Green Roof Design Techniques to Improve Water Use under Mediterranean Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
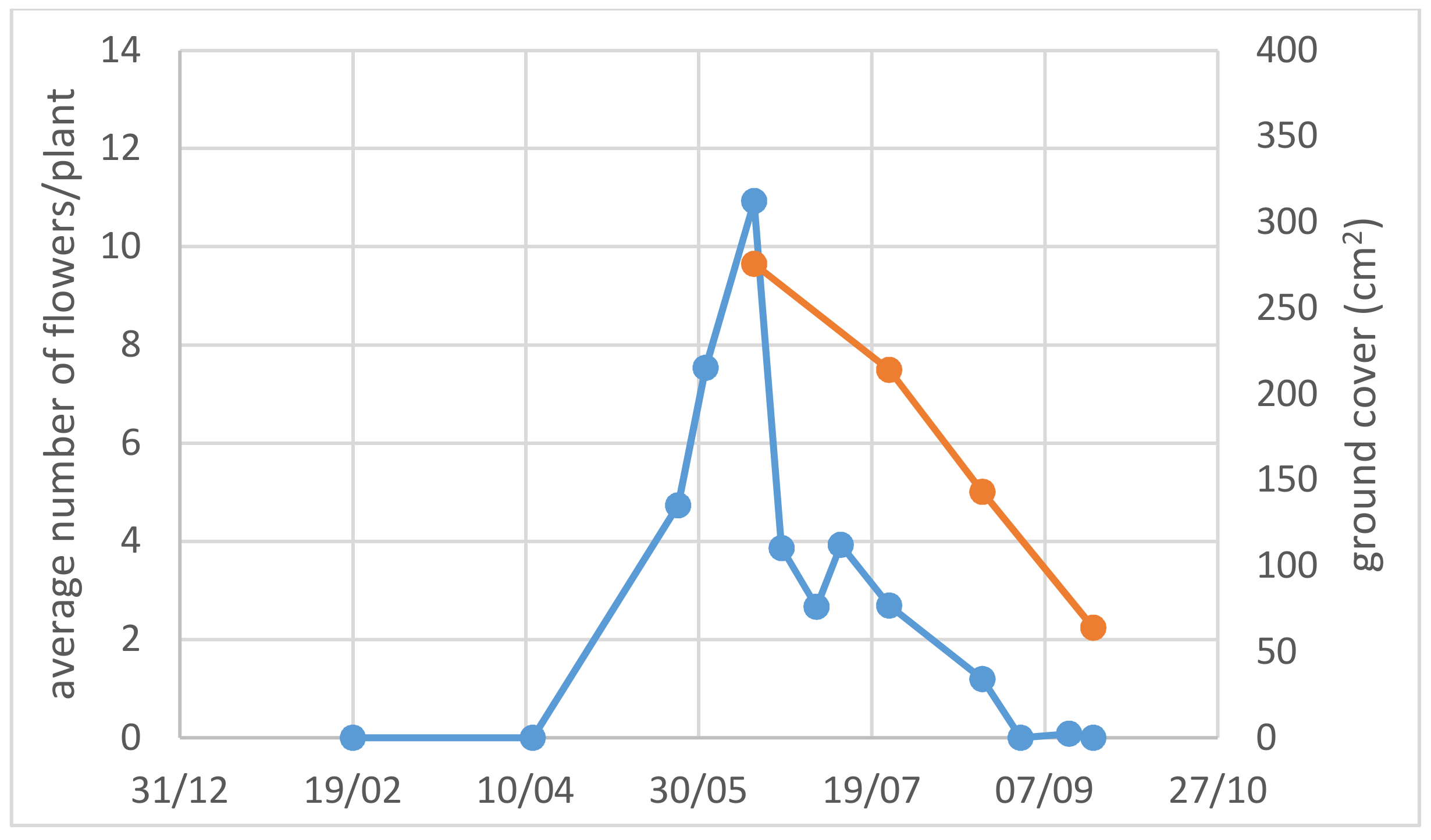
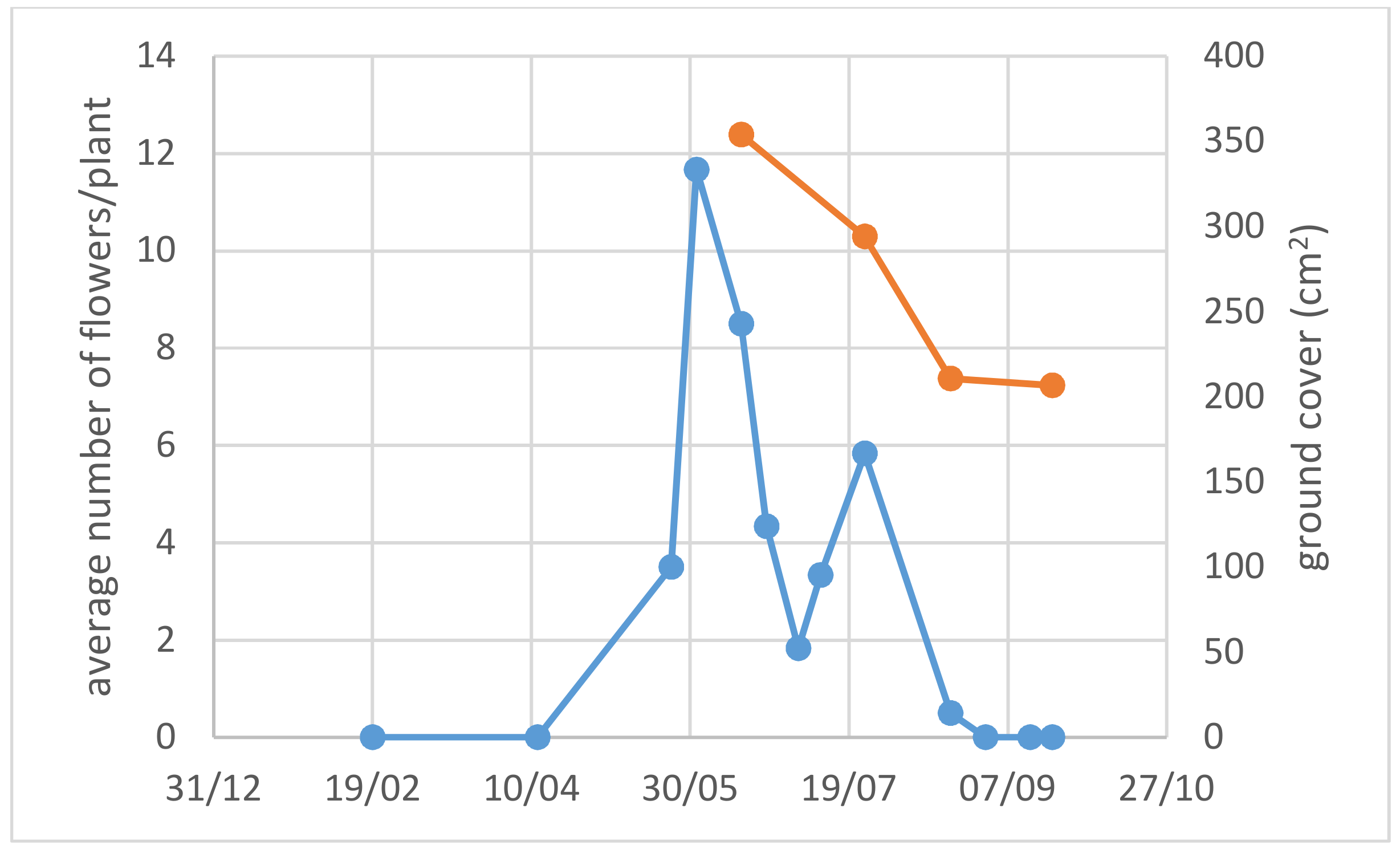
3.1. Use of Drought-Adapted Native Species
3.2. Use of Structural Materials with Water Retention Capacity
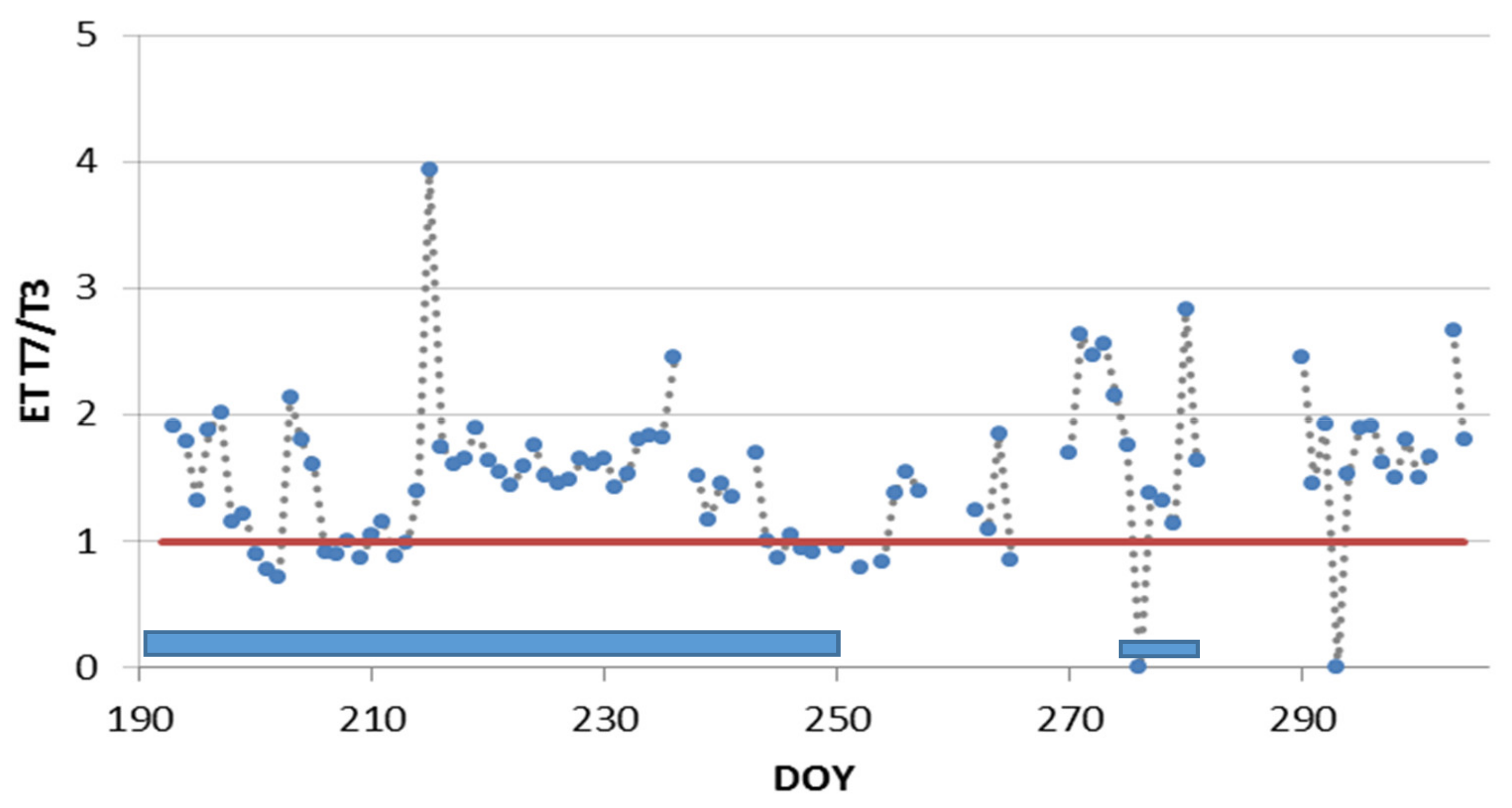
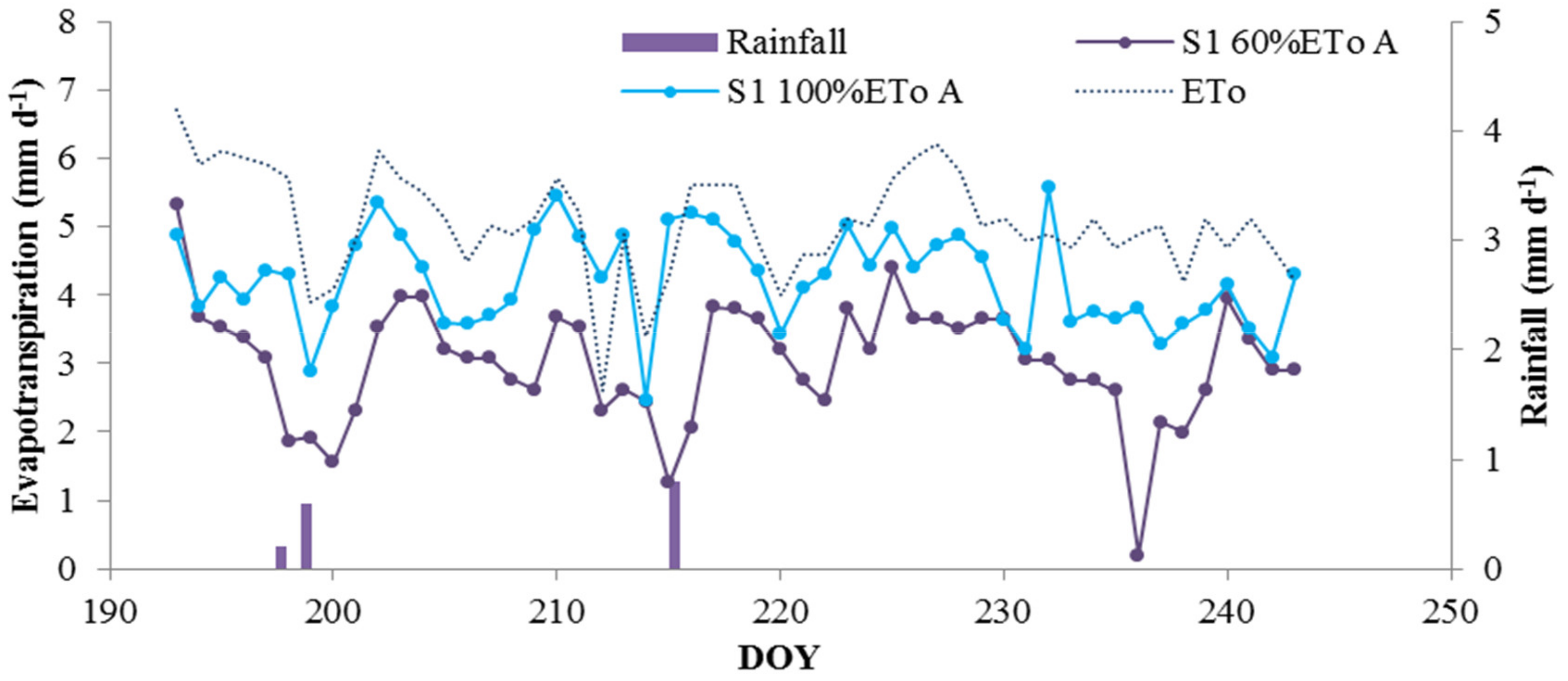
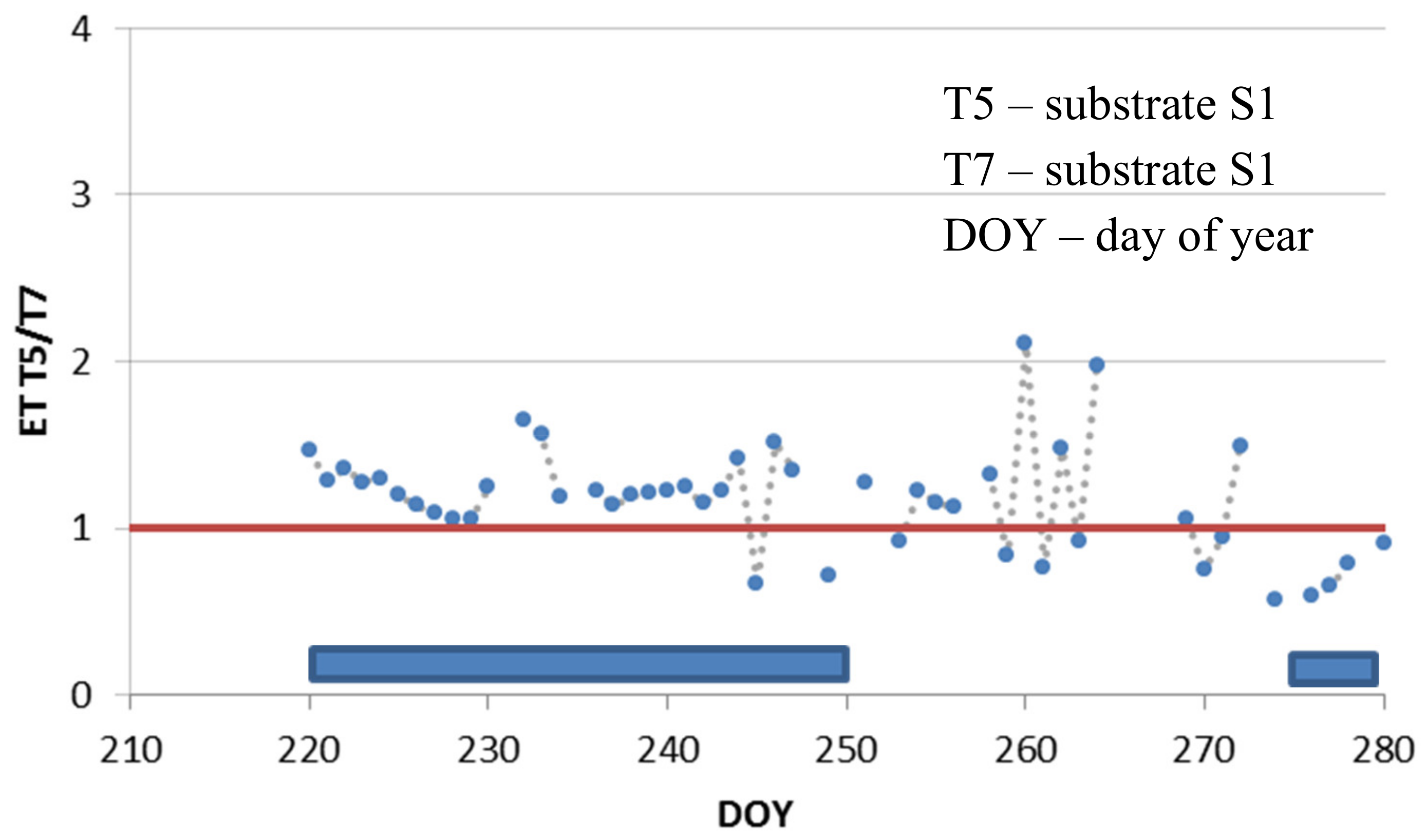
3.3. Deficit Irrigation vs. Aesthetic Value
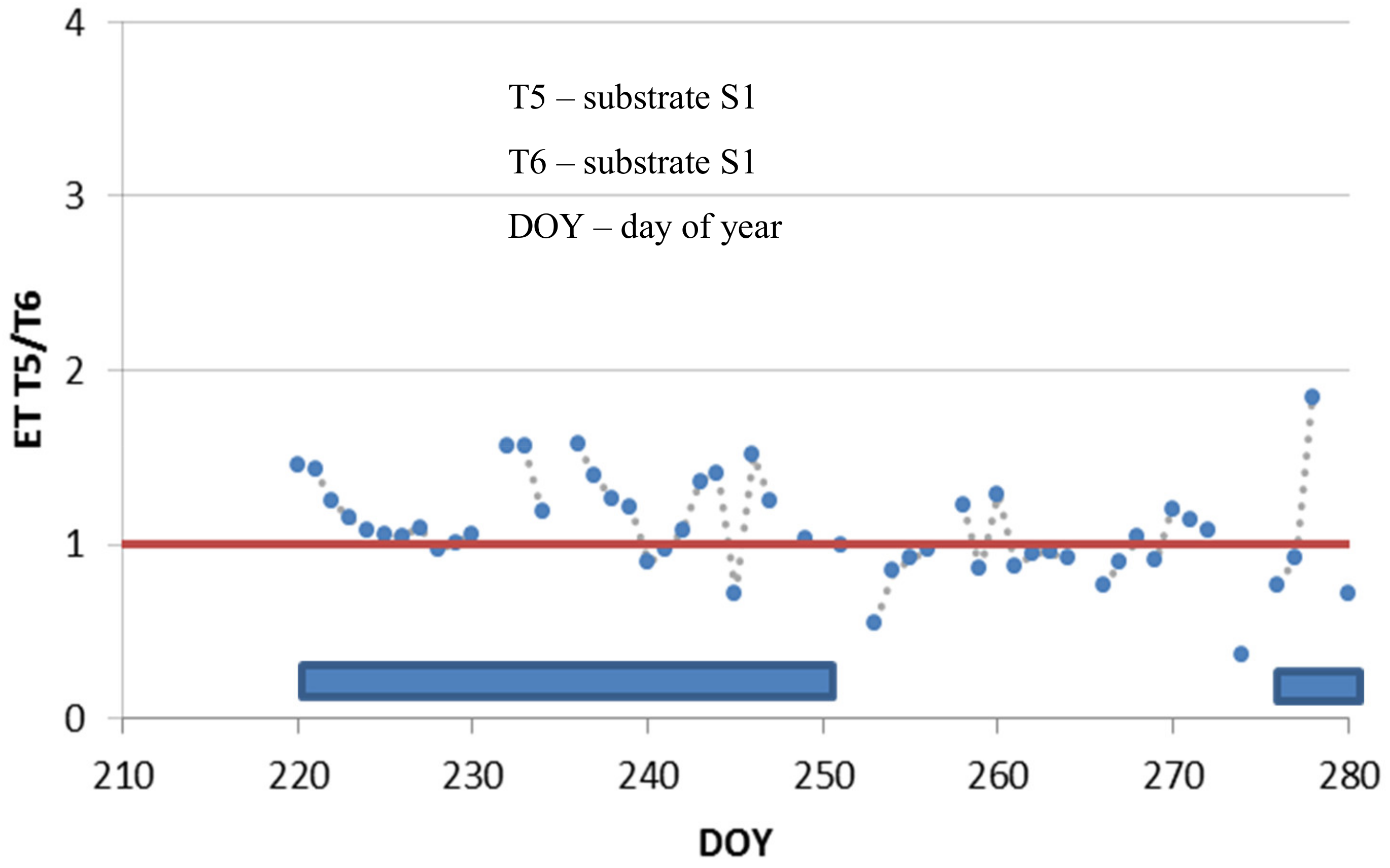
3.4. Mixtures of Vascular Plants and Mosses
3.5. Use of Moss-Dominated Biocrust Roofs
3.6. Precultivated Vegetation Blankets for Roofs
3.7. Wall Plants Transplanted to Roofs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fioretti, R.; Palla, A.; Lanza, L.G.; Principi, P. Green roof energy and water related performance in the Mediterranean climate. Build. Environ. 2010, 45, 1890–1904. [Google Scholar] [CrossRef]
- Van Mechelen, C.; Dutoit, T.; Hermy, M. Mediterranean open habitat vegetation offers great potential for extensive green roof design. Landsc. Urban Plan. 2014, 121, 81–91. [Google Scholar] [CrossRef]
- Lundholm, J.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Rowe, B.; Oberndorfer, E.; Bass, B.; Köhler, M.; Liu, K.K.Y. Green roofs as urban ecosystems: Ecological structures, functions, and services. Bioscience 2007, 57, 823–833. [Google Scholar]
- Ascione, F.; Bianco, N.; de’Rossi, F.; Turni, G.; Vanoli, G.P. Green roofs in European climates. Are effective solutions for the energy savings in air-conditioning? Appl. Energy 2013, 104, 845–859. [Google Scholar] [CrossRef]
- Susca, T.; Gaffin, S.R.; Dell’Osso, G.R. Positive effects of vegetation: Urban heat island and green roofs. Environ. Pollut. 2011, 159, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Santamouris, M. Cooling the cities—A review of reflective and green roof mitigation technologies to fight heat island and improve comfort in urban environments. Sol. Energy 2014, 103, 682–703. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B. The Role of Extensive Green Roofs in Sustainable Development. HortScience 2006, 41, 1276–1285. [Google Scholar]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Mini, C.; Hogue, T.S.; Pincetl, S. The effectiveness of water conservation measures on summer residential water use in Los Angeles, California. Resour. Conserv. Recycl. 2015, 94, 136–145. [Google Scholar] [CrossRef]
- Azenas, V.; Janner, I.; Medrano, H.; Gulias, J. Performance evaluation of five Mediterranean species to optimize ecosystem services of green roofs under water-limited conditions. J. Environ. Manag. 2018, 212, 236–247. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Kotzen, B. Chapter 4.2—Green Roofs Social and Aesthetic Aspects. In Nature Based Strategies for Urban and Building Sustainability; Pérez, G., Perini, K., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 273–281. [Google Scholar]
- Sutton, R. Aesthetics for green roofs and green walls. J. Living Archit. 2014, 1, 1–20. [Google Scholar]
- Shafique, M.; Kim, R.; Rafiq, M. Green roof benefits, opportunities and challenges—A review. Renew. Sustain. Energy Rev. 2018, 90, 757–773. [Google Scholar] [CrossRef]
- Andres-Domenech, I.; Perales-Momparler, S.; Morales-Torres, A.; Escuder-Bueno, I. Hydrological Performance of Green Roofs at Building and City Scales under Mediterranean Conditions. Sustainability 2018, 10, 3105. [Google Scholar] [CrossRef]
- Akther, M.; He, J.X.; Chu, A.; Huang, J.; van Duin, B. A Review of Green Roof Applications for Managing Urban Stormwater in Different Climatic Zones. Sustainability 2018, 10, 2864. [Google Scholar] [CrossRef]
- Salman, I.N.A.; Blaustein, L. Vegetation Cover Drives Arthropod Communities in Mediterranean/Subtropical Green Roof Habitats. Sustainability 2018, 10, 4209. [Google Scholar] [CrossRef]
- Vanstockem, J.; Vranken, L.; Bleys, B.; Somers, B.; Hermy, M. Do Looks Matter? A Case Study on Extensive Green Roofs Using Discrete Choice Experiments. Sustainability 2018, 10, 309. [Google Scholar] [CrossRef]
- Aksoy, Y. Examining the Ecological Quality of Kucukcekmece District Parks in Istanbul in Terms of Permeability and Natural Vegetation. Ekoloji 2010, 19, 181–189. [Google Scholar] [CrossRef]
- Cook, E.M.; Hall, S.J.; Larson, K.L. Residential landscapes as social-ecological systems: A synthesis of multi-scalar interactions between people and their home environment. Urban Ecosyst. 2012, 15, 19–52. [Google Scholar] [CrossRef]
- Katti, M.; Jones, A.R.; Caglar, D.O.; Delcore, H.D.; Gupta, K.K. The Influence of Structural Conditions and Cultural Inertia on Water Usage and Landscape Decision-Making in a California Metropolitan Area. Sustainability 2017, 9, 1746. [Google Scholar] [CrossRef]
- Kokkinou, I.; Ntoulas, N.; Nektarios, P.A.; Varela, D. Response of Native Aromatic and Medicinal Plant Species to Water Stress on Adaptive Green Roof Systems. Hortscience 2016, 51, 608–614. [Google Scholar]
- Anico, A. Plantas Autóctones em Coberturas Verdes: Avaliação do Desenvolvimento e Valor Estético Vs. Rega e Tipo de Substrato; Instituto Superior de Agronomia, Universidade de Lisboa: Lisbon, Portugal, 2015. [Google Scholar]
- Papafotiou, M.; Pergialioti, N.; Tassoula, L.; Massas, I.; Kargas, G. Growth of Native Aromatic Xerophytes in an Extensive Mediterranean Green Roof as Affected by Substrate Type and Depth and Irrigation Frequency. Hortscience 2013, 48, 1327–1333. [Google Scholar]
- Farrell, C.; Szota, C.; Williams, N.S.G.; Arndt, S.K. High water users can be drought tolerant: Using physiological traits for green roof plant selection. Plant Soil 2013, 372, 177–193. [Google Scholar] [CrossRef]
- Rayner, J.P.; Farrell, C.; Raynor, K.J.; Murphy, S.M.; Williams, N.S.G. Plant establishment on a green roof under extreme hot and dry conditions: The importance of leaf succulence in plant selection. Urban For. Urban Green. 2016, 15, 6–14. [Google Scholar] [CrossRef]
- Piro, P.; Carbone, M.; De Simone, M.; Maiolo, M.; Bevilacqua, P.; Arcuri, N. Energy and Hydraulic Performance of a Vegetated Roof in Sub-Mediterranean Climate. Sustainability 2018, 10, 3473. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Hui, S.; Chan, H.-M. Development of Modular Green Roofs for High-Density Urban Cities; World Green Roof Congress: London, UK, 2008. [Google Scholar]
- Clark, M.J.; Zheng, Y.B. Evaluating Fertilizer Influence on Overwintering Survival and Growth of Sedum Species in a Fall-installed Green Roof. Hortscience 2012, 47, 1775–1781. [Google Scholar]
- Savi, T.; Dal Borgo, A.; Love, V.L.; Andri, S.; Tretiach, M.; Nardini, A. Drought versus heat: What’s the major constraint on Mediterranean green roof plants? Sci. Total Environ. 2016, 566–567, 753–760. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilò, P.; Lo Gullo, M.A.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. Aob Plants 2015, 7, plv007. [Google Scholar] [CrossRef]
- Laranjeira, R. Simulação Energética de Coberturas Verdes Semi-Intensivas. Master’s Thesis, Instituto Superior Técnico, University of Lisbon, Lisbon, Portugal, 2018. (In Portuguese). [Google Scholar]
- De Cuyper, K.; Dinne, K.; van de Vel, L. Rainwater Discharge from Green Roofs; Dinne, K., Ed.; CIB: Delft, The Netherlands, 2004; Available online: http://www.irbnet.de/daten/iconda/CIB10549.pdf (accessed on 7 December 2018).
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H.C. The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Ingerpuu, N.; Liira, J.; Partel, M. Vascular plants facilitated bryophytes in a grassland experiment. Plant Ecol. 2005, 180, 69–75. [Google Scholar] [CrossRef]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Gornall, J.L.; Woodin, S.J.; Jonsdottir, I.S.; van der Wal, R. Balancing positive and negative plant interactions: How mosses structure vascular plant communities. Oecologia 2011, 166, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, R.D.; Branquinho, C.; Marques da Silva, J. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta 2011, 234, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, R.D.; Catalá, M.; Marques da Silva, J.; Branquinho, C.; Barreno, E. The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Ann. Bot. 2012, 110, 1007–1016. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.D.; Silva, A.B.; Soares, R.; Almeida, A.; Coelho, A.V.; Marques da Silva, J.; Branquinho, C. Differential proteomics of dehydration and rehydration in bryophytes: Evidence towards a common desiccation tolerance mechanism. Plant Cell Environ. 2014, 37, 1499–1515. [Google Scholar] [CrossRef]
- Stark, L.R.; Brinda, J.C.; McLetchie, D.N. Effects of increased summer precipitation and N deposition on Mojave Desert populations of the biological crust moss Syntrichia caninervis. J. Arid Environ. 2011, 75, 457–463. [Google Scholar] [CrossRef]
- Antoninka, A.; Bowker, M.A.; Reed, S.C.; Doherty, K. Production of greenhouse-grown biocrust mosses and associated cyanobacteria to rehabilitate dryland soil function. Restor. Ecol. 2016, 24, 324–335. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Santos, P.; Branquinho, C. Production of moss-dominated biocrusts to enhance the stability and function of the margins of artificial water bodies. Restor. Ecol. 2018, 26, 419–421. [Google Scholar] [CrossRef]



| Test Beds | Vegetation | I | S | |||
|---|---|---|---|---|---|---|
| L. luisieri | R. oficinalis | B. phoenicoides | Mosses | |||
| T1 | ✓ | ✓ | ✓ | ✓ | 60% ETo | S2 |
| T2 | ✓ | S3 | ||||
| T3 | ✓ | S2 | ||||
| T4 | ✓ | S1 + S3 | ||||
| T5 | ✓ | ✓ | ✓ | ✓ | S1 | |
| T6 | ✓ | S1 | ||||
| T7 | ✓ | S1 | ||||
| T8 | ✓ | 100% ETo | S1 | |||
| T9 | ✓ | S1 | ||||
| T10 | ✓ | S3 | ||||
| T11 | S3 | |||||
| T12 | ✓ | No I | S3 | |||
| Test Beds | Vegetation | I | S | ||||
|---|---|---|---|---|---|---|---|
| A. fistulosus | C. ruber | A. linkianum | S. sediforme | Mosses | |||
| T1 | 1 | 2 | 4 | 3 | ✓ | 60% ETo | S4 |
| T2 | 1 | 2 | 2 | 2 | S4 | ||
| T3 | 1 | 2 | 2 | 2 | S4 | ||
| T4 | 1 | 2 | 2 | 2 | S4 | ||
| T5 | 1 | 5 | 3 | ✓ | S4 | ||
| T6 | S4 | ||||||
| T7 | S4 | ||||||
| T8 | 1 | 2 | 2 | 2 | 100% ETo | S4 | |
| T9 | 1 | 2 | 2 | 2 | S4 | ||
| T10 | 1 | 2 | 2 | 2 | S4 | ||
| T11 | ✓ | S4 | |||||
| T12 | ✓ | No I | S4 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paço, T.A.; Cruz de Carvalho, R.; Arsénio, P.; Martins, D. Green Roof Design Techniques to Improve Water Use under Mediterranean Conditions. Urban Sci. 2019, 3, 14. https://doi.org/10.3390/urbansci3010014
Paço TA, Cruz de Carvalho R, Arsénio P, Martins D. Green Roof Design Techniques to Improve Water Use under Mediterranean Conditions. Urban Science. 2019; 3(1):14. https://doi.org/10.3390/urbansci3010014
Chicago/Turabian StylePaço, Teresa A., Ricardo Cruz de Carvalho, Pedro Arsénio, and Diana Martins. 2019. "Green Roof Design Techniques to Improve Water Use under Mediterranean Conditions" Urban Science 3, no. 1: 14. https://doi.org/10.3390/urbansci3010014
APA StylePaço, T. A., Cruz de Carvalho, R., Arsénio, P., & Martins, D. (2019). Green Roof Design Techniques to Improve Water Use under Mediterranean Conditions. Urban Science, 3(1), 14. https://doi.org/10.3390/urbansci3010014







