, and Sóter EP, the concentrations are lower than the values established by Conama/Brasil [33] and the USEPA (2003) [64]. On the other hand, all Ni concentration values in the parks (Table 3) are below those obtained for the soils around an industrial zone (37.66 mg/kg) in Neyshabur, Iran [17]. In addition, in the study by Mohammadi et al. (2020) [17], the concentrations of Ni are higher than those reported in other countries. Different soil types exhibit varying capacities for nickel adsorption, influenced by properties such as cation-exchange capacity and organic matter content [17,74]. All the parks analyzed in Table 3 are located in different urban areas and are diverse anthropogenic conditions; hence, the variations in the Ni concentrations are mentioned.
Concentrations of Pb in the soils of Anhanduí EP, Águas do Prosa EP, Sóter EP, and Lago do Amor EP are below the values set by Conama/Brazil (Conama, 2009) [33] and the Canadian soil quality guidelines [65]. On the other hand, the concentrations of this element in the soils of all parks are higher than the values reported by the USEPA (2003) [64]. The concentrations of Pb in all parks are below those values obtained in soils around an industrial zone in Neyshabur, Iran, which was 57.33 mg/kg [17]. According to Mohammadi et al. (2020) [17], the high value of Pb concentrations in their study is due to the presence of soils collected in an industrial zone. However, studies indicate that heavy metals in industrial soils often exceed permissible levels, with Pb frequently identified as a major pollutant.
The concentrations of Se in soils were above the levels compared to the Canadian soil quality guidelines value for soil and the USEPA (Table 3). The level of Se in the soil of parks is much higher than those obtained in soil samples from selected playgrounds (2.60 mg/kg) in the City of Sarajevo, Bosnia and Herzegovina [73]. According to Zhao et al. (2022) [79], the excess of Se in the soil of karstic urban parks poses health risks due to contamination from various sources. In fact, according to Sapcanin et al. (2017) [73], the major polluting source of pollutants, such as heavy metals and metalloids in the City of Sarajevo, are vehicles because of high traffic density.
In addition, the concentration values of Zn in soil were above those obtained by Conama/Brazil [33] and the USEPA (2003) [64]; however, these values are below the values compared to the Canadian soil quality guidelines [65] (Table 3). All concentrations of Zn in parks (Table 3) are above the values obtained in the soil of the City of Sarajevo, Bosnia and Herzegovina (89.0 ± 6.5 mg/kg) [73], where the values of Zn concentrations do not exceed the intervention values provided by international regulations. Thus, according to the authors in Ref. [73], the Zn content does not exceed the intervention values provided by international regulations and can lead to the conclusion that the soils of Sarajevo playgrounds are not polluted by this element. According to Rosa et al. (2022) [25], the concentration of Zn in soil has different pollution sources. The soil samples studied in our research were carried out near urban regions; therefore, soils can be contaminated.
The results show that besides Mg (Table 3), the most abundant metals in the study area were Zn and Mn. Mn and Mg can be naturally present in the soil, but in urban areas, they can also come from anthropogenic sources such as fertilizers, concrete dust, construction waste, and even asphalt wear. Mn concentrations are influenced by climatic factors such as precipitation and temperature, with higher concentrations found in mesic areas with substantial rainfall and poor drainage. In addition, in urban areas, soil Mn levels can reflect pollution from heavy metals, with concentrations shaped more by soil lithology than anthropogenic factors. On the other hand, Mg concentrations in soils vary significantly based on geographic and environmental factors. Research indicates that Mg levels can be influenced by soil type, climate, and agricultural practices [80,81,82,83,84]. For this study, the soil samples were collected from public parks located in different urban areas of Campo Grande, Brazil, in which the concentrations of chemical elements may depend on the various conditions mentioned above, such as vehicle traffic or soil types.
The concentration of metals in soils depends on several factors, which makes comparing results from different countries complex. According to some authors in Refs. [80,81,82,83,84], Zn is the most abundant heavy metal in urban soil, while in some studies, Pb, Ni, Cr, Cu, As, and Cd are found in the lowest concentration [85]. However, Chen et al. (2021) [86] reported Cr as the most abundant toxic metal in soil. For some authors, high levels of Zn and Pb are influenced by human activities, and their dispersion over the sampling area is less uniform [85].
Since the data in Table 3 do not have a normal distribution (Shapiro–Wilk test, p = 0.05), the non-parametric Kruskal–Wallis test (multiple and independent variables) was used and revealed that there are no significant differences between the medians of the soil samples from the respective studied locations. Therefore, principal component analysis (PCA) was used for reducing and interpreting the datasets in Table 3, and for discovering which urban parks have the highest concentration of heavy metals. Using principal component analysis, we examined the relationship between metal and these urban parks. The eigenvalues gave a measure of the variance accounted for by the corresponding eigenvectors (components). The elements were correlated with three principal components (PCs) in which 99.999% of the total variance in the data was found. As a result, the principal component PC1, with an eigenvalue of 342.673, explains the results with approximately 67.757% variance. On the other hand, the main component PC2, with an eigenvalue of 124.421, explains the results with a variance of 24.602%. In this case, both components, PC1 and PC2, explain approximately 92.359% of the variances (results), and the third component, PC3, with an eigenvalue of 38.6447, explains the results with 7.6412% of the variance.
From Figure 2, it is observed that the values of As, Cd, Co, Cr, Mo, Ni, Se, and Pb contribute positively to the PC1 component, while the concentration of Al contributes negatively to this component. According to the results obtained from the PC analysis, a positive score implies that the concentration of variables increases along the PC axis, on the other hand, a negative score implies that the concentration of variables decreases along the axis. The length of the arrows reflects the variance explained by each component, with the longer arrows indicating greater importance. That is, the arrows in the PCA plots represent the direction and magnitude of the influence of each variable (Al, As, Cd, Co, Cr, Mo, Ni, Se, and Pb) on the principal components, allowing for a visual understanding of the relationships between metal(loid)s and their collection sites.
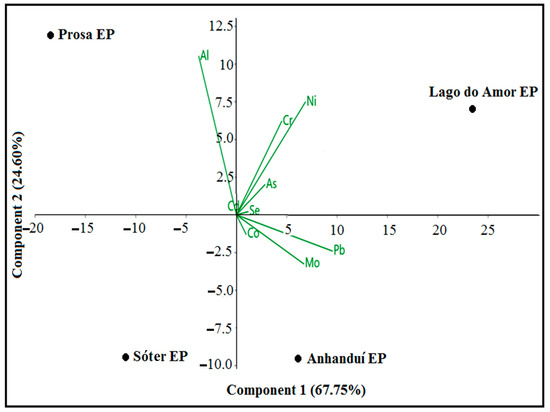
Figure 2.
Scatter plot of PC1 versus PC2 components: Al, As, Cd, Co, Cr, Mo, Ni, Se, and Pb are variables used in PCA. Points referring to the figure outline the soils of the studied places.
It is worth mentioning that in the PC1 component (Figure 2), the Pb element is the one that most contributes. On the other hand, the As, Cd, Cr, Ni, Se, and Pb concentration values contribute positively to the PC2 values; however, Co, Mo, and Pb contribute negatively to this component. The data in Figure 2 come from the values presented in Table 3, which can be considered as a measure of the relative importance of each variable. In Figure 2, in parks such as Águas do Prosa, greater influences of Al concentrations predominate. On the other hand, elements such as Se, As, Ni, Cd, and Cr predominate in greater concentration in places such as Lago do Amor EP. About the Anhanduí EP, there is a greater predominance of concentrations of Co, Mo, and Pb.
The chemical components, such as Cd, Cr, Co, Cu, Mn, Ni, Pb, Zn, and the metalloid As, are prevalent pollutants in the air. These substances are primarily released into the atmosphere due to human activities. Despite their low concentrations in the air, they contribute to the deposition and accumulation in soils [87]. The presence of these metal(loid)s in the soil, as well as the presence of Fe and Al, is largely attributed to the intensity of vehicular traffic, as fuels, lubricating oils, tires, and vehicle brake discs serve as the primary sources of certain chemical elements [88,89,90].
3.3. Contamination Factor and the Pollution Load Index (PLI)
The contamination factors (CFs) calculated based on the concentration of heavy metals in the soil of the ecological parks are presented in Table 4. The CF results indicate that the ecological parks presented low contamination for the elements As, Co, Cr, and Pb (with CF < 1). According to the results obtained from ecological parks in Saudi Arabia [91], a CF of less than one for the elements Cr, Co, and Pb suggests that the source of these elements comes mainly from the local soil and not anthropogenic contamination in public parks such as industrial, traffic, and agricultural sources. Studies carried out with agricultural soils quantified the presence of several elements, where the CF values of Ni and Zn of farmlands used for saffron cultivation were above 1, showing moderate contamination conditions [18].

Table 4.
Soil contamination factor (CF) values in urban ecological parks in Campo Grande/MS, Brazil.
In Table 4, soil samples taken from EP (Anhanduí CF = 1.018; Sóter CF = 1.474; Lago do Amor, CF = 2.108) had contamination factors demonstrating moderate (1–3) contamination of Cd.
The data represented in Table 4 indicate that the CF for Mo is 1.089 in Anhanduí EP and 1.157 for Lago do Amor EP, which resides in moderate contamination. In addition, the CF for Ni was low (CF < 1) in all of the studied locations except those sites close to Lago do Amor EP, which was in the moderate category. According to the data represented in Table 3, the CF for Se in all the parks studied had moderate contamination (1–3) due to the presence of this element. As the regulatory values sometimes vary from country to country, CF values may be different, even if the metal concentrations are identical. Therefore, comparisons involving other countries were not performed.
Regarding the level of soil contamination based on the calculation of the pollution load index (PLI), the Sóter EP (PLI = 0.4557) and the Águas do Prosa EP (PLI = 0.4636) have a safety domain classification with a PLI < 0.7, while Anhanduí EP (PLI = 0.7023) and Lago do Amor EP (PLI = 0.9103) are classified as a caution region (0.7 < PLI < 1).
3.4. Geo-Accumulation Index (Igeo)
For the comprehensive assessment of soil pollution degree in the study area, the geo-accumulation index was used. The Igeo values for the metals evaluated in soil samples from the ecological parks of Campo Grande/MS, Brazil, are shown in Table 5. The Igeo values of Al, Cu, Fe, Mg, Mn, P, and Zn were not calculated because there are no geochemical background concentration values of the average established by Conama (2009) [33]. With Igeo < 0, the ecological parks Anhanduí, Águas do Prosa, Sóter, and Lago do Amor are uncontaminated by As, Co, Cr, Mo, Ni, and Pb. In particular, Lago do Amor presented a Igeo (0 < 0.491 < 1) class 1 value for Cd; that is, the environment is uncontaminated to moderately contaminated by this element.

Table 5.
Index of geo-accumulation (Igeo) of heavy metal in the soil of the ecological parks of Campo Grande/MS, Brazil.
Our results of park soil uncontaminated by Cr and Pb when compared with those published by China, which observed urban park soils in Shanghai, were largely polluted by Cr and Pb with varying degrees [10], revealing that soils of urban ecological parks in Campo Grande, Brazil, suffer little anthropological influence. On the other hand, the Igeo of Se (from 0.419 to 0.981) in all parks was considered uncontaminated to moderately contaminated by this element. The Igeo values of Cd in Lago do Amor EP and Se in all parks were higher than those obtained for the other parks (Table 5), respectively, indicating that Cd and Se may be the main enriched pollutants in park soils.
3.5. Human Health Risk Analysis
Advanced average doses (ADD) decrease in the following order: ADDoral > ADDderm > ADDinal, both for children and adults, in all parks studied. The highest ADD values were found for Cu (ADDoral = 1.64 10−3 to 3.99 10−3), Fe (ADDoral = 1.51 10−3 to 3.80 10−3), Mn (ADDoral = 1.59 10−3 to 4.15 10−3), and Zn (ADDoral = 2.94 10−3 to 4.29 10−3) for the oral route of exposure in children. Thus, oral ingestion was the main route of contamination by heavy metals present in the soil of ecological parks. A similar result was found by Javed et al. (2019) [91] in Saudi Arabia, Penteado et al. (2021) [37] in southern Brazil, and Wang et al. (2021) in China [92].
Children aged 1 to 6 years are more exposed to contamination by metals through the soil due to greater contact with the ground and the practice of taking dirty hands and objects to the mouth, in addition to the indirect ingestion associated with the consumption of water and food [70]. Our results corroborate those published by Mohammadi et al. (2020) [17], where the highest levels of risk were also observed in children of the same age (6 years). According to the risk quotient (HQ < 1) values and the hazard index (HI < 1), it seems unlikely that the oral, dermal, and inhalation exposure of children and adults to heavy metals present in the soil of the studied urban parks represents any risk. In fact, according to studies utilizing models recommended by the USEPA to evaluate health risks due to ingestion, dermal contact, and inhalation pathways due to metal exposure in urban soil for children is considered low, suggesting that the current metal concentrations are within acceptable safety limits [93]. However, the impacts of exposure to any hazardous substance depend on the dosage, duration, how you are exposed, individual characteristics and propensities, and whether other chemicals are present. These assessments are crucial due to children’s heightened vulnerability to pollutants, which stem from their behaviors and physiological characteristics. Metals such as Pb, Hg, Cd, and metalloids (As) can lead to severe health issues, including neurocognitive impairments, respiratory problems, and increased susceptibility to infections like tuberculosis [94,95].
The total carcinogenic risks for children were above the acceptable range of 1.0 × 10−4 for the elements As, Cr, and Ni (Table 6). The TCR among children registered a maximum value of 5.80 10−4 for Ni in Lago do Amor, while the minimum value was 2.02 10−4 (also for Ni in Anhanduí EP). The TCR of exposure to heavy metal through the soil of urban parks decreased in the order As > Cr > Ni in the Anhanduí EP, Cr > Ni > As in the Águas do Prosa EP, Ni > Cr in the Sóter EP, and Ni > As > Cr in the Lago do Amor, demonstrating a risk for the development of cancer in children exposed in the long term to heavy metals present in the soil of ecological parks. Counting this result, Liu et al. (2020) [9] found TCR values for Cd, As, Cr, and Ni lower than 10−6 when assessing the risk of exposure to these metals through the soil of Chinese urban parks, as well as Penteado et al. (2021) [37] when assessing the risk due to exposure to Pb and Ni in Brazilian parks in the Rio Grande. On the other hand, the carcinogenic risk for adults considering the routes of oral, dermal, and inhalation exposure due to the presence of As, Cr, and Ni are greater than 10−6 (Table 6); however, according to the USEPA (1989) [23], there is no concern for an increased cancer risk. Our results are in agreement with those published by Taghavi et al. (2023) [18], which found no significant carcinogenic health hazards for both adults via ingestion, skin contact, and inhalation exposure pathways in agricultural soils used for saffron cultivation. However, children face significant carcinogenic risks from exposure to metalloids and heavy metals found in contaminated soils. Studies have established that children are particularly vulnerable to the adverse health effects associated with soil contamination due to their developing bodies and behaviors that increase exposure, such as playing in soil and frequent hand-to-mouth activities. For instance, Gong et al. (2022) [96] highlighted that the health risk index for children is consistently higher than that for adults, indicating a greater susceptibility to soil heavy metal(loid) exposure. Similarly, She et al. (2022) [97] confirmed that health risks, including both carcinogenic and non-carcinogenic risks, are generally elevated for children compared to adults.

Table 6.
Total carcinogenic risk for children and adults, considering the routes of oral, dermal, and inhalation exposure.
According to a study conducted in China considering children aged 3 to 12 years, hand-to-mouth contact was an important route for children’s exposure to Cr, Mn, Ni, Cu, Zn, As, Cd, and Pb, with the risk of oral exposure cancer corresponding to Cr already exceeding the maximum acceptable level [98]. The ingestion of arsenic (As) and nickel (Ni) through contaminated crops poses carcinogenic risks to all populations, with teenagers and children being particularly vulnerable, as indicated by the study’s findings on total carcinogenic risk (TCR). Children are particularly susceptible to the neurocognitive and developmental impacts of heavy metals and metalloids, which can lead to long-term health issues, including cancer [95]. The specific metalloids and heavy metals of concern include arsenic, cadmium, chromium, and lead, all of which have been shown to pose significant health risks. For example, arsenic is recognized as a potent carcinogen, and its presence in soil has been linked to increased cancer risks in children [10].
While the ingestion of heavy metals poses serious health risks, it is essential to consider that not all children or adults will develop cancer or severe health issues. Factors such as genetic predisposition, overall health, and environmental conditions also play critical roles in determining individual outcomes. The cumulative evidence suggests that children living in areas with heavy metal and metalloid contamination are at an increased risk of developing cancer and other serious health issues, necessitating urgent public health interventions and soil remediation efforts to mitigate these risks.
The interpretation of the results presented in this work has limitations, such as the values of the contamination factors (FCs) where the reference concentration (Cb) varies from country to country, as well as the values of the geo-accumulation index (Igeo) where Bn is the value of the background of the corresponding metal and vary between countries.
4. Conclusions
This study revealed that different soil types from ecological parks of Campo Grande, Brazil, exhibit varying capacities for metal(loid) retention. The concentration values of Al and Mn in the soils of the ecological parks studied here are below the USEPA permissible level for soils. The contents of As, Cd, Co, Pb, Cr, Cu, Mo, Ni, Se, and Zn in the soils of some urban parks were above the national and international permissible levels. In addition, the ecological parks presented low contamination for the elements As, Co, Cr, and Pb. The pollution load index (PLI) showed that the two parks are classified as a caution region. The geo-accumulation index of Se in all parks was from uncontaminated to moderately contaminated by this element.
According to our study, it is unlikely that an adult would develop cancer from exposure to As, Cr, and Ni in park dust; however, children showed more severe carcinogenic risks. It is important to emphasize that this study focused only on soil heavy metal levels and did not take into account other potential sources of exposure to contamination, such as air, water, and food. Future studies should explore these additional sources of contamination to gain a more comprehensive understanding of the potential health risks associated with exposure to ecological parks.
Future research on the pollution and contamination indices of hazardous heavy metal ions should be conducted in urban ecological parks to monitor and minimize the impact of these elements on human health and the environment in various countries. Our findings can be used to inform future policies and practices designed to protect the health and well-being of the public, especially children, the elderly, and pregnant women who attend ecological parks.
Author Contributions
Conceptualization, F.G.d.P. and G.M.S.; methodology, I.D.d.S. and E.S.d.P.M.; validation, D.A.Z.G.; formal analysis, F.G.d.P.; data curation, F.G.d.P.; writing—original draft preparation, V.A.d.N.; writing—review and editing, F.G.d.P., D.A.Z.G., D.B., R.d.C.A.G., K.d.C.F.G., R.J.O., P.A.H., and V.A.d.N.; visualization, F.G.d.P. and M.A.P.A.; supervision, V.A.d.N.; project administration, V.A.d.N.; funding acquisition, V.A.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) (grant number 314551/2023-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) (Finance Code 001).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors thank the Federal University of Mato Grosso do Sul, Faculty of Medicine for their scientific support, as well as the Municipal Secretariat for the Environment and Urban Management, the National Sports Foundation/MS, and the Mato Grosso do Sul Environmental Institute (IMASUL) for permission and authorization to enter the parks to collect samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ouyang, T.; Guo, Y.; Peng, S.; He, C.; Zhu, Z. Assessment of soil heavy metal pollution and its ecological risk for city parks, Vicinity of a Landfill, and an industrial area within Guangzhou, South China. App. Sci. 2022, 12, 9345. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Behbehani, M.; Lee, J.Y. Is atmospheric pathway a significant contributor to microplastics in the marine environment? Emerg. Contam. 2024, 10, 100297. [Google Scholar] [CrossRef]
- Kumari, S.; Mishra, A. Heavy metal contamination. In Soil Contamination—Threats and Sustainable Solutions; IntechOpen: Rijeka, Croatia, 2021; p. 298. [Google Scholar]
- Timonen, H.; Mylläri, F.; Simonen, P.; Aurela, M.; Maasikmets, M.; Bloss, M.; Kupri, H.-L.; Vainumäe, K.; Lepistö, T.; Salo, L.; et al. Household solid waste combustion with wood increases particulate trace metal and lung deposited surface area emissions. J. Environ. Manag. 2021, 293, 112793. [Google Scholar] [CrossRef]
- Banu, Z. Contamination and ecological risk assessment of heavy metal in the sediment of Turag River, Bangladesh: An index analysis approach. J. Water Resour. Prot. 2013, 5, 239–248. [Google Scholar] [CrossRef]
- Khan, M.D.H.; Talukder, A.; Rahman, M.S. Spatial distribution and contamination assessment of heavy metals in urban road dusts from Dhaka city, Bangladesh. IOSR J. Appl. Chem. 2018, 11, 90–99. [Google Scholar]
- Gope, M.; Masto, R.E.; George, J.; Hoque, R.R.; Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Saf. 2017, 138, 231–241. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Ma, J.; Wu, H.; Qu, Y.; Gong, Y.; Yang, S.; An, Y.; Zhou, Y. Heavy metal(loid)s in the topsoil of urban parks in Beijing, China: Concentrations, potential sources, and risk assessment. Environ. Pollut. 2020, 260, 114083. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Sun, J.; Li, X.; Geng, X.; Zhao, M.; Sun, T.; Fan, Z. Health risk assessment of heavy metal(loid)s in park soils of the largest megacity in China by using Monte Carlo simulation coupled with Positive matrix factorization model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Lei, K.; Wang, W.; Guan, Y. Trace metal element pollution of soil and water resources caused by small-scale metallic ore mining activities: A case study from a sphalerite mine in North China. Environ. Sci. Pollut. Res. Int. 2019, 26, 24630–24644. [Google Scholar] [CrossRef]
- Tong, S.; Yang, L.; Gong, H.; Wang, L.; Li, H.; Yu, J.; Li, Y.; Deji, Y.; Nima, C.; Zhao, S.; et al. Bioaccumulation characteristics, transfer model of heavy metals in soil-crop system and health assessment in plateau region, China. Ecotoxicol. Environ. Saf. 2022, 241, 113733. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, L.; Wang, H.; Martín, J.D. Bioavailability and health risk of toxic heavy metals (As, Hg, Pb and Cd) in urban soils: A Monte Carlo simulation approach. Environ. Res. 2022, 214, 113772. [Google Scholar] [CrossRef] [PubMed]
- Peña-Fernández, A.; González-Muñoz, M.J.; Lobo-Bedmar, M.C. Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ. Int. 2014, 72, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Aja, D.; Okolo, C.C.; Nwite, N.J.; Njoku, C. Environmental risk assessment in selected dumpsites in Abakaliki metropolis, Ebonyi state, southeastern Nigeria. Environ. Chall. 2021, 4, 100143. [Google Scholar] [CrossRef]
- Mavakala, B.K.; Sivalingam, P.; Laffite, A.; Mulaji, C.K.; Giuliani, G.; Mpiana, P.T.; Poté, J. Evaluation of heavy metal content and potential ecological risks in soil samples from wild solid waste dumpsites in developing country under tropical conditions. Environ. Chall. 2022, 7, 100461. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Zarei, A.; Esmaeilzadeh, M.; Taghavi, M.; Yousefi, M.; Yousefi, Z.; Sedighi, F.; Javan, S. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol. Trace Elem. Res. 2020, 195, 343–352. [Google Scholar] [CrossRef]
- Taghavi, M.; Darvishiyan, M.; Momeni, M.; Eslami, H.; Fallahzadeh, R.A.; Zarei, A. Ecological risk assessment of trace elements (TEs) pollution and human health risk exposure in agricultural soils used for saffron cultivation. Sci. Rep. 2023, 13, 4556. [Google Scholar] [CrossRef]
- Peirovi-Minaee, R.; Alami, A.; Moghaddam, A.; Zarei, A. Determination of concentration of metals in grapes grown in Gonabad Vineyards and assessment of associated health risks. Biol. Trace Elem. Res. 2023, 201, 3541–3552. [Google Scholar] [CrossRef]
- Sobhanardakani, S. Potential health risk assessment of heavy metals via consumption of caviar of Persian sturgeon. Mar. Pollut. Bull. 2017, 123, 34–38. [Google Scholar] [CrossRef]
- Sobhanardakani, S. Tuna fish and common kilka: Health risk assessment of metal pollution through consumption of canned fish in Iran. J. Consum. Prot. Food. Saf. 2017, 12, 157–163. [Google Scholar] [CrossRef]
- Sobhanardakani, S. Ecological and human health risk assessment of heavy metal content of atmospheric dry deposition, a case study: Kermanshah, Iran. Biol. Trace. Elem. Res. 2019, 187, 602–610. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). Assessing Human Health Risks from Chemically Contaminated Fish and Shellfish: A Guidance Manual; U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int. J. Environ. Res. Public Health 2019, 16, 4064. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.G.; Melo, E.S.P.; Junior, A.S.A.; Gondim, J.M.S.; de Sousa, A.G.; Cardoso, C.A.L.; Viana, L.F.; Carvalho, A.M.A.; Machate, D.J.; do Nascimento, V.A. Transfer of metal(loid)s from soil to leaves and trunk xylem sap of medicinal plants and possible health risk assessment. Int. J. Environ. Res. Public Health 2022, 19, 660. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.B.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B.; Cunha, T.G.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, DF, Brazil, 2018; ISBN 978-85-7035-800-4. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Long, G.L.; Winefordner, J.D. Limit of detection: A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Martin, J.-M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Förstner, U.; Ahlf, W.; Calmano, W. Studies on the transfer of heavy metals between sedimentary phases with a multi-chamber device: Combined effects of salinity and redox variation. Mar. Chem. 1989, 28, 145–158. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Rubio, B.; Nombela, M.A.; Vilas, F. Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): An assessment of metal pollution. Mar. Pollut. Bull. 2000, 40, 968–980. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. Conselho Nacional do Meio Ambiente. Resolução Nº 420, de 28 de Dezembro de 2009, Brasil. Available online: http://hab.eng.br/wp-content/uploads/2017/09/resolucao-conama-420-2009-gerenciamento-de-acs.pdf (accessed on 3 June 2024).
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Muller, G. Dei Schwermetallbelstung der sedimente des Neckars and seiner Nebenfusse: Eine estandsaufnahme. Chem. Ztg. 1981, 105, 157–164. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Penteado, J.O.; Brum, R.d.L.; Ramires, P.F.; Garcia, E.M.; dos Santos, M.; da Silva Júnior, F.M.R. Health risk assessment in urban parks soils contaminated by metals, Rio Grande city (Brazil) case study. Ecotoxicol. Environ. Saf. 2021, 208, 111737. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund (RAGS) Volume III: Part A, Process for Conducting Probabilistic Risk Assessment; U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- USEPA (United States Environmental Protection Agency). Volume II of Remedial Investigation. In Human Health Risk Assessment, Revision 2; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. Available online: https://semspub.epa.gov/work/01/550299.pdf (accessed on 3 June 2024).
- USEPA (United States Environmental Protection Agency). Exposure Factors Handbook: 2011 Edition; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Ocampos, M.S.; Leite, L.C.S.; de Pádua Melo, E.S.; de Cássia Avellaneda Guimarães, R.; Oliveira, R.J.; de Cássia Freitas, K.; Hiane, P.A.; Karuppusamy, A.; do Nascimento, V.A. Indirect methods to determine the risk of damage to the health of firefighters and children due to exposure to smoke emission from burning wood/coal in a controlled environment. Int. J. Environ. Res. Public Health 2023, 20, 5607. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). Exposure Factors Handbook Chapter 5 (Update): Soil and Dust Ingestion; U.S. Environmental Protection Agency: Washington, DC, USA, 2017.
- USEPA (United States Environmental Protection Agency). Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). In Risk Assessment Guidance for Superfund; U.S. Environmental Protection Agency: Washington, DC, USA, 2004. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-e (accessed on 25 March 2024).
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef]
- Van der Berg, R. Human Exposure to Soil Contamination: A Qualitative and Quantitative Analysis Towards Proposals for Human Toxicological Intervention Values; RIVM Report no. 72520101; National Institute of Public Health and Environmental Protection (RIVM): Bilthoven, The Netherlands, 1995. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Regional Screening Levels (RSLs)—Generic Tables—Summary Table. [WWW Document]. Regional Screening Levels (RSLs)—Generic Tables—Summary Table. 2023. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 29 April 2023).
- Shomar, B.; Rashkeev, S.N. A comprehensive risk assessment of toxic elements in international brands of face foundation powders. Environ. Res. 2021, 192, 110274. [Google Scholar] [CrossRef]
- Cu, Y.C.; Lin, Q.; Cao, Y.P. Metals in exposed lawn soils from 18 urban parks and its human health implications in southern China’s largest city, Guangzhou. J. Clean. Prod. 2016, 115, 122–129. [Google Scholar] [CrossRef]
- De Miguel, E.; Iribarren, I.; Chacón, E.; Ordoñez, A.; Charlesworth, S. Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 2007, 66, 505–513. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, B.; Asad, N.; Mian, I.A.; Jamil, M. Traffic-related lead pollution in roadside soils and plants in Khyber Pakhtunkhwa, Pakistan: Implications for human health. Int. J. Environ. Sci. Technol. 2019, 16, 8015–8022. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency), Integrated Risk Information System (IRIS). Chemical Search. Oral Slope Factor. 1995. Available online: https://cfpub.epa.gov/ncea/iris/search/index.cfm (accessed on 18 April 2023).
- Xia, Q.; Zhang, J.; Chen, Y.; Ma, Q.; Peng, J.; Rong, G.; Tong, Z.; Liu, X. Pollution, sources and human health risk assessment of potentially toxic elements in different land use types under the background of industrial cities. Sustainability 2020, 12, 2121. [Google Scholar] [CrossRef]
- USDOE, The Risk Assessment Information System (RAIS). RAIS Toxicity Values and Physical Parameters Search. 2011. The Risk Assessment Information System. Available online: https://rais.ornl.gov/cgi-bin/tools/TOX_search (accessed on 18 April 2023).
- Zhang, R.; Chen, T.; Zhang, Y.; Hou, Y.; Chang, Q. Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in Northwest China. Chemosphere 2020, 252, 126591. [Google Scholar] [CrossRef]
- Konstantinova, E.; Minkina, T.; Mandzhieva, S.; Nevidomskaya, D.; Bauer, T.; Zamulina, I.; Sushkova, S.; Lychagin, M.; Rajput, V.D.; Wong, M.H. Ecological and human health risks of metal–PAH combined pollution in riverine and coastal soils of Southern Russia. Water 2023, 15, 234. [Google Scholar] [CrossRef]
- Chen, R.; Han, L.; Liu, Z.; Zhao, Y.; Li, R.; Xia, L.; Fan, Y. Assessment of soil-heavy metal pollution and the health risks in a mining area from Southern Shaanxi Province, China. Toxics 2022, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Usman, K.; Al Jabri, H.; Rizwan, M. Ecological and health risks assessment of potentially toxic metals and metalloids contaminants: A case study of agricultural soils in Qatar. Toxics 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Korna, R.; Fan, J.; Ke, W.; Lou, W.; Wang, J.; Zhu, F. Spatial distribution, environmental risks, and sources of potentially toxic elements in soils from a typical abandoned antimony smelting site. J. Environ. Sci. 2023, 127, 780–790. [Google Scholar] [CrossRef]
- Abreu Junior, C.H.; Muraoka, T.; Lavorante, A.F.; Villanueva, F.C.A. Condutividade elétrica, reação do solo e acidez potencial em solos adubados com composto de lixo. Rev. Bras. Ciência Do Solo 2000, 24, 635–647. [Google Scholar] [CrossRef]
- Sintorini, M.M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 737, 012071. [Google Scholar] [CrossRef]
- Pikuła, D. Effect of the degree of soil contamination with Cd, Zn, Cu i Zn on its content in the forder crops and mobility in the soil Profile. In Soil Contamination—Recent Advances and Future Perspectives; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Díez, M.; Simón, M.; García, I.; Martín, F. Assessment of the critical load of trace elements in soils polluted by pyrite tailings. A laboratory experiment. Water Air Soil Pollut. 2009, 199, 381–387. [Google Scholar] [CrossRef]
- Kazlauskaitė-Jadzevičė, A.; Volungevičius, J.; Gregorauskienė, V.; Marcinkonis, S. The role of pH in heavy metal contamination of urban soil. J. Environ. Eng. Land. Manag. 2014, 22, 311–318. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Guidance for Developing Ecological Soil Screening Levels; Office of Emergency and Remedial Response, OSWER Directive 9285.77-55, U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- Canadian Council of Ministers of the Environment. Canadian soil quality guidelines for the protection of environmental and human health: Summary tables. In Canadian Environmental Quality Guidelines, 1999; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Zoz, T.; Lana, M.; Steiner, F.; Frandoloso, J.F.; Fey, R. Influência do pH do solo e de fertilizantes fosfatados sobre a adsorção de fósforo em latossolo vermelho. Agric. Food Sci. Environ. Sci. 2009, 4, Synergismus scyentifica UTFPR. [Google Scholar]
- Pandey, B.; Agrawal, M.; Singh, S. Ecological risk assessment of soil contamination by trace elements around coal mining area. J. Soils Sediments 2016, 16, 159–168. [Google Scholar] [CrossRef]
- Setälä, H.; Francini, G.; Allen, J.A.; Jumpponen, A.; Hui, N.; Kotze, D.J. Urban parks provide ecosystem services by retaining metals and nutrients in soils. Environ. Pollut. 2017, 231, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Flues, M.; Sato, I.M.; Cotrim, M.B.; Salvador, V.L.; Ranzani, A.C.; Vallilob, M.I.; Oliveira, E. Soil Characterization in a subtropical forest crossed by highways (Cantareira State Park, SP, Brazil). J. Braz. Chem. Soc. 2004, 15, 496–503. [Google Scholar] [CrossRef]
- Ljung, K.; Selinus, O.; Otabbong, E. Metals in soils of children’s urban environments in the small northern European city of Uppsala. Sci. Total Environ. 2006, 366, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Rahmonov, O.; Kowal, A.; Rahmonov, M.; Pytel, S. Variability of concentrations of potentially toxic metals in the topsoil of urban forest parks (Southern Poland). Forests 2024, 15, 1020. [Google Scholar] [CrossRef]
- Gąsiorek, M.; Kowalska, J.; Mazurek, R.; Pająk, M. Comprehensive assessment of heavy metal pollution in topsoil of historical urban park on an example of the Planty Park in Krakow (Poland). Chemosphere 2017, 179, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Sapcanin, A.; Cakal, M.; Jacimovic, Z.; Pehlic, E.; Jancan, G. Soil pollution fingerprints of children playgrounds in Sarajevo city, Bosnia and Herzegovina. Environ. Sci. Pollut. Res. 2017, 24, 10949–10954. [Google Scholar] [CrossRef]
- Madrid, L.; Diaz-Barrientos, E.; Ruiz-Cortés, E.; Reinoso, R.; Biasioli, M.; Davidson, C.M.; Duarte, A.C.; Grcman, H.; Hossack, I.; Hursthouse, A.S.; et al. Variability in concentrations of potentially toxic elements in urban parks from six European cities. J. Environ. Monit. 2006, 8, 1158–1165. [Google Scholar] [CrossRef]
- Wu, X.; Du, E.; Guo, Y.; Xia, N.; Tang, Y.; Wang, Y.; Guo, H. Climate control of topsoil potassium, calcium, and magnesium concentrations in urban forests across eastern China. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006230. [Google Scholar] [CrossRef]
- Khorshid, M.S.H.; Kruse, J.; Semella, S.; Vohland, M.; Wagner, J.F. Phosphorus fractions and speciation in rural and urban calcareous soils in the semiarid region of Sulaimani city, Kurdistan, Iraq. Environ. Earth Sci. 2019, 78, 531. [Google Scholar] [CrossRef]
- Ng, S.L.; Chan, L.S.; Lam, K.C.; Chan, W.K. Heavy metal contents and magnetic properties of playground dust in Hong Kong. Environ. Monit. Assess. 2004, 89, 221–232. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Yenokyan, T.; Sahakyan, L. Geospatial patterns and geochemical compositional characteristics of molybdenum in different mediums of an urban environment. Environ. Res. 2023, 239, 117340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hao, M.; Li, Y.; Li, S. Contamination, sources and health risks of toxic elements in soils of karstic urban parks based on Monte Carlo simulation combined with a receptor model. Sci. Total Environ. 2022, 839, 156223. [Google Scholar] [CrossRef] [PubMed]
- Egbueri, J.C.; Ukah, B.U.; Ubido, O.E.; Unigwe, C.O. A chemometric approach to source apportionment, ecological and health risk assessment of heavy metals in industrial soils from southwestern Nigeria. J. Environ. Anal. Chem. 2022, 102, 3399–3417. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Hu, G.C.; Chen, L.G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lv, J. Integrated receptor models and multivariate geostatistical simulation for source apportionment of potentially toxic elements in soils. Catena 2020, 194, 104638. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Singh, V.K.; Li, J.; Zhang, G. Spatial distribution, source analysis, and health risk assessment of heavy metals contamination in house dust and surface soil from four major cities of Nepal. Chemosphere 2019, 218, 1100–1113. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, W.; Qiu, Q.; Ye, Z.; Li, Y.; Tunney, H.; Dou, C.; Zhou, K.; Qian, X. Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma 2019, 337, 453–462. [Google Scholar] [CrossRef]
- Radomirović, M.; Ćirović, Ž.; Maksin, D.; Bakić, T.; Lukić, J.; Stanković, S.; Onjia, A. Ecological risk assessment of heavy metals in the soil at a former painting industry facility. Front. Environ. Sci. 2020, 8, 560415. [Google Scholar] [CrossRef]
- Chen, B.; Liu, K.; Liu, Y.; Qin, J.; Peng, Z. Source identification, spatial distribution pattern, risk assessment and influencing factors for soil heavy metal pollution in a high-tech industrial development zone in Central China. Hum. Ecol. Risk Assess. 2021, 27, 560–574. [Google Scholar] [CrossRef]
- Dahmardeh Behrooz, R.; Kaskaoutis, D.G.; Grivas, G.; Mihalopoulos, N. Human health risk assessment for toxic elements in the extreme ambient dust conditions observed in Sistan, Iran. Chemosphere 2021, 262, 127835. [Google Scholar] [CrossRef]
- Falahi-Ardakani, A. Contamination of environment with heavy metals emitted from automotives. Ecotoxicol. Environ. Saf. 1984, 8, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hulskotte, J.H.J.; Roskam, G.D.; Denier van der Gon, H.A.C. Elemental composition of current automotive braking materials and derived air emission factors. Atmos. Environ. 2014, 99, 436–445. [Google Scholar] [CrossRef]
- Proshad, R.; Dey, H.C.; Ritu, S.A.; Baroi, A.; Khan, M.S.U.; Islam, M.; Idris, A.M. A review on toxic metal pollution and source-oriented risk apportionment in road dust of a highly polluted megacity in Bangladesh. Environ. Geochem. Health 2023, 45, 2729–2762. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.A.; Al-Bratty, M.; Al-Rajab, A.J.; Alhazmi, H.A.; Ahsan, W.; Abdelwahab, S.I.; Thangavel, N. Risk-based exposure assessment for multiple toxic elements encountered by children in school playgrounds and parks in the southwest region of Saudi Arabia. Environ. Monit. Assess. 2019, 191, 549. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Walker, T.R.; Wang, Y.; Luo, Q.; Wu, H.; Wang, X. Distribution characteristics, chemical speciation and human health risk assessment of metals in surface dust in Shenyang City, China. Appl. Geochem. 2021, 131, 105031. [Google Scholar] [CrossRef]
- Mugosa, B.; Djurovic, D.; Pirnat, A.; Bulat, Z.; Barjaktarovic-Labovic, S. Children’s health risk assessment based on the content of toxic metals Pb, Cd, Cu and Zn in urban soil samples of Podgorica, Montenegro. Vojnosanit. Pregl. 2015, 72, 807–812. [Google Scholar] [CrossRef]
- Kishore, S.; Kumari, M.; Panda, I.; Gupta, S.; Priya, S.; Mondal, S.; Malik, S.; Lata, S. Health Effects of Heavy Metals Contamination in Children. In Advances in Environmental Engineering and Green Technologies Book Series; IGI Global Scientific Publishing: Hershey, PA, USA, 2024; pp. 254–275. [Google Scholar] [CrossRef]
- Haque, R.; Chisti, M.J.; Rahman, M.; Islam, M.d.S.; Moniruzzaman, M.; Rahman, S.M.; Raqib, R. Assessing the Effect of Heavy Metal Exposures on Pediatric Tuberculosis. In Proceedings of the ISEE 2022: 34th Annual Conference of the International Society of Environmental Epidemiology, Athens, Greece, 18–21 September 2022; ISEE Conference Abstracts. Volume 2022. [Google Scholar] [CrossRef]
- Gong, C.; Lu, H.; Zhang, Z.; Wang, L.; Xia, X.; Wang, L.; Xiang, Z.; Shuai, L.; Ding, Y.; Chen, Y.; et al. Spatial distribution characteristics of heavy metal(loid)s health risk in soil at scale on town level. Sci. Rep. 2022, 12, 19195. [Google Scholar] [CrossRef]
- She, W.; Guo, L.; Gao, J.; Zhang, C.; Wu, S.; Jiao, Y.; Zhu, G. Spatial Distribution of Soil Heavy Metals and Associated Environmental Risks near Major Roads in Southern Tibet, China. Int. J. Environ. Res. Public Health 2022, 19, 8380. [Google Scholar] [CrossRef]
- Wang, B.; Gao, F.; Li, Y.; Lin, C.; Cheng, H.; Duan, X. Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers. Int. J. Envrion. Res. Public Health 2022, 19, 14614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).