Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. General Experimental Procedures
2.3. Extraction, Fractionation, and Isolation
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.5. Liquid Chromatography High-Resolution Quadrupole Time-of-Flight Mass Spectrometry (LC-Q-TOF-MS/MS)
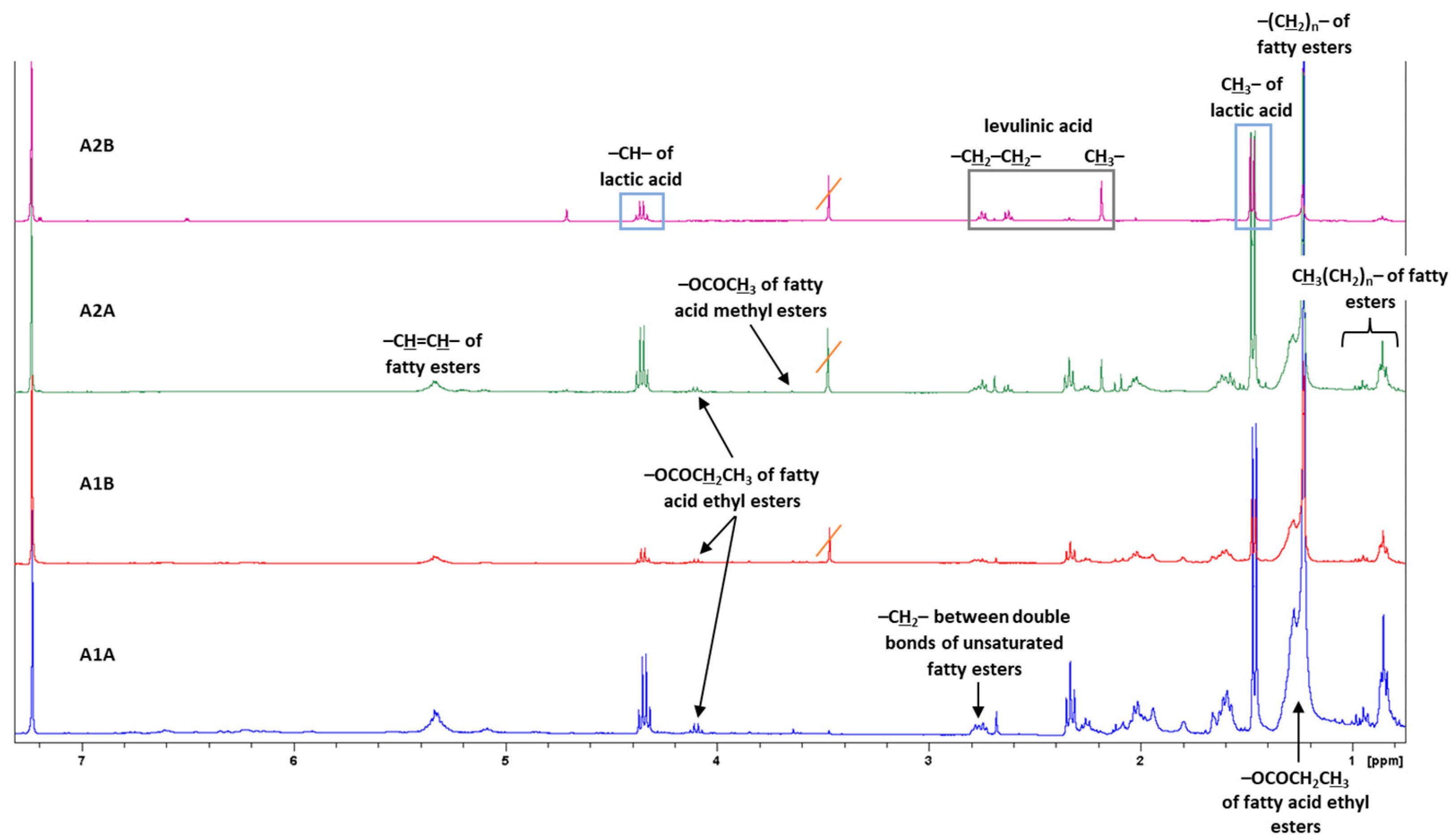
2.6. Nuclear Magnetic Resonance Spectroscopy (NMR) Spectroscopy
2.7. Identification of Cutibacterium acnes Strain ATCC 6919 by 16S rRNA Gene Sequencing
2.8. Bacterial Strains and Growth Conditions
2.9. Determination of Minimum Inhibitory Concentration (MIC)
2.10. Determination of Minimum Bactericidal Concentration (MBC)
3. Results and Discussion
3.1. Chemical Composition by GC-MS Analyses in Various Fractions of Dioscorea communis Berry Juice
3.2. NMR Analyses in Non-Polar Fractions of Dioscorea communis Berry Juice
3.3. LC-MS/MS Analysis of n-Butanol Extract of Dioscorea communis Berry Juice
3.4. Isolated Compounds of Dioscorea communis Berry Juice
3.5. Antibacterial Activity of Dioscorea communis Berry Juice and Selected Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferrer-Gallego, P.P.; Boisset, F. Typification of Dioscorea communis and Its Synonym Tamus communis var. subtriloba (Dioscoreaceae). Phytotaxa 2016, 260, 258–266. [Google Scholar] [CrossRef]
- Carnoy, A. Dictionnaire Étymologique des Noms Grecs de Plantes; Publications Universitaires: Louvain, Belgium, 1959; Volume 46, p. 109. [Google Scholar]
- Caddick, L.R.; Wilkin, P.; Rudall, P.J.; Hedderson, T.A.J.; Chase, M.W. Yams Reclassified: A Recircumscription of Dioscoreaceae and Dioscoreales. Taxon 2002, 51, 103–114. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1980; Volume 5, pp. 84–85. [Google Scholar]
- Caddick, L.R.; Rudall, P.J.; Wilkin, P.; Hedderson, T.A.J.; Chase, M.W. Phylogenetics of Dioscoreales Based on Combined Analyses of Morphological and Molecular Data. Bot. J. Linn. Soc. 2002, 138, 123–144. [Google Scholar] [CrossRef][Green Version]
- De Cortes Sánchez-Mata, M.; Tardío, J. Mediterranean Wild Edible Plants; Springer: New York, NY, USA, 2016; pp. 436–441. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Moult, S.P. The Dermatitic Properties of Black Bryony (Tamus communis L.). Contact Derm. 1983, 9, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kavvadas, D. Botanical Dictionary; Pelekanos Publications: Athens, Greece, 1956; Volume 10, pp. 3859–3860. [Google Scholar]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; duCellier, J.; Duke, P.-A.K. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002; Volume 2, p. 85. [Google Scholar]
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical Study of Medicinal Plants Traditionally Used in the Arribes Del Duero, Western Spain. J. Ethnopharmacol. 2010, 131, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Styczyński, M.; Pączkowski, C.; Szakiel, A.; Pinheiro de Carvalho, M.Â.A. GC-MS Analysis of Steroids and Triterpenoids Occurring in Leaves and Tubers of Tamus edulis Lowe. Phytochem. Lett. 2019, 30, 231–234. [Google Scholar] [CrossRef]
- Aquino, R.; Behar, I.; De Simone, F.; Pizza, C.; Senatore, F. Phenanthrene Derivatives from Tamus communis. Biochem. Syst. Ecol. 1985, 13, 251–252. [Google Scholar] [CrossRef]
- Shaheen, F.; Ali, L.; Ali, S.; Erdemoglu, N.; Sener, B. Antioxidant Flavonoids from Tamus communis ssp. cretica. Chem. Nat. Compd. 2009, 45, 346–349. [Google Scholar] [CrossRef]
- Rafael, M.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Topical Anti-Inflammatory Plant Species: Bioactivity of Bryonia dioica, Tamus communis and Lonicera periclymenum Fruits. Ind. Crops Prod. 2011, 34, 1447–1454. [Google Scholar] [CrossRef]
- Morales, P.; Carvalho, A.M.; de Cortes Sánchez-Mata, M.; Cámara, M.; Molina, M.; Ferreira, I.C.F.R. Tocopherol Composition and Antioxidant Activity of Spanish Wild Vegetables. Genet. Resour. Crop Evol. 2012, 59, 851–863. [Google Scholar] [CrossRef]
- Capasso, F.; Mascolo, N.; Autore, G.; De Simone, F.; Senatore, F. Anti-Inframmatory and Analgesic Activity in Alcoholic Extract of Tamus Communis L. J. Ethnopharmacol. 1983, 8, 321–325. [Google Scholar] [CrossRef]
- Schmidt, R.J.; De Corrés, L.F. An Unusual Case of Delayed Contact Sensitivity to Black Bryony Berries (Tamus communis L.). Contact Derm. 1990, 23, 260–261. [Google Scholar] [CrossRef]
- Chiu, C.-S.; Deng, J.-S.; Chang, H.-Y.; Chen, Y.-C.; Lee, M.-M.; Hou, W.-C.; Lee, C.-Y.; Huang, S.-S.; Huang, G.-J. Antioxidant and Anti-Inflammatory Properties of Taiwanese Yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and Its Reference Compounds. Food Chem. 2013, 141, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Hidayat, A.F.; Chan, C.K.; Mohamad, J.; Abdul Kadir, H. Dioscorea Bulbifera Induced Apoptosis through Inhibition of ERK 1/2 and Activation of JNK Signaling Pathways in HCT116 Human Colorectal Carcinoma Cells. Biomed. Pharmacother. 2018, 104, 806–816. [Google Scholar] [CrossRef]
- Yang, M.H.; Yoon, K.D.; Chin, Y.-W.; Park, J.H.; Kim, S.H.; Kim, Y.C.; Kim, J. Neuroprotective Effects of Dioscorea opposita on Scopolamine-Induced Memory Impairment in in Vivo Behavioral Tests and in Vitro Assays. J. Ethnopharmacol. 2009, 121, 130–134. [Google Scholar] [CrossRef]
- Kuete, V.; Betrand Teponno, R.; Mbaveng, A.T.; Tapondjou, L.A.; Meyer, J.J.M.; Barboni, L.; Lall, N. Antibacterial Activities of the Extracts, Fractions and Compounds from Dioscorea bulbifera. BMC Complement. Altern. Med. 2012, 12, 228. [Google Scholar] [CrossRef]
- Neu, R. Chelate von Diarylborsäuren mit aliphatischen Oxyalkylaminen als Reagenzien für den Nachweis von Oxyphenyl-benzo-γ-pyronen. Naturwissenschaften 1957, 44, 181–183. [Google Scholar] [CrossRef]
- Grafakou, M.E.; Barda, C.; Heilmann, J.; Skaltsa, H. Macrocyclic Diterpenoid Constituents of Euphorbia deflexa, an Endemic Spurge from Greece. J. Nat. Prod. 2021, 84, 2893–2903. [Google Scholar] [CrossRef]
- Grafakou, M.E.; Diamanti, A.; Simirioti, E.; Terezaki, A.; Barda, C.; Sfiniadakis, I.; Rallis, M.; Skaltsa, H. Wound Healing Effects from 3 Hypericum spp. Essential Oils. Planta Medica Int. Open 2021, 8, e69–e77. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P.M. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
- Tremmel, M.; Paetz, C.; Heilmann, J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules 2021, 26, 6632. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. 16S/23S rRNA Sequencing. Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit rRNA Sequence Analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Didaras, N.A.; Kafantaris, I.; Dimitriou, T.G.; Mitsagga, C.; Karatasou, K.; Giavasis, I.; Stagos, D.; Amoutzias, G.D.; Hatjina, F.; Mossialos, D. Biological Properties of Bee Bread Collected from Apiaries Located across Greece. Antibiotics 2021, 10, 555. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. In Honey Analysis; InTech: London, UK, 2017. [Google Scholar]
- Réthy, B.; Kovács, A.; Zupkó, I.; Forgo, P.; Vasas, A.; Falkay, G.; Hohmann, J. Cytotoxic Phenanthrenes from the Rhizomes of Tamus communis. Plant Med. 2006, 72, 767–770. [Google Scholar] [CrossRef]
- Zerargui, F.; Boumerfeg, S.; Charef, N.; Baghiani, A.; Djarmouni, M.; Khennouf, S.; Arrar, L.; Abu Zarga, M.; Mubarak, M. Antioxidant Potentials and Xanthine Oxidase Inhibitory Effect of Two Furanocoumarins Isolated from Tamus Communis L. Med. Chem. 2015, 11, 506–513. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.; Carvalho, A.; Sánchez-Mata, M.; Cámara, M.; Tardío, J. Fatty Acids Profiles of Some Spanish Wild Vegetables. Food Sci. Technol. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef] [PubMed]
- García-Herrera, P.; De Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J.; Olmedilla-Alonso, B. Carotenoid content of wild edible young shoots traditionally consumed in Spain (Asparagus acutifolius L., Humulus lupulus L., Bryonia dioica Jacq. and Tamus communis L.). J. Sci. Food Agr. 2012, 93, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Pereira, E.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bryonia Dioica, Tamus communis and Lonicera Periclymenum Fruits: Characterization in Phenolic Compounds and Incorporation of Their Extracts in Hydrogel Formulations for Topical Application. Ind. Crop Prod. 2013, 49, 169–176. [Google Scholar] [CrossRef]
- Takisawa, K.; Kanemoto, K.; Miyazaki, T.; Kitamura, Y. Hydrolysis for Direct Esterification of Lipids from Wet Microalgae. Bioresour. Technol. 2013, 144, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Jing, S.; Sun, J.; Li, X.; Man, S.; Wang, Y.; Gao, W. Novel Phenanthrene and Isocoumarin from the Rhizomes of Dioscorea nipponica Makino subsp. rosthornii (Prain et Burkill) C. T. Ting (Dioscoreaceae). Bioorg. Medl. Chem. Lett. 2017, 27, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; El-Mekkawy, S. New Sulphide Derivative from Ferula rutabensis. Nat. Prod. Res. 2009, 23, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Bederska-Łojewska, D.; Pieszka, M.; Marzec, A.; Rudzi′nska, M.; Grygier, A.; Siger, A.; Cie′slik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical Properties, Fatty Acid Composition, Volatile Compounds of Blueberries, Cranberries, Raspberries, and Cuckooflower Seeds Obtained Using Sonication Method. Molecules 2021, 26, 7446. [Google Scholar] [CrossRef] [PubMed]
- Griebel, T.; Zeier, J. A role for beta-sitosterol to stigmasterol conversion in plant-pathogen interactions. Plant J. 2010, 63, 254–268. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Rev. Bras. Farmacogn. 2020, 30, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.L.; Shestak, O.P. Reactions of Hydrogen Peroxide with Acetylacetone and 2-Acetylcyclopentanone. Russ. Chem. Bull. 2013, 62, 2171–2190. [Google Scholar] [CrossRef]
- Chaudhari, D.A.; Fernandes, R.A. Hypervalent Iodine as a Terminal Oxidant in Wacker-Type Oxidation of Terminal Olefins to Methyl Ketones. J. Org. Chem. 2016, 81, 2113–2121. [Google Scholar] [CrossRef]
- Puls, F.; Linke, P.; Kataeva, O.; Knölker, H. Iron-Catalyzed Wacker-type Oxidation of Olefins at Room Temperature with 1,3-Diketones or Neocuproine as Ligands. Angew. Chem. Int. Ed. 2021, 60, 14083–14090. [Google Scholar] [CrossRef]
- Armstrong, K.M.; Kilian, P. Catalytic Synthesis of Triaryl Phosphates from White Phosphorus. Eur. J. Inorg. Chem. 2011, 2011, 2138–2147. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, G.; Ma, Z.; Liu, Q.; Gao, X.; Tang, X.; Gao, Z.; Li, C.; Sun, T. Cichoric Acid from Witloof Inhibit Misfolding Aggregation and Fibrillation of HIAPP. Internat. J. Bioll. Macromol. 2020, 148, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Tapondjou, A.L.; Gatsing, D.; Djoukeng, J.D.; Abou-Mansour, E.; Tabacchi, R.; Tane, P.; Stoekli-Evans, H.; Lontsi, D. Bafoudiosbulbins A, and B, Two Anti-Salmonellal Clerodane Diterpenoids from Dioscorea bulbifera L. var. sativa. Phytochemistry 2006, 17, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mahanti, P.; Singh, N.R.; Rath, S.K.; Jena, P.K.; Patra, J.K. Antioxidant Activity, Antibacterial Potential and Characterization of Active Fraction of Dioscorea pentaphylla L. Tuber Extract Collected from Similipal Biosphere Reserve, Odisha, India. Braz. J. Pharm. Sci. 2018, 53, e17006. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yoon, J.Y.; Kwon, H.H.; Min, S.; Moon, J.; Suh, D.H. Seeking New Acne Treatment from Natural Products, Devices and Synthetic Drug Discovery. Derm.-Endocrinol. 2017, 9, e1356520. [Google Scholar] [CrossRef]

| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 39.611 | 6.3 | 1950 | 1964 | 1,2-benzenedicarboxylic acid, dibutyl ester [dibutyl phthalate] | C16H22O4 | 278 |
| 2 | 41.018 | 3.0 | 1993 | 1992 | hexadecanoic acid, ethyl ester [ethyl palmitate] | C18H36O2 | 284 |
| 3 | 44.032 | 2.1 | 2089 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester [methyl linoleate] | C19H34O2 | 294 |
| 4 | 46.097 | 45.2 | 2157 | 2159 | 9Z,12Z-octadecadienoic acid, ethyl ester [ethyl linoleate] | C20H36O2 | 308 |
| 5 | 46.272 | 36.9 | 2163 | 2173 | 9Z,12Z,5Z-octadecatrienoic acid, ethyl ester [ethyl linolenate] | C20H34O2 | 306 |
| 6 | 46.515 | 3.7 | 2171 | 2179 | 9Z-octadecenoic acid, ethyl ester [ethyl oleate] | C20H38O2 | 310 |
| 7 | 70.332 | 2.8 | 3130 | α-tocopherol | C29H50O2 | 430 | |
| Total | 100.0 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 41.014 | 3.3 | 1993 | 1992 | hexadecanoic acid, ethyl ester (ethyl palmitate) | C18H36O2 | 284 |
| 2 | 44.025 | 2.7 | 2089 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester (methyl linoleate) | C19H34O2 | 294 |
| 3 | 44.206 | tr | 2094 | 2105 | 9Z,12Z,15Z-octadecatrienoic acid, methyl ester (methyl linolenate) | C19H32O2 | 292 |
| 4 | 46.095 | 46.7 | 2157 | 2159 | 9Z,12Z-octadecadienoic acid, ethyl ester (ethyl linoleate) | C20H36O2 | 308 |
| 5 | 46.271 | 38.2 | 2163 | 2173 | 9Z,12Z,15Z-octadecatrienoic acid, ethyl ester (ethyl linolenate) | C20H34O2 | 306 |
| 6 | 46.509 | 3.6 | 2170 | 2179 | 9Z-octadecenoic acid, ethyl ester (ethyl oleate) | C20H38O2 | 310 |
| 7 | 70.328 | 2.9 | 3130 | α-tocopherol | C29H50O2 | 430 | |
| 8 | 73.784 | 2.6 | 3203 | (3β)-stigmast-5-en-3-ol (β-sitosterol) | C29H50O | 414 | |
| Total | 100.0 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 37.969 | tr | 1900 | 1890 | 9Z-hexadecenoic acid, methyl ester (methyl palmitoleate) | C17H32O2 | 268 |
| 2 | 38.823 | 53.3 | 1926 | 1921 | hexadecanoic acid, methyl ester (methyl palmitate) | C17H34O2 | 270 |
| 3 | 44.038 | 20.3 | 2089 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester (methyl linoleate) | C19H34O2 | 294 |
| 4 | 44.202 | 14.8 | 2094 | 2105 | 9Z,12Z,15Z-octadecatrienoic acid, methyl ester (methyl linolenate) | C19H32O2 | 292 |
| 5 | 44.435 | 1.3 | 2102 | 2103 | 9Z-octadecenoic acid, methyl ester (methyl oleate) | C19H36O2 | 296 |
| 6 | 45.142 | 3.5 | 2125 | 2124 | octadecanoic acid, methyl ester (methyl stearate) | C19H38O2 | 298 |
| 7 | 46.087 | 3.2 | 2156 | 2159 | 9Z,12Z-octadecadienoic acid, ethyl ester (ethyl linoleate) | C20H36O2 | 308 |
| 8 | 46.274 | 2.2 | 2163 | 2173 | 9Z,12Z,15Z-octadecatrienoic acid, ethyl ester (ethyl linolenate) | C20H34O2 | 306 |
| 9 | 47.570 | 1.5 | 2206 | unknown (m/z = 278.3) | |||
| 10 | 51.039 | tr | 2324 | 2329 | eicosanoic acid, methyl ester (methyl arachidate) | C21H42O2 | 326 |
| Total | 98.5 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 38.823 | 33.2 | 1926 | 1921 | hexadecanoic acid, methyl ester (methyl palmitate) | C17H34O2 | 270 |
| 2 | 41.061 | tr | 1995 | 1992 | hexadecanoic acid, ethyl ester (ethyl palmitate) | C18H36O2 | 284 |
| 3 | 44.059 | 7.7 | 2090 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester (methyl linoleate) | C19H34O2 | 294 |
| 4 | 44.280 | 6.8 | 2097 | 2105 | 9Z,12Z,15Z-octadecatrienoic acid, methyl ester (methyl linolenate) | C19H32O2 | 292 |
| 5 | 45.217 | tr | 2128 | 2124 | octadecanoic acid, methyl ester (methyl stearate) | C19H38O2 | 298 |
| 6 | 46.117 | 28.1 | 2157 | 2159 | 9Z,12Z-octadecadienoic acid, ethyl ester (ethyl linoleate) | C20H36O2 | 308 |
| 7 | 46.315 | 24.2 | 2164 | 2173 | 9Z,12Z,15Z-octadecatrienoic acid, ethyl ester (ethyl linolenate) | C20H34O2 | 306 |
| Total | 100.0 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 7.787 | 2.3 | 1102 | 1100 | nonanal (pelargonaldehyde) | C9H18O | 142 |
| 2 | 27.237 | 5.3 | 1598 | 1600 | hexadecane | C16H34 | 226 |
| 3 | 34.204 | 1.9 | 1791 | 1803 | 3-hexadecanone | C16H32O | 240 |
| 4 | 34.930 | 4.9 | 1812 | 1811 | hexadecanal (palmitaldehyde) | C16H32O | 240 |
| 5 | 35.956 | 1.6 | 1842 | 1845 | 6,10,14-trimethyl-2-pentadecanone (hexahydrofarnesyl acetone) | C18H36O | 268 |
| 6 | 38.238 | 9.4 | 1909 | 1902 | 2E-nonadecene | C19H38 | 266 |
| 7 | 38.398 | 5.0 | 1914 | unknown (m/z = 266.1) | |||
| 8 | 38.750 | 2.4 | 1924 | 1921 | hexadecanoic acid, methyl ester (methyl palmitate) | C17H34O2 | 270 |
| 9 | 41.213 | 34.0 | 1999 | 2004 | 2-octadecanone | C18H36O | 268 |
| 10 | 41.724 | 3.0 | 2016 | 2013 | octadecanal (stearaldehyde) | C18H36O | 268 |
| 11 | 43.737 | 1.3 | 2079 | 2077 | 1-octadecanol (stearyl alcohol) | C18H38O | 270 |
| 12 | 44.068 | 1.7 | 2090 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester (methyl linoleate) | C19H34O2 | 294 |
| 13 | 44.242 | 2.6 | 2095 | 2103 | 9Z-octadecenoic acid, methyl ester (methyl oleate) | C19H36O2 | 296 |
| 14 | 45.053 | 3.0 | 2122 | unknown (m/z = 282.1) | |||
| 15 | 45.133 | 2.2 | 2125 | 2124 | octadecanoic acid, methyl ester (methyl stearate) | C19H38O2 | 298 |
| 16 | 45.667 | 1.5 | 2143 | 2141 | 9Z-octadecenoic acid (oleic acid) | C18H34O2 | 282 |
| 17 | 46.618 | 2.9 | 2174 | unknown (m/z = 283.3) | |||
| 18 | 47.256 | 2.3 | 2195 | 2196 | octadecanoic acid, ethyl ester (ethyl stearate) | C20H40O2 | 312 |
| 19 | 47.395 | 6.3 | 2200 | 2200 | docosane | C22H46 | 310 |
| 20 | 50.300 | 1.2 | 2298 | 2300 | tricosane | C23H48 | 324 |
| 21 | 51.307 | 0.9 | 2332 | 2329 | eicosanoic acid, methyl ester (methyl arachidate) | C21H42O2 | 326 |
| 22 | 51.720 | 2.0 | 2347 | unknown (m/z = 323.3) | |||
| 23 | 53.078 | 2.3 | 2393 | 2400 | tetracosane | C24H50 | 338 |
| Total | 89.1 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 34.435 | 7.1 | 1797 | 1800 | 2-hexadecanone | C16H32O | 240 |
| 2 | 35.344 | 1.7 | 1824 | 1826 | pentadecanoic acid, methyl ester | C16H32O2 | 256 |
| 3 | 37.890 | 3.2 | 1898 | 1901 | 2-heptadecanone | C17H34O | 254 |
| 4 | 38.767 | 3.8 | 1925 | 1921 | hexadecanoic acid, methyl ester (methyl palmitate) | C17H34O2 | 270 |
| 5 | 41.437 | 49.4 | 2007 | 2004 | 2-octadecanone | C18H36O | 268 |
| 6 | 42.043 | 1.7 | 2026 | 2028 | heptadecanoic acid, methyl ester (methyl margarate) | C18H38O2 | 284 |
| 7 | 44.112 | 4.2 | 2091 | 2095 | 9Z,12Z-octadecadienoic acid, methyl ester (methyl linoleate) | C19H34O2 | 294 |
| 8 | 45.180 | 20.8 | 2126 | 2124 | octadecanoic acid, methyl ester (methyl stearate) | C19H38O2 | 298 |
| 9 | 45.713 | 4.8 | 2144 | 2141 | 9Z-octadecenoic acid (oleic acid) | C18H34O2 | 282 |
| 10 | 47.281 | 3.3 | 2196 | unknown (m/z = 313.2) | |||
| Total | 96.7 |
| No. | Retention Time | % Area | KI | AI | Name of Compound | Molecular Formula | MW |
|---|---|---|---|---|---|---|---|
| 1 | 34.928 | tr | 1812 | 1811 | hexadecanal (palmitaldehyde) | C16H32O | 240 |
| 2 | 37.855 | 0.6 | 1897 | 1901 | 2-heptadecanone | C17H34O | 254 |
| 3 | 38.228 | 2.5 | 1909 | 1902 | 2E-nonadecene | C19H38 | 266 |
| 4 | 38.358 | 1.6 | 1913 | unknown (m/z = 266.1) | |||
| 5 | 38.734 | 1.3 | 1924 | 1921 | hexadecanoic acid, methyl ester (methyl palmitate) | C17H34O2 | 270 |
| 6 | 40.385 | 1.0 | 1975 | unknown (m/z = 285.1) | |||
| 7 | 41.007 | tr | 1994 | 1992 | hexadecanoic acid, ethyl ester (ethyl palmitate) | C18H36O2 | 284 |
| 8 | 41.372 | 59.7 | 2005 | 2004 | 2-octadecanone | C18H36O | 268 |
| 9 | 41.690 | 2.4 | 2015 | 2013 | octadecanal (stearaldehyde) | C18H36O | 268 |
| 10 | 42.013 | 0.7 | 2025 | 2028 | heptadecanoic acid, methyl ester (methyl margarate) | C18H38O2 | 284 |
| 11 | 42.778 | 1.4 | 2050 | unknown (m/z = 282.1) | |||
| 12 | 43.568 | 1.6 | 2075 | unknown (m/z = 299.1) | |||
| 13 | 45.163 | 11.5 | 2126 | 2124 | octadecanoic acid, methyl ester (methyl stearate) | C19H38O2 | 298 |
| 14 | 46.080 | 0.4 | 2157 | 2159 | 9Z,12Z-octadecadienoic acid, ethyl ester (ethyl linoleate) | C20H36O2 | 308 |
| 15 | 46.646 | 11.6 | 2175 | unknown (m/z = 313.3) | |||
| 16 | 47.386 | 0.5 | 2200 | 2200 | docosane | C22H46 | 310 |
| 17 | 71.869 | 0.5 | 3131 | (3β,24R)-ergost-5-en-3-ol (campesterol) | C28H48O | 410 | |
| 18 | 72.575 | 0.8 | 3170 | (3β,22E)-stigmasta-5,22-dien-3-ol (stigmasterol) | C29H48O | 412 | |
| 19 | 73.781 | 1.9 | 3203 | (3β)-stigmast-5-en-3-ol (β-sitosterol) | C29H50O | 414 | |
| Total | 82.8 |
| Positive Ion Mode | Negative Ion Mode | Molecular Formula | Metabolite Name | |||
|---|---|---|---|---|---|---|
| RT | Found | Calcd | Found | Calcd | ||
| 0.324 | 203.0530 [M + Na]+ | 203.0526 | 179.0563 [M − H]− | 179.0561 | C6H12O6 | hexose |
| 0.337 | 365.1059 [M + Na]+ | 365.1054 | 341.1093 [M − H]− | 341.1089 | C12H22O11 | hexose-pentose |
| 0.488 | 113.0217 [M + Na]+ | 113.0209 | 89.0243 [M − H]− | 89.0244 | C3H6O3 | lactic acid |
| 0.490 | 349.1121 [M + Na]+ | 349.1105 | 371.1189 [M + HCOO]− | 371.1195 | C12H22O10 | hexose-pentose |
| 0.559 | 175.025 [M − H]− | 175.0248 | C6H8O6 | ascorbic acid | ||
| 0.565 | 117.0182 [M + H]+ | 117.0182 | C4H4O4 | fumaric acid | ||
| 0.605 | 121.0652 [M + H]+ | 121.0648 | C8H8O | 2-methylbenzaldehyde | ||
| 0.917 | 166.0866 [M + H]+ | 166.0863 | 164.0716 [M − H]− | 164.0717 | C9H11NO2 | phenylalanine |
| 1.208 | 146.0604 [M + H]+ | 146.0600 | C9H7NO | 4-formyl indole | ||
| 1.210 | 208.0609 [M + H]+ | 208.0604 | 206.0458 [M − H]− | 206.0459 | C10H9NO4 | pyranonigrin S |
| 1.303 | 239.1489 [M + H]+ | 239.1489 | C10H22O6 | unknown | ||
| 1.404 | 188.0707 [M + H]+ | 188.0706 | C11H9NO2 | unknown | ||
| 1.725 | 247.1080 [M + H]+ | 247.1077 | C13H14N2O3 | unknown | ||
| 1.794 | 217.0977 [M + H]+ | 217.0972 | C12H12N2O2 | unknown | ||
| 1.973 | 231.1131 [M + H]+ | 231.1128 | 229.0983 [M − H]− | 229.0983 | C13H14N2O2 | unknown |
| 1.983 | 611.1609 [M + H]+ | 611.1607 | 609.1462 [M − H]− | 609.1461 | C27H30O16 | rutin |
| 2.046 | 181.0498 [M + H]+ | 181.0495 | 179.0350 [M − H]− | 179.0350 | C9H8O4 | caffeic acid |
| 2.175 | 275.1032 [M + H]+ | 275.1026 | C14H14N2O4 | unknown | ||
| 2.183 | 595.1668 [M + H]+ | 595.1657 | 593.1516 [M − H]− | 593.1512 | C27H30O15 | kaempferol 3-O-rutinoside |
| 2.490 | 165.0556 [M − H]− | 165.0557 | C9H10O3 | phenolic | ||
| 3.006 | 349.1646 [M + H]+ | 349.1646 | 347.1498 [M − H]- | 347.1500 | C19H24O6 | unknown |
| 3.248 | 345.1341 [M − H]− | 345.1644 | C19H22O6 | unknown | ||
| 4.183 | 883.4695 [M + H]+ | 883.4686 | C45H70O17 | 7-oxodioscin | ||
| 4.192 | 399.1781 [M + Na]+ | 399.1778 | 375.181 [M − H]− | 375.1813 | C21H28O6 | 3,5-dihydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)heptane |
| 4.204 | 303.0502 [M + H]+ | 303.0499 | 301.0353 [M − H]− | 301.0354 | C15H10O7 | quercetin |
| 4.714 | 279.2325 [M + H]+ | 279.2319 | C18H30O2 | linolenic acid | ||
| 4.874 | 287.0551 [M + H]+ | 287.0550 | 285.0406 [M − H]− | 285.0405 | C15H10O6 | kaempferol |
| 5.050 | 329.2333 [M − H]− | 329.2333 | C18H34O5 | 9,10,11-trihydroxy-12-octadecenoic acid | ||
| 6.060 | 312.2533 [M + H]+ | 312.2533 | C18H33NO3 | unknown | ||
| 6.458 | 885.4842 [M + H]+ | 885.4842 | 929.4747 [M + HCOO]− | 929.4752 | C45H72O17 | gracillin |
| 6.524 | 315.2537 [M + H]+ | 315.2530 | 313.2383 [M − H]− | 313.2384 | C18H34O4 | lipid derivative |
| 6.805 | 415.2117 [M + H]+ | 415.2115 | C24H30O6 | bersenogenin | ||
| 6.899 | 478.2943 [M + H]+ | 478.2952 | 476.2794 [M − H]− | 476.2806 | C30H39NO4 | 18-deoxycytochalasin H |
| 7.044 | 339.2509 [M + Na]+ | 339.2506 | 315.2541 [M − H]− | 315.2541 | C18H36O4 | lipid derivative |
| 7.086 | 310.2381 [M + H]+ | 310.2377 | 308.2232 [M − H]− | 308.2231 | C18H31NO3 | lipid amide |
| 7.296 | 492.1134 [M+NH4]+ | 492.1137 | 473.0724 [M − H]− | 473.0725 | C22H18O12 | cichoric acid |
| 7.539 | 891.4708 [M + Na]+ | 891.4713 | 913.4790 [M + HCOO]− | 913.4802 | C45H72O16 | dioscin |
| 7.657 | 280.2640 [M + H]+ | 280.2635 | C18H33NO | linoleamide | ||
| 7.672 | 449.3732 [M + Na]+ | 449.3757 | C30H50O | amyrin | ||
| 7.782 | 295.2280 [M − H]− | 295.2279 | C18H32O3 | 9-hydroxy-10,12-octadecadienoic acid | ||
| 7.887 | 257.2478 [M + H]+ | 257.2475 | 255.2329 [M − H]− | 255.2330 | C16H32O2 | palmitic acid |
| 7.970 | 358.2595 [M − H]− | 358.2599 | C19H37NO5 | unknown | ||
| 7.990 | 296.2592 [M + H]+ | 296.2584 | C18H33NO2 | stearimide | ||
| 8.076 | 437.3729 [M + Na]+ | 437.3754 | C29H50O | β-sitosterol | ||
| 8.235 | 685.4366 [M + Na]+ | 685.4356 | C42H63O4P | tris-(2,4-di-tert-butylphenyl)phosphate | ||
| 8.251 | 239.2377 [M + H]+ | 239.2397 | C16H30O | 2-hexadecenal | ||
| 8.248 | 281.2481 [M + H]+ | 281.2475 | 279.2327 [M − H]− | 279.2330 | C18H32O2 | linoleic acid |
| 8.400 | 453.3685 [M + Na]+ | 453.3703 | C29H50O2 | α-tocopherol | ||
| 8.704 | 228.2329 [M + H]+ | 228.2322 | C14H29NO | myristamide | ||
| 9.025 | 254.2484 [M + H]+ | 254.2478 | C16H31NO | palmitoleamide | ||
| 9.550 | 271.2277 [M − H]− | 271.2279 | C16H32O3 | unknown | ||
| 9.689 | 307.2628 [M + H]+ | 307.2632 | 351.2539 [M + HCOO]− | 351.2541 | C20H34O2 | ethyl linolenate |
| 9.868 | 256.2638 [M + H]+ | 256.2625 | C16H33NO | palmitamide | ||
| 10.109 | 282.2791 [M + H]+ | 282.2791 | C18H35NO | 9-octadecenamide | ||
| 10.147 | 309.2787 [M + H]+ | 309.2788 | 353.2694 [M + HCOO]− | 353.2697 | C20H36O2 | ethyl linoleate |
| 11.58 | 285.2794 [M + H]+ | 285.2788 | 283.2643 [M − H]− | 283.2643 | C18H36O2 | ethyl palmitate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsami, K.; Barda, C.; Ladopoulos, G.; Didaras, N.A.; Grafakou, M.-E.; Heilmann, J.; Mossialos, D.; Rallis, M.C.; Skaltsa, H. Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice. Sci 2022, 4, 21. https://doi.org/10.3390/sci4020021
Tsami K, Barda C, Ladopoulos G, Didaras NA, Grafakou M-E, Heilmann J, Mossialos D, Rallis MC, Skaltsa H. Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice. Sci. 2022; 4(2):21. https://doi.org/10.3390/sci4020021
Chicago/Turabian StyleTsami, Konstantina, Christina Barda, George Ladopoulos, Nikos Asoutis Didaras, Maria-Eleni Grafakou, Jörg Heilmann, Dimitris Mossialos, Michail Christou Rallis, and Helen Skaltsa. 2022. "Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice" Sci 4, no. 2: 21. https://doi.org/10.3390/sci4020021
APA StyleTsami, K., Barda, C., Ladopoulos, G., Didaras, N. A., Grafakou, M.-E., Heilmann, J., Mossialos, D., Rallis, M. C., & Skaltsa, H. (2022). Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice. Sci, 4(2), 21. https://doi.org/10.3390/sci4020021








