Ultrasound-Assisted Production of Virgin Olive Oil: Effects on Bioactive Compounds, Oxidative Stability, and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
- -
- Sigma-Aldrich (St. Louis, MO, USA): benzamide, butylhydroxytoluene (BHT), L-dithiothreitol, ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), triton X-100, polyvinylpyrrolidone (PVP), guaiacol, catechol, coomassie brilliant blue G 250, linoleic acid, sodium acetate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), diethyl ether, ethyl acetate, acetonitrile, standards for phenolic compounds (hydroxytyrosol, tyrosol, p-coumaric acid, hydroxytyrosol acetate, oleacein, methyl hemiacetal of oleocanthal, oleuropein aglycone, ligstroside aglycone and oleocanthal), standards for oleuropein, 4-methyl-2-pentanol and methyl pentadecanoate.
- -
- Kemika (Zagreb, Croatia): boric acid, anhydrous disodium hydrogen phosphate, potassium chloride, potassium phosphate, sodium dihydrogen phosphate-2-hydrate, isopropanol, isooctane, acetic acid, potassium hydroxide, sodium chloride, sodium thiosulfate, anhydrous sodium hydrogen sulphate and acetone.
- -
- Alfa Aesar (Haverhill, MA, USA): D-norleucine.
- -
- Lach-Ner (Neratovice, Czech Republic): ethanol and potassium iodide.
- -
- Fluka (Buchs, Switzerland): tween-40 and orthophosphoric acid.
- -
- T.T.T. (Sveta Nedjelja, Croatia): hydrogen peroxide and formic acid.
- -
- Honeywell (Offenbach, Germany): methanol, hexane and heptane.
- -
- Thermo Fischer Scientific (Waltham, MA, USA): starch and hydrochloric acid.
- -
- Santa Cruz Biotechnology (Dallas, TX, USA): bovine serum albumin.
- -
- LGC (Teddington, UK): α-tocopherol standard.
- -
- Millipore (Burlington, MA, USA): β-, γ- and δ-tocopherol standards.
- -
- Larodan (Solna, Sweden): standards for hydroperoxyoctadecatrienoic acid (HPOT).
2.2. Plant Material
2.3. Virgin Olive Oil Production
2.4. Determination of Oil Yield
2.5. Basic Quality Parameters
2.6. Determination of Fatty Acid Composition
2.7. Enzyme Activity Assay
2.7.1. Olive Paste and Acetone Powders
2.7.2. Lipoxygenase Extraction and Activity Assay
2.7.3. β-Glucosidase Extraction and Activity Assay
2.7.4. Polyphenol Oxidase Extraction and Activity Assay
2.7.5. Peroxidase Extraction and Activity Assay
2.7.6. Total Protein Content
2.8. Determination of Volatile Compounds
2.9. Determination of Phenolic Compounds
2.10. Determination of Tocopherols
2.11. Determination of Oxidative Stability
2.12. Determination of Antioxidative Capacity
2.13. Statistical Analysis
3. Results and Discussion
3.1. Influence of Olive Variety
3.1.1. Oil Yield
3.1.2. Fatty Acid Composition
3.1.3. Endogenous Enzymes
3.1.4. Volatile Compounds
3.1.5. Phenolic Compounds and Tocopherols
3.1.6. Oxidative Stability and Antioxidant Capacity
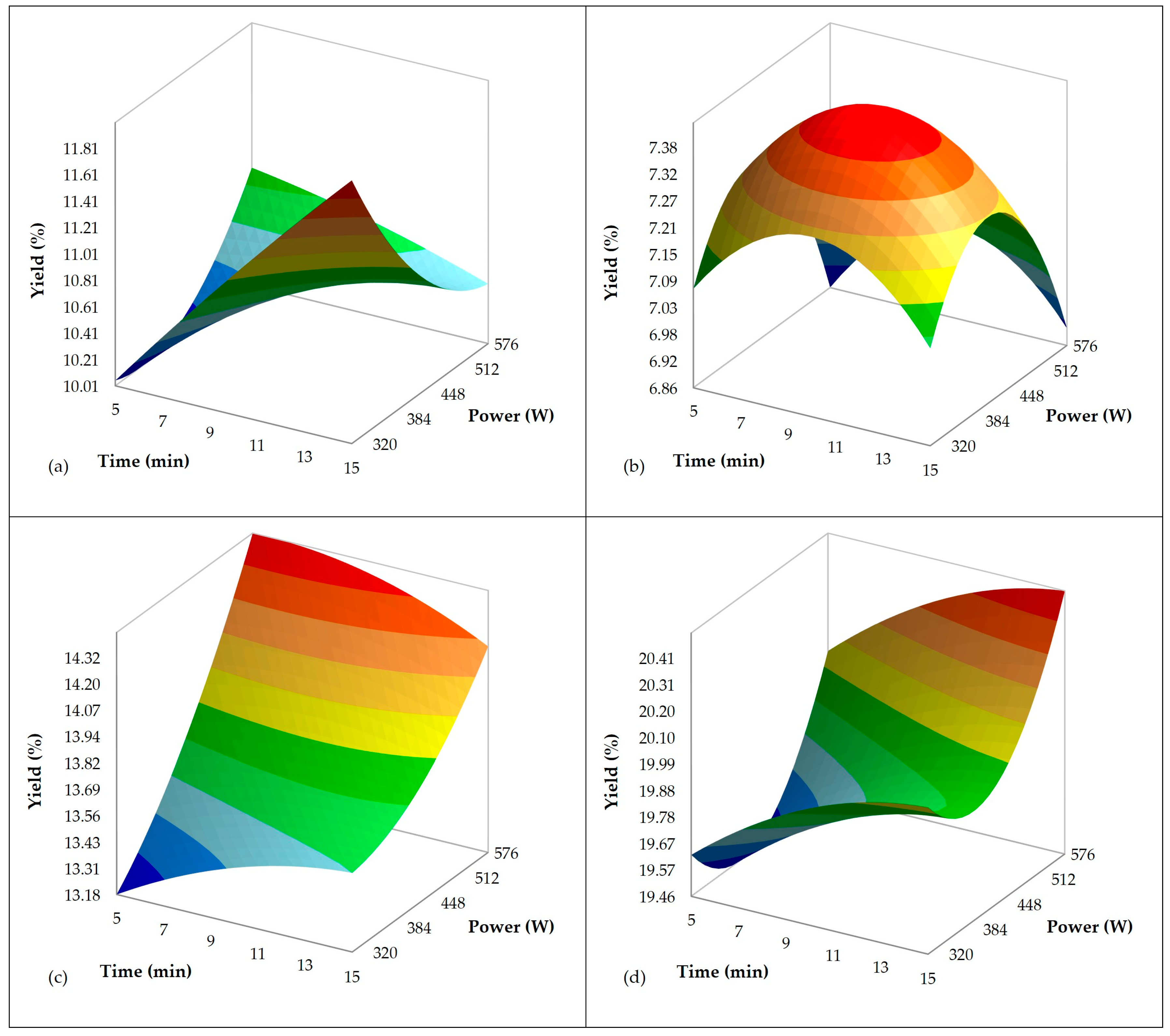
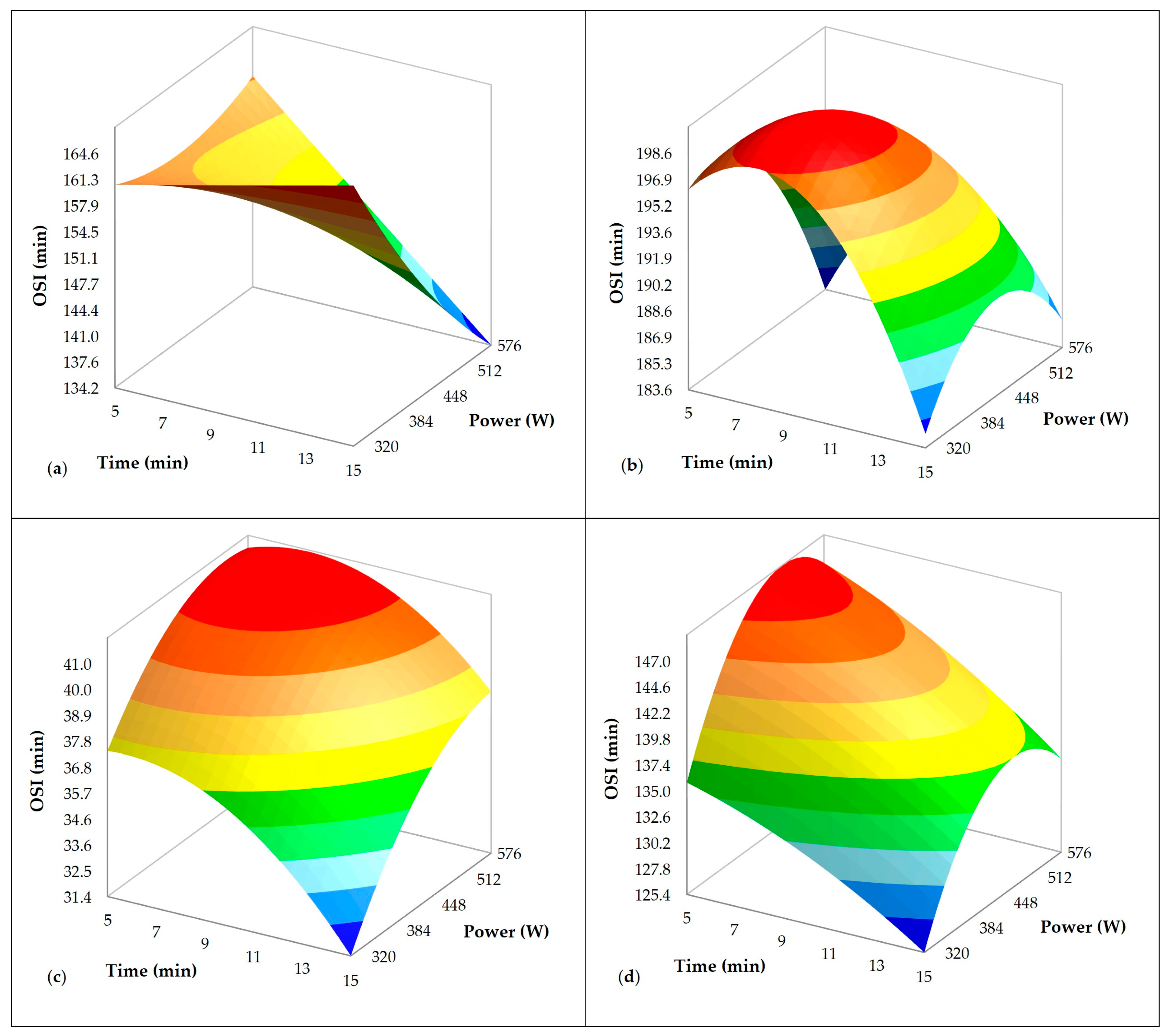
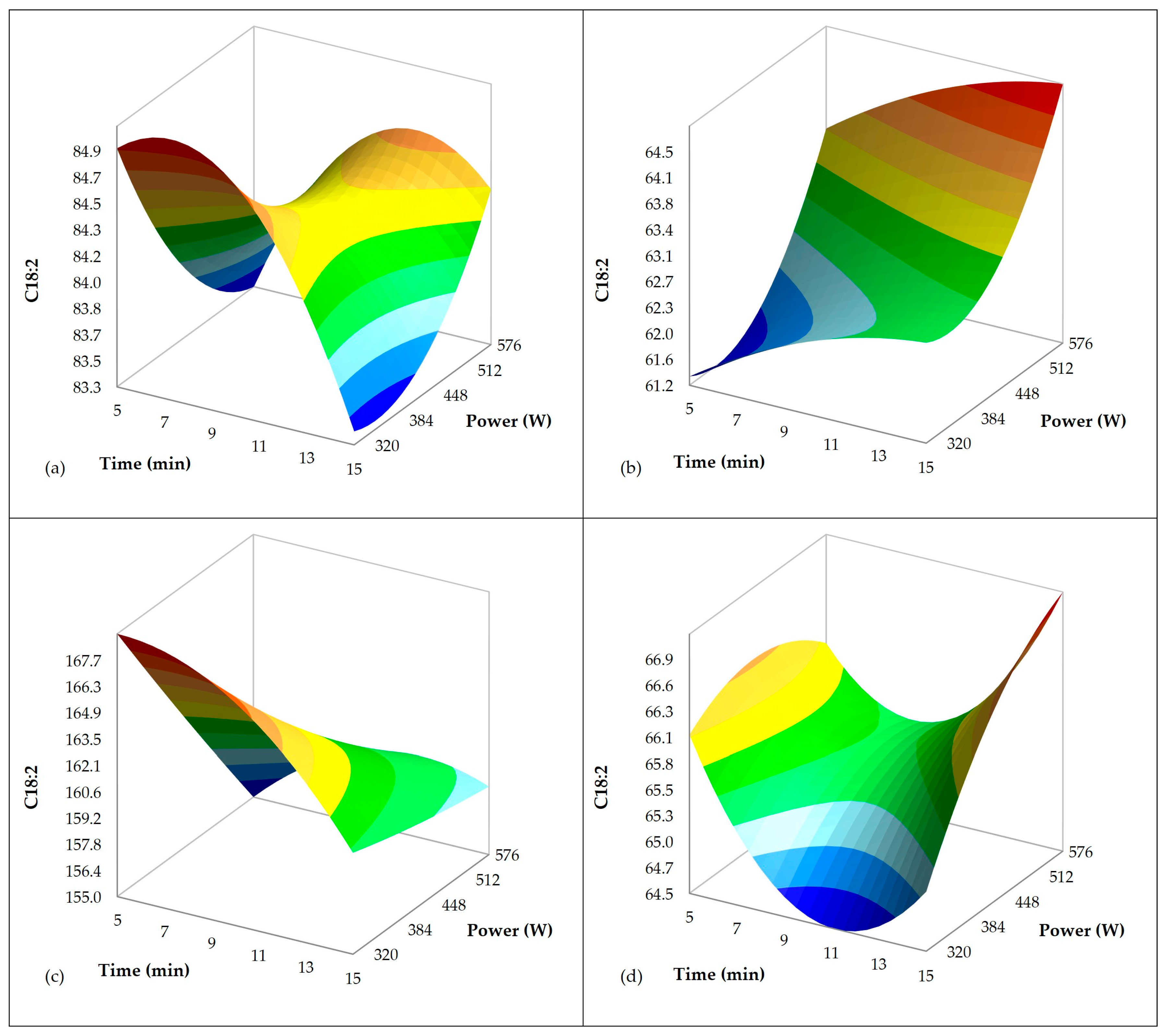
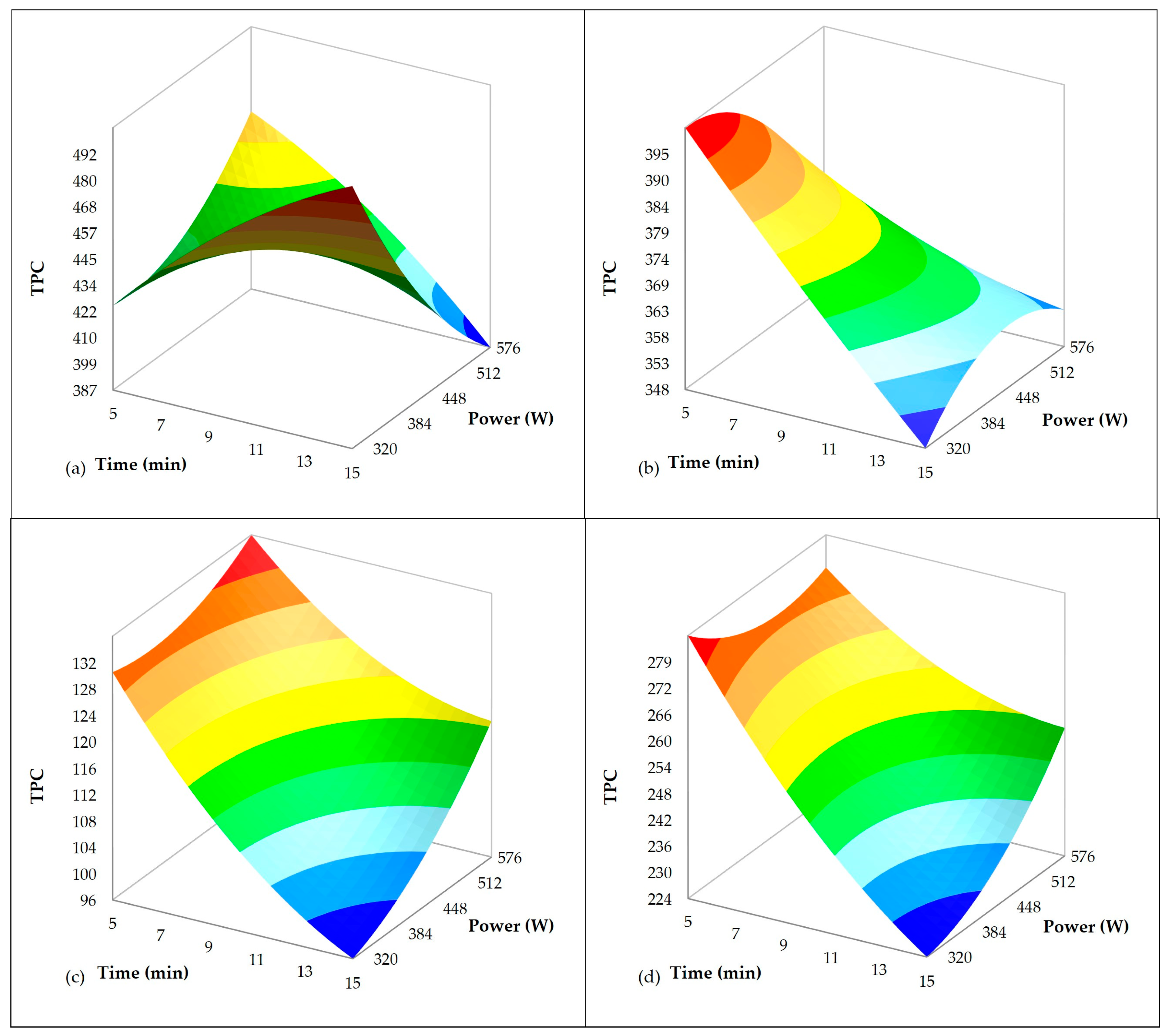
3.2. Influence of Ultrasound
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Y | Yield |
| VOO | Virgin olive oil |
| OSI | Oxidative stability index |
| AC | Antioxidant capacity |
| EE | Endogenous enzymes of the olive fruit |
| LOX | Lipoxygenase |
| Β-GLU | β-Glucosidase |
| PPO | Polyphenol oxidase |
| POX | Peroxidase |
| BHT | Butylhydroxytoluene |
| EDTA | Ethylenediaminetetraacetic acid |
| PMSF | Phenylmethylsulfonylfluoride |
| PVP | Polyvinylpyrrolidone |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HPOT | Hydroperoxyoctadecatrienoic acid |
| GC | Gas chromatography |
| FID | Flame ionization detector |
| FAME | Fatty acid methyl ester |
| DAD | Diode array detector |
| DSC | Differential scanning calorimeter |
| EPR | Electron paramagnetic resonance |
| ANOVA | Analysis of variance |
| RSM | Response surface methodology |
| SFA | Saturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| MBA | Microbiological activity |
| OX | Oxidation |
| TPC | Total phenolic content |
References
- Nardella, M.; Moscetti, R.; Bedini, G.; Bandiera, A.; Chakravartula, S.S.N.; Massantini, R. Impact of Traditional and Innovative Malaxation Techniques and Technologies on Nutritional and Sensory Quality of Virgin Olive Oil—A Review. Food Chem. Adv. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Polari, J.J.; Wang, S.C. Comparative Effect of Hammer Mill Screen Size and Cell Wall-Degrading Enzymes During Olive Oil Extraction. ACS Omega 2020, 5, 6074–6081. [Google Scholar] [CrossRef]
- Nardella, M.; Moscetti, R.; Chakravartula, S.S.N.; Bedini, G.; Massantini, R. A Review on High-Power Ultrasound-Assisted Extraction of Olive Oils: Effect on Oil Yield, Quality, Chemical Composition and Consumer Perception. Foods 2021, 10, 2743. [Google Scholar] [CrossRef]
- Amirante, R.; Paduano, A. Ultrasound in Olive Oil Extraction. In Products from Olive Tree; Boskou, D., Clodoveo, M.L., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 43–47. [Google Scholar] [CrossRef]
- Cecchi, L.; Bellumori, M.; Corbo, F.; Milani, G.; Clodoveo, M.L.; Mulinacci, N. Implementation of the Sono-Heat-Exchanger in the Extra Virgin Olive Oil Extraction Process: End-User Validation and Analytical Evaluation. Molecules 2019, 24, 2379. [Google Scholar] [CrossRef]
- Hachicha Hbaieb, R.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring Endogenous Enzymes during Olive Fruit Ripening and Storage: Correlation with Virgin Olive Oil Phenolic Profiles. Food Chem. 2015, 174, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L. Industrial Ultrasound Applications in the Extra-Virgin Olive Oil Extraction Process: History, Approaches, and Key Questions. Foods 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Sordini, B.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Servili, M. Improvement of Olive Oil Mechanical Extraction: New Technologies, Process Efficiency, and Extra Virgin Olive Oil Quality. In Products from Olive Tree; Boskou, D., Clodoveo, M.L., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 21–42. [Google Scholar] [CrossRef]
- Tamborrino, A.; Taticchi, A.; Romaniello, R.; Perone, C.; Esposto, S.; Leone, A.; Servili, M. Assessment of the Olive Oil Extraction Plant Layout Implementing a High-Power Ultrasound Machine. Ultrason. Sonochem. 2021, 73, 105505. [Google Scholar] [CrossRef]
- Pagano, M.; Tomasone, R.; Cedrola, C.; Fedrizzi, M.; Veneziani, G.; Servili, M. Use of Ultrasound in the Extraction Process of Virgin Olive Oil and Influence on Malaxation Time. In Innovative Biosystems Engineering for Sustainable Agriculture, Forestry and Food Production; Coppola, A., Di Renzo, G.C., Altieri, G., D’Antonio, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 67, pp. 703–712. [Google Scholar] [CrossRef]
- Manganiello, R.; Pagano, M.; Nucciarelli, D.; Ciccoritti, R.; Tomasone, R.; Di Serio, M.G.; Giansante, L.; Del Re, P.; Servili, M.; Veneziani, G. Effects of Ultrasound Technology on the Qualitative Properties of Italian Extra Virgin Olive Oil. Foods 2021, 10, 2884. [Google Scholar] [CrossRef]
- Paié-Ribeiro, J.; Baptista, F.; Teixeira, J.; Guedes, C.; Gomes, M.J.; Teixeira, A.; Barros, A.N.; Pinheiro, V.; Outor-Monteiro, D. From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients. Appl. Sci. 2024, 14, 5586. [Google Scholar] [CrossRef]
- Fainassi, F.; Taarji, N.; Benkhalti, F.; Hafidi, A.; Neves, M.A.; Isoda, H.; Nakajima, M. Emulsion Formation and Stabilizing Properties of Olive Oil Cake Crude Extracts. Processes 2021, 9, 633. [Google Scholar] [CrossRef]
- Suchintita Das, R.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of Ultrasound Processing on Alternative Protein Systems: Protein Extraction, Nutritional Effects and Associated Challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, S.; Pradhan, R.C. Ultrasound-Assisted Hydration of Finger Millet (Eleusine coracana) and Its Effects on Starch Isolates and Antinutrients. Ultrason. Sonochem. 2021, 73, 105542. [Google Scholar] [CrossRef]
- Aktı, N.; Yildiz, S.; Aktı, N.; Yildiz, S.; Yildiz, S. Exploring Ultrasound-Induced Free Radical Formation: A Comparative Study in Water and Sour Cherry Juice Using Glutathione and Terephthalic Acid Indicators. Ultrason. Sonochem. 2025, 112, 107193. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical Characterization of Virgin Olive Oil Obtained Using an Ultrasound-Assisted Extraction at an Industrial Scale: Influence of Olive Maturity Index and Malaxation Time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Gila, A.; Sánchez-Ortiz, A.; Jiménez, A.; Beltrán, G. The Ultrasound Application Does Not Affect to the Thermal Properties and Chemical Composition of Virgin Olive Oils. Ultrason. Sonochem. 2021, 70, 105320. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Mostafa, H.; Goodrich-Schneider, R.; Baker, G.L.; Welt, B.; Marshall, M.R. High Power Ultrasound: Impact on Olive Paste Temperature, Malaxation Time, Extraction Efficiency, and Characteristics of Extra Virgin Olive Oil. Food Bioprocess. Technol. 2018, 11, 634–644. [Google Scholar] [CrossRef]
- Jiménez, A.; Beltrán, G.; Uceda, M. High-Power Ultrasound in Olive Paste Pretreatment. Effect on Process Yield and Virgin Olive Oil Characteristics. Ultrason. Sonochem. 2007, 14, 725–731. [Google Scholar] [CrossRef]
- Juliano, P.; Bainczyk, F.; Swiergon, P.; Supriyatna, M.I.M.; Guillaume, C.; Ravetti, L.; Canamasas, P.; Cravotto, G.; Xu, X.Q. Extraction of Olive Oil Assisted by High-Frequency Ultrasound Standing Waves. Ultrason. Sonochem. 2017, 38, 104–114. [Google Scholar] [CrossRef]
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-Frequency, High-Power Ultrasound Treatment at Different Pressures for Olive Paste: Effects on Olive Oil Yield and Quality. Ultrason. Sonochem. 2019, 59, 104747. [Google Scholar] [CrossRef]
- Almeida, B.; Valli, E.; Bendini, A.; Gallina Toschi, T. Semi-Industrial Ultrasound-Assisted Virgin Olive Oil Extraction: Impact on Quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1–7. [Google Scholar] [CrossRef]
- Bejaoui, M.A.; Sánchez-Ortiz, A.; Aguilera, M.P.; Ruiz-Moreno, M.J.; Sánchez, S.; Jiménez, A.; Beltrán, G. High Power Ultrasound Frequency for Olive Paste Conditioning: Effect on the Virgin Olive Oil Bioactive Compounds and Sensorial Characteristics. Innov. Food Sci. Emerg. Technol. 2018, 47, 136–145. [Google Scholar] [CrossRef]
- Yahyaoui, A.; Rigane, G.; Mnif, S.; Salem, R.B.; Acar, A.; Arslan, D. Ultrasound Technology Parameters: Effects on Phenolics in Olive Paste and Oil in Relation to Enzymatic Activity. Eur. J. Lipid Sci. Technol. 2019, 121, 1–9. [Google Scholar] [CrossRef]
- IOC—Economic Affairs & Promotion Unit. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 25 August 2025).
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Bubola, K.B. Inter-Varietal Diversity of Typical Volatile and Phenolic Profiles of Croatian Extra Virgin Olive Oils as Revealed by GC-IT-MS and UPLC-DAD Analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef]
- Hrvatska Enciklopedija, Leksikografski Zavod Miroslav Krleža, 2013–2025. Available online: https://www.enciklopedija.hr/clanak/hrvatska-drzava (accessed on 25 August 2025).
- Žanetić, M.; Špika, M.J.; Ožić, M.M.; Bubola, K.B. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Strikić, F.; Klepo, T.; Rošin, J.; Radunić, M. Udomaćene Sorte Masline u Republici Hrvatskoj; Institut za Jadranske Kulture i Melioraciju Krša: Split, Croatia, 2010. [Google Scholar]
- International Olive Council. Guide for the Determination of the Characteristics of Oil-Olives; COI/OH/Doc. No 1; International Olive Council: Madrid, Spain, 2011. [Google Scholar]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-Scale Optimization of Olive Oil Extraction: Simultaneous Addition of Enzymes and Microtalc Improves the Yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneve, Switzerland, 2017.
- International Olive Council. Determination of Free Fatty Acids, Cold Method; COI/T.20/Doc. No 34/Rev. 1; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- International Olive Council. Spectrophotometric Investigation in the Ultraviolet; COI/T.20/Doc. No 19/Rev. 5; International Olive Council: Madrid, Spain, 2019. [Google Scholar]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneve, Switzerland, 2017.
- Romero-Segura, C.; Sanz, C.; Perez, A.G. Purification and Characterization of an Olive Fruit β-Glucosidase Involved in the Biosynthesis of Virgin Olive Oil Phenolics. J. Agric. Food Chem. 2009, 57, 7983–7988. [Google Scholar] [CrossRef]
- Luaces, P.; Pérez, A.G.; García, J.M.; Sanz, C. Effects of Heat-Treatments of Olive Fruit on Pigment Composition of Virgin Olive Oil. Food Chem. 2005, 90, 169–174. [Google Scholar] [CrossRef]
- Soldo, B.; Šprung, M.; Mušac, G.; Pavela-Vrančić, M.; Ljubenkov, I. Evaluation of Olive Fruit Lipoxygenase Extraction Protocols on 9- and 13-Z,E-HPODE Formation. Molecules 2016, 21, 506. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Antunes, P.; Ferreira-Dias, S. Phenolic Compounds of “Galega Vulgar” and “Cobrançosa” Olive Oils Along Early Ripening Stages. Food Chem. 2016, 211, 51–58. [Google Scholar] [CrossRef]
- Caponio, F.; Squeo, G.; Curci, M.; Silletti, R.; Paradiso, V.M.; Summo, C.; Crecchio, C.; Pasqualone, A. Calcium Carbonate Effect on Alkyl Esters and Enzymatic Activities during Olive Processing. Ital. J. Food Sci. 2018, 30, 381–392. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- International Olive Council. Determination of Biophenols in Olive Oils by HPLC; COI/T.20/Doc. No 29/Rev.1; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Škevin, D.; Balbino, S.; Žanetić, M.; Jukić Špika, M.; Koprivnjak, O.; Filipan, K.; Obranović, M.; Žanetić, K.; Smajić, E.; Radić, M.; et al. Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process. Foods 2025, 14, 2564. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High Performance Liquid Chromatography. ISO: Geneve, Switzerland, 2016.
- Tan, C.P.; Man, Y.B.C.; Selamat, J.; Yusoff, A. Analytical, Nutritional and Clinical Methods Section Comparative Studies of Oxidative Stability of Edible Oils by Differential Scanning Calorimetry and Oxidative Stability Index Methods. Food Chem. 2002, 76, 385–389. [Google Scholar] [CrossRef]
- European Union Commission. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union. 2022, 65, 1–22. [Google Scholar]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The Influence of the Malaxation Temperature on the Activity of Polyphenoloxidase and Peroxidase and on the Phenolic Composition of Virgin Olive Oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Simal-Gandara, J. Characterization of Virgin Olive Oils Produced with Autochthonous Galician Varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef]
- Bubola, K.B.; Lukić, M.; Novoselić, A.; Krapac, M.; Lukić, I. Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom. Foods 2020, 9, 1445. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Kriško, A.; Valić, S.; Carić, D.; Krapac, M.; Poljuha, D. Antioxidants, Radical-Scavenging and Protein Carbonylation Inhibition Capacity of Six Monocultivar Virgin Olive Oils in Istria (Croatia). Acta Aliment. 2016, 45, 427–433. [Google Scholar] [CrossRef]
- Franić, M.; Pasković, I.; Goreta Ban, S.; Marcelić, Š.; Lukić, M.; Rončević, S.; Nemet, I.; Kosić, U.; Soldo, B.; Polić Pasković, M. Cultivar-Dependent Effect of Silicon Foliar Application on Olive Fruit Yield, Morphology, and Olive Oil Quality Parameters. Appl. Sci. 2024, 14, 11500. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Belobrajić, I. Influence of Storage Temperature on Quality Parameters, Phenols and Volatile Compounds of Croatian Virgin Olive Oils. Grasas Aceites 2014, 65, e034. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Vivaldi, G.A.; Nicolás, E.N.; Paduano, A.; Salcedo, F.P.; Camposeo, S. Ripening Indices, Olive Yield and Oil Quality in Response to Irrigation with Saline Reclaimed Water and Deficit Strategies. Front. Plant Sci. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Kaniewski, D.; Marriner, N.; Morhange, C.; Khater, C.; Terral, J.F.; Besnard, G.; Otto, T.; Luce, F.; Couillebault, Q.; Tsitsou, L.; et al. Climate Change Threatens Olive Oil Production in the Levant. Nat. Plants 2023, 9, 219–227. [Google Scholar] [CrossRef]
- Juliano, P.; Gaber, M.A.F.M.; Romaniello, R.; Tamborrino, A.; Berardi, A.; Leone, A. Advances in Physical Technologies to Improve Virgin Olive Oil Extraction Efficiency in High-Throughput Production Plants. Food Eng. Rev. 2023, 15, 625–642. [Google Scholar] [CrossRef]
- Rotondo, A.; Bartolomeo, G.; Spanò, I.M.; La Torre, G.L.; Pellicane, G.; Molinu, M.G.; Culeddu, N. Comparison between Traditional and Novel NMR Methods for the Analysis of Sicilian Monovarietal Extra Virgin Olive Oils: Metabolic Profile Is Influenced by Micro-Pedoclimatic Zones. Molecules 2024, 29, 4532. [Google Scholar] [CrossRef]
- Špika, M.J.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef]
- European Union Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union. 2012, 55, 1–40. [Google Scholar]
- Soldo, B.; Jukić Špika, M.; Pasković, I.; Vuko, E.; Polić Pasković, M.; Ljubenkov, I. The Composition of Volatiles and the Role of Non-Traditional LOX on Target Metabolites in Virgin Olive Oil from Autochthonous Dalmatian Cultivars. Molecules 2024, 29, 1696. [Google Scholar] [CrossRef]
- Susamcı, E.; Tuncay, Ö.; Bayraktar, H.; Önal, S. Phenol Content and β-Glucosidase Activity during the Ripening Period of Olive Fruits (Erkence Cv.) from Different Locations. Grasas Aceites 2024, 75, 2017. [Google Scholar] [CrossRef]
- Vaz, J.E.; Rabelo, L.; Zaiter, M.A.; Pereira, W.E.S.; Metzker, G.; Boscolo, M.; da Silva, R.; Gomes, E.; da Silva, R.R. Functional Properties and Potential Application of Ethanol Tolerant β-Glucosidases from Pichia Ofunaensis and Trichosporon Multisporum Yeasts. 3 Biotech. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Bhagwat, P.; Pillai, S. Biochemical and in Silico Structural Properties of a Thermo-Acid Stable β-Glucosidase from Beauveria Bassiana. Heliyon 2024, 10, 28667. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Brkic Bubola, K.; Koprivnjak, O.; Sladonja, B.; Škevin, D.; Belobrajić, I. Influence of Harvest Time on the Composition and Quality of Rosinjola Cultivar Virgin Olive Oils. Croat. J. Food Sci. Technol. 2012, 4, 9–18. [Google Scholar]
- Malheiro, R.; Rodrigues, N.; Pereira, J.A. Olive Oil Phenolic Composition as Affected by Geographic Origin, Olive Cultivar, and Cultivation Systems. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 93–121. [Google Scholar] [CrossRef]
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C.I.G.; Jerković, I. Phytochemicals and Other Characteristics of Croatian Monovarietal Extra Virgin Olive Oils from Oblica, Lastovka and Levantinka Varieties. Molecules 2015, 20, 4395–4409. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Kraljić, K.; Koprivnjak, O.; Škevin, D.; Žanetić, M.; Katalinić, M. Effect of Agronomical Factors and Storage Conditions on the Tocopherol Content of Oblica and Leccino Virgin Olive Oils. J. Am. Oil Chem. Soc. 2015, 92, 1293–1301. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Ouzounidou, G.; Tsantili, E. Fruit Ripening, Antioxidants and Oil Composition in Koroneiki Olives (Olea Europea l.) at Different Maturity Indices. Agronomy 2021, 11, 122. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Vrhovnik, I.; Hladnik, T.; Prgomet, Ž.; Hlevnjak, B.; Majetić, V. Characteristics of nutritive value of virgin olive oils from Buža, Istarska bjelica, Leccino and Rosulja cultivars. Croat. J. Food Sci. Technol. 2012, 7, 172–178. [Google Scholar]
- European Union Commission. Commission Regulation (EU) No 1924/2006 of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union. 2006, 49, 9–25. [Google Scholar]
- European Union Commission. Commission Regulation (EU) No 1169/2011 of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union. 2011, 54, 18–63. [Google Scholar]
- Olmo-Cunillera, A.; Pérez, M.; López-Yerena, A.; Abuhabib, M.M.; Ninot, A.; Romero-Aroca, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Oleacein and Oleocanthal: Key Metabolites in the Stability of Extra Virgin Olive Oil. Antioxidants 2023, 12, 1776. [Google Scholar] [CrossRef]
- Bilušić, T.; Žanetić, M.; Ljubenkov, I.; Generalić Mekinić, I.; Štambuk, S.; Bojović, V.; Soldo, B.; Magiatis, P. Molecular Characterization of Dalmatian Cultivars and the Influence of the Olive Fruit Harvest Period on Chemical Profile, Sensory Characteristics and Oil Oxidative Stability. Eur. Food Res. Technol. 2018, 244, 281–289. [Google Scholar] [CrossRef]
- Bohn, V.F.; Schappo, F.B.; Braga Alves, A.S.; Ferreira Ribeiro, C.D.; Nunes, I.L. Polyphenol antioxidants in vegetable oils: A scientific and technological prospecting. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands; London, UK; Cambridge, MA, USA, 2024; Volume 80, pp. 407–436. [Google Scholar] [CrossRef]
- Rigane, G.; Yahyaoui, A.; Acar, A.; Mnif, S.; Salem, R.B.; Arslan, D. Change in Some Quality Parameters and Oxidative Stability of Olive Oils with Regard to Ultrasound Pretreatment, Depitting and Water Addition. Biotechnol. Rep. 2020, 26, 4–9. [Google Scholar] [CrossRef]
- Aydar, A.Y.; Bağdatlıoğlu, N.; Köseoğlu, O. Effect of ultrasound on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM). Grasas Aceites 2017, 68, 189. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-Virgin Olive Oil Extracted Using Pulsed Electric Field Technology: Cultivar Impact on Oil Yield and Quality. Front. Nutr. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-Flow Industrial Olive-Oil Production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef]
- Boffa, L.; Calcio Gaudino, E.; Grillo, G.; Binello, A.; Capaldi, G.; Rego, D.; Pereira, M.; Cravotto, G. Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment. Foods 2024, 13, 2613. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Lukić, I.; Brkić Bubola, K. Evolution of phenolic compounds and quality parameters after storage of Istarska bjelica and Buža cv. virgin olive oil. In Proceedings of the 55th Croatian & 15th International Symposium on Agriculture, Vodice, Croatia, 16–21 February 2020. [Google Scholar]
- Chemat, F.; Grondin, I.; Costes, P.; Moutoussamy, L.; Sing, A.S.C.; Smadja, J. High Power Ultrasound Effects on Lipid Oxidation of Refined Sunflower Oil. Ultrason. Sonochem. 2004, 11, 281–285. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Durante, V.; La Notte, D.; Punzi, R.; Gambacorta, G. Ultrasound-Assisted Extraction of Virgin Olive Oil to Improve the Process Efficiency. Eur. J. Lipid Sci. Technol. 2013, 115, 1062–1069. [Google Scholar] [CrossRef]



| Sample | Treatment Duration (min) | Ultrasonic Bath Power (W) | ΔT During the Treatment (°C) |
|---|---|---|---|
| 1-control | 0 | 0 | 28.3 ± 1.7 * |
| 2 | 10 | 256 | −1.2 |
| 3 | 5 | 320 | −1.4 |
| 4 | 15 | 320 | 1.9 |
| 5 | 3 | 448 | −1.4 |
| 6-central | 10 | 448 | 1.4 |
| 7 | 17 | 448 | 3.9 |
| 8 | 5 | 576 | −0.3 |
| 9 | 15 | 576 | 4.4 |
| 10 | 10 | 640 | 2.4 |
| Fatty Acids/Fatty Acid Groups (mg/g) | p-Value | Variety | |||
|---|---|---|---|---|---|
| Istarska Bjelica | Rosulja | Oblica | Levantinka | ||
| C16:0 | <0.0001 | 13.4 ± 0.2 b | 14.1 ± 0.1 a | 13.8 ± 0.2 a | 11.5 ± 0.1 c |
| C18:0 | <0.0001 | 3.1 ± 0.1 b | 2.3 ± 0.0 c | 2.4 ± 0.1 c | 4.1 ± 0.1 a |
| C18:1 | <0.0001 | 70.1 ± 0.2 c | 72.5 ± 0.2 b | 63.7 ± 0.5 d | 73.6 ± 0.6 a |
| C18:2 | <0.0001 | 8.6 ± 0.2 b | 6.6 ± 0.0 c | 16.1 ± 0.4 a | 6.9 ± 0.3 c |
| SFA | <0.0001 | 17.2 ± 0.3 a | 17.0 ± 0.0 ab | 16.7 ± 0.1 b | 16.3 ± 0.2 c |
| MUFA | <0.0001 | 71.6 ± 0.2 b | 74.3 ± 0.2 a | 64.9 ± 0.4 c | 74.7 ± 0.6 a |
| PUFA | <0.0001 | 9.3 ± 0.3 b | 7.4 ± 0.0 c | 16.9 ± 0.4 a | 7.5 ± 0.4 c |
| MUFA/PUFA | <0.0001 | 7.7 ± 0.2 b | 10.0 ± 0.0 a | 3.8 ± 0.1 c | 10.0 ± 0.5 a |
| Activity of Endogenous Enzymes (μmol/mg min) | p-Value | Variety | |||
|---|---|---|---|---|---|
| Istarska Bjelica | Rosulja | Oblica | Levantinka | ||
| LOX | <0.0001 | 0.120 ± 0.012 b | 1.638 ± 0.072 a | 0.132 ± 0.004 b | 0.190 ± 0.016 b |
| β-GLU | <0.0001 | <LOD *b | 0.031 ± 0.001 a | <LOD *b | <LOD *b |
| PPO | <0.0001 | 1.593 ± 0.112 a | <LOD *c | 0.016 ± 0.021 c | 0.459 ± 0.026 b |
| POX | <0.0001 | <LOD *b | <LOD *b | <LOD *b | 0.007 ± 0.002 a |
| Groups of Volatile Compounds (mg/kg) | p-Value | Variety | |||
|---|---|---|---|---|---|
| Istarska Bjelica | Rosulja | Oblica | Levantinka | ||
| 2-Methylbutanal | 0.126 | 0.22 ± 0.04 | 0.04 ± 0.03 | 0.08 ± 0.02 | 0.38 ± 0.33 |
| Pentanal | 0.154 | 0.29 ± 0.30 | 0.35 ± 0.30 | <LOD * | <LOD * |
| 2-Pentenal | 0.834 | 0.24 ± 0.03 | 0.16 ± 0.11 | 0.18 ± 0.03 | 0.19 ± 0.19 |
| 2-Hexenal | <0.0001 | 15.27 ± 1.31 b | 37.18 ± 4.21 a | 9.42 ± 0.95 b | 10.92 ± 5.43 b |
| 2,4-Hexadienal | 0.005 | 2.92 ± 0.23 a | 3.28 ± 0.26 a | 2.97 ± 0.27 a | 1.54 ± 0.76 b |
| 4-Oxohex-2-enal | <0.0001 | 3.54 ± 0.40 a | 2.27 ± 0.25 b | 2.10 ± 0.24 b | 0.52 ± 0.38 c |
| Nonanal | <0.0001 | 0.32 ± 0.05 a | 0.36 ± 0.06 a | 0.12 ± 0.00 b | 0.21 ± 0.03 b |
| 3-Hexenal + 2-Methyl-4-pentenal | <0.0001 | 14.31 ± 0.77 a | 7.20 ± 0.89 c | 10.32 ± 0.94 b | 1.81 ± 0.98 d |
| Total aldehydes | <0.0001 | 37.11 ± 1.90 b | 50.84 ± 4.77 a | 25.19 ± 2.43 bc | 15.57 ± 7.42 c |
| 1-Penten-3-ol | 0.111 | 0.39 ± 0.41 | 1.49 ± 1.46 | 2.51 ± 2.51 | 5.42 ± 3.42 |
| (E)-2-Penten-1-ol | 0.560 | 0.16 ± 0.01 | 0.05 ± 0.06 | 0.04 ± 0.04 | 0.50 ± 0.86 |
| (Z)-2-Penten-1-ol | 0.192 | 1.93 ± 0.06 | 1.32 ± 0.08 | 0.91 ± 0.19 | 1.19 ± 1.03 |
| (Z)-3-Hexen-1-ol | 0.338 | 2.20 ± 0.26 | 1.17 ± 2.02 | 3.39 ± 0.10 | 2.13 ± 1.86 |
| 2-Hexen-1-ol | 0.483 | <LOD * | 0.10 ± 0.02 | 0.05 ± 0.05 | 0.90 ± 1.56 |
| 1-Hexanol | 0.512 | 0.10 ± 0.02 | <LOD * | 0.57 ± 0.11 | 1.09 ± 1.89 |
| Total alcohols | 0.006 | 4.77 ± 0.62 b | 4.13 ± 1.66 b | 7.48 ± 2.62 ab | 11.23 ± 1.92 a |
| 1-Penten-3-one | 0.003 | 6.11 ± 0.23 a | 1.93 ± 1.67 b | 2.02 ± 1.95 b | <LOD *b |
| Total ketones | 0.003 | 6.11 ± 0.23 a | 1.93 ± 1.67 b | 2.02 ± 1.95 b | <LOD *b |
| 3-Hexenyl acetate | <0.0001 | <LOD *b | 0.44 ± 0.25 b | <LOD *b | 2.69 ± 0.42 a |
| Hexyl acetate | <0.0001 | <LOD *b | <LOD *b | <LOD *b | 1.62 ± 0.10 a |
| Total esters | <0.0001 | <LOD *b | 0.44 ± 0.25 b | <LOD *b | 4.31 ± 0.46 a |
| ΣLOX § | <0.0001 | 40.75 ± 2.44 b | 51.05 ± 2.86 a | 29.42 ± 2.58 c | 28.45 ± 4.86 c |
| ΣOX ¥ | <0.0001 | 7.07 ± 0.48 a | 6.26 ± 0.42 ab | 5.19 ± 0.50 b | 2.27 ± 1.13 c |
| ΣMBA £ | 0.126 | 0.22 ± 0.04 | 0.04 ± 0.03 | 0.08 ± 0.02 | 0.38 ± 0.33 |
| Phenolic Compounds (mg/kg) | p-Value | Variety | |||
|---|---|---|---|---|---|
| Istarska Bjelica | Rosulja | Oblica | Levantinka | ||
| Hydroxytyrosol | 0.157 | 2 ± 0 | 7 ± 2 | <LOD * | 14 ± 22 |
| Tyrosol | 0.112 | 4 ± 0 | <LOD * | 4 ± 0 | 11 ± 15 |
| p-Coumaric acid | <0.0001 | 3 ± 0 b | <LOD *c | 7 ± 1 a | 3 ± 0 b |
| Hydroxytyrosol acetate | 0.089 | <LOD * | <LOD * | <LOD * | 1 ± 1 |
| Oleacein | <0.0001 | 136 ± 23 a | 145 ± 4 a | 38 ± 8 c | 71 ± 18 b |
| Methyl hemiacetal of oleocanthal | <0.0001 | 19 ± 1 b | 32 ± 2 a | 7 ± 4 c | 15 ± 3 b |
| Oleuropein aglycone | <0.0001 | 85 ± 6 b | 110 ± 5 a | 24 ± 11 d | 46 ± 6 c |
| Ligstroside aglycone | <0.0001 | 78 ± 12 a | 32 ± 1 b | 35 ± 3 b | 41 ± 3 b |
| Oleocanthal | <0.0001 | 94 ± 2 a | 42 ± 1 d | 46 ± 1 c | 60 ± 4 b |
| Total | <0.0001 | 420 ± 12 a | 368 ± 9 b | 161 ± 26 d | 263 ± 30 c |
| α-tocopherol (mg/kg) | <0.0001 | 332 ± 52 a | 272 ± 10 b | 273 ± 22 b | 367 ± 18 a |
| Parameter | p-Value | Variety | |||
|---|---|---|---|---|---|
| Istarska Bjelica | Rosulja | Oblica | Levantinka | ||
| OSI (min) | <0.0001 | 155.2 ± 4.6 b | 206.3 ± 0.6 a | 55.1 ± 2.7 d | 138.8 ± 4.0 c |
| AC (% DPPH radical reduction) | <0.0001 | 58.33 ± 1.89 b | 73.28 ± 2.12 a | 39.09 ± 3.35 c | 56.61 ± 4.28 b |
| Model Parameter | Y (%) | OSI (min) | AC (% of DPPH Radical Reduction) | Linoleic Acid (mg/g) | TPC (mg/kg) | α-Tocopherol (mg/kg) |
|---|---|---|---|---|---|---|
| Intercept | 12.923 | 130.94 | 53.037 | 93.6 | 296.586 | 287.106 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Time | 0.159 | −3.908 | −2.012 | −0.166 | −12.653 | −2.557 |
| p-value | 0.093 | 0.004 | <0.0001 | 0.603 | 0.000 | 0.375 |
| Power | 0.105 | −0.879 | −0.453 | −0.649 | −3.305 | −0.228 |
| p-value | 0.247 | 0.477 | 0.272 | 0.037 | 0.275 | 0.935 |
| Variety-Istarska Bjelica | −2.167 | 24.252 | 8.156 | −9.462 | 147.029 | 13.317 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 |
| Variety-Levantinka | 7.05 | 7.46 | −3.413 | −27.823 | −41.035 | 35.01 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Variety-Oblica | 0.9 | −92.602 | −22.275 | 68.004 | −179.161 | −33.683 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Variety-Rosulja | −5.783 | 60.89 | 17.533 | −30.719 | 73.168 | −14.644 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0 |
| Time * Power | −0.184 | −1.325 | 0.4 | 1.066 | −3.992 | 16.688 |
| p-value | 0.164 | 0.462 | 0.505 | 0.019 | 0.365 | <0.0001 |
| Time * Variety-Istarska Bjelica | 0.217 | −1.066 | −0.403 | 0.053 | 12.657 | −6.509 |
| p-value | 0.182 | 0.63 | 0.585 | 0.923 | 0.021 | 0.193 |
| Time * Variety-Levantinka | 0.064 | −1.879 | −1.322 | 0.191 | −8.421 | −5.966 |
| p-value | 0.691 | 0.398 | 0.076 | 0.729 | 0.122 | 0.233 |
| Time * Variety-Oblica | −0.131 | 1.542 | −0.204 | −1.087 | −0.853 | 1.509 |
| p-value | 0.419 | 0.487 | 0.783 | 0.051 | 0.875 | 0.762 |
| Time * Variety-Rosulja | −0.151 | 1.403 | 1.929 | 0.842 | −3.382 | 10.966 |
| p-value | 0.352 | 0.527 | 0.01 | 0.129 | 0.532 | 0.03 |
| Power * Variety-Istarska Bjelica | −0.276 | −7.309 | −2.812 | 0.493 | −15.387 | 0.169 |
| p-value | 0.082 | 0.001 | 0 | 0.356 | 0.004 | 0.972 |
| Power * Variety-Levantinka | 0.115 | 5.803 | 1.782 | 1.131 | 8.789 | −11.743 |
| p-value | 0.461 | 0.009 | 0.014 | 0.036 | 0.095 | 0.016 |
| Power * Variety-Oblica | 0.363 | 3.574 | 2.376 | −3.343 | 9.826 | −1.184 |
| p-value | 0.024 | 0.1 | 0.001 | <0.0001 | 0.062 | 0.806 |
| Power * Variety-Rosulja | −0.202 | −2.068 | −1.347 | 1.719 | −3.229 | 12.757 |
| p-value | 0.199 | 0.336 | 0.061 | 0.002 | 0.537 | 0.009 |
| R2 | 0.991 | 0.988 | 0.958 | 0.997 | 0.966 | 0.653 |
| R2 adjusted | 0.988 | 0.985 | 0.952 | 0.996 | 0.962 | 0.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipan, K.; Kraljić, K.; Žanetić, M.; Jukić Špika, M.; Herceg, Z.; Vukušić Pavičić, T.; Stulić, V.; Ivanov, M.; Obranović, M.; Hojka, I.; et al. Ultrasound-Assisted Production of Virgin Olive Oil: Effects on Bioactive Compounds, Oxidative Stability, and Antioxidant Capacity. Sci 2025, 7, 135. https://doi.org/10.3390/sci7040135
Filipan K, Kraljić K, Žanetić M, Jukić Špika M, Herceg Z, Vukušić Pavičić T, Stulić V, Ivanov M, Obranović M, Hojka I, et al. Ultrasound-Assisted Production of Virgin Olive Oil: Effects on Bioactive Compounds, Oxidative Stability, and Antioxidant Capacity. Sci. 2025; 7(4):135. https://doi.org/10.3390/sci7040135
Chicago/Turabian StyleFilipan, Katarina, Klara Kraljić, Mirella Žanetić, Maja Jukić Špika, Zoran Herceg, Tomislava Vukušić Pavičić, Višnja Stulić, Mia Ivanov, Marko Obranović, Ivana Hojka, and et al. 2025. "Ultrasound-Assisted Production of Virgin Olive Oil: Effects on Bioactive Compounds, Oxidative Stability, and Antioxidant Capacity" Sci 7, no. 4: 135. https://doi.org/10.3390/sci7040135
APA StyleFilipan, K., Kraljić, K., Žanetić, M., Jukić Špika, M., Herceg, Z., Vukušić Pavičić, T., Stulić, V., Ivanov, M., Obranović, M., Hojka, I., Tokić, M., Škevin, D., & Balbino, S. (2025). Ultrasound-Assisted Production of Virgin Olive Oil: Effects on Bioactive Compounds, Oxidative Stability, and Antioxidant Capacity. Sci, 7(4), 135. https://doi.org/10.3390/sci7040135










