HPLC–NMR-Based Chemical Profiling of Matricaria pubescens (Desf.) Schultz and Matricaria recutita and Their Protective Effects on UVA-Exposed Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Samples
2.3. Isolation of the Compounds from M. pubescens
2.4. Sample Preparation for HPLC Quantitative Analysis of Methanol and Hydromethanolic Extracts
2.5. Chemicals and Standards
2.6. HPLC–PDA–MS Analysis Instrumentation
2.7. Qualitative and Quantitative Determination of Flavonoids
2.8. Activity of M. pubescens and M. recutita Extracts on Fibroblasts
3. Results
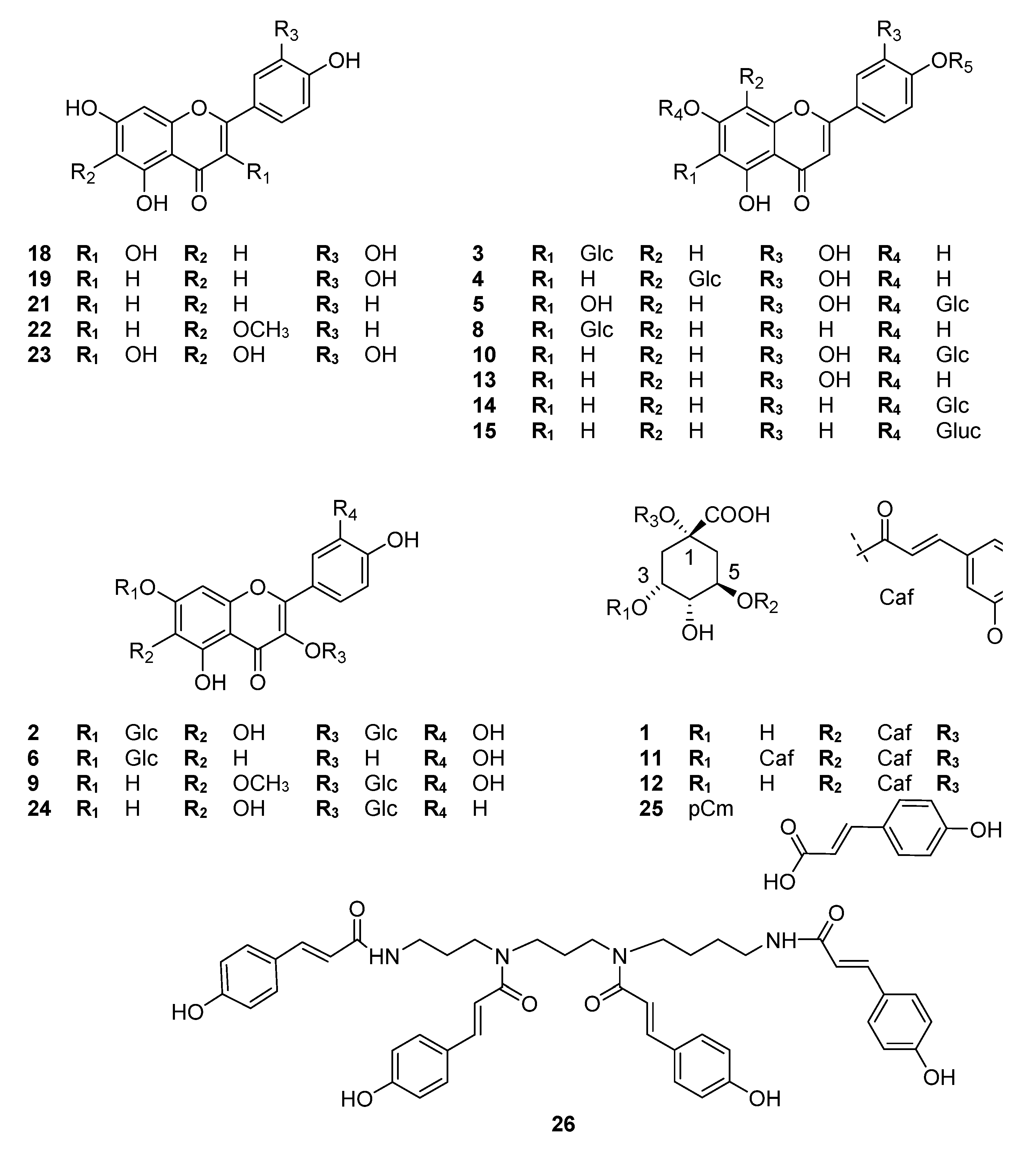
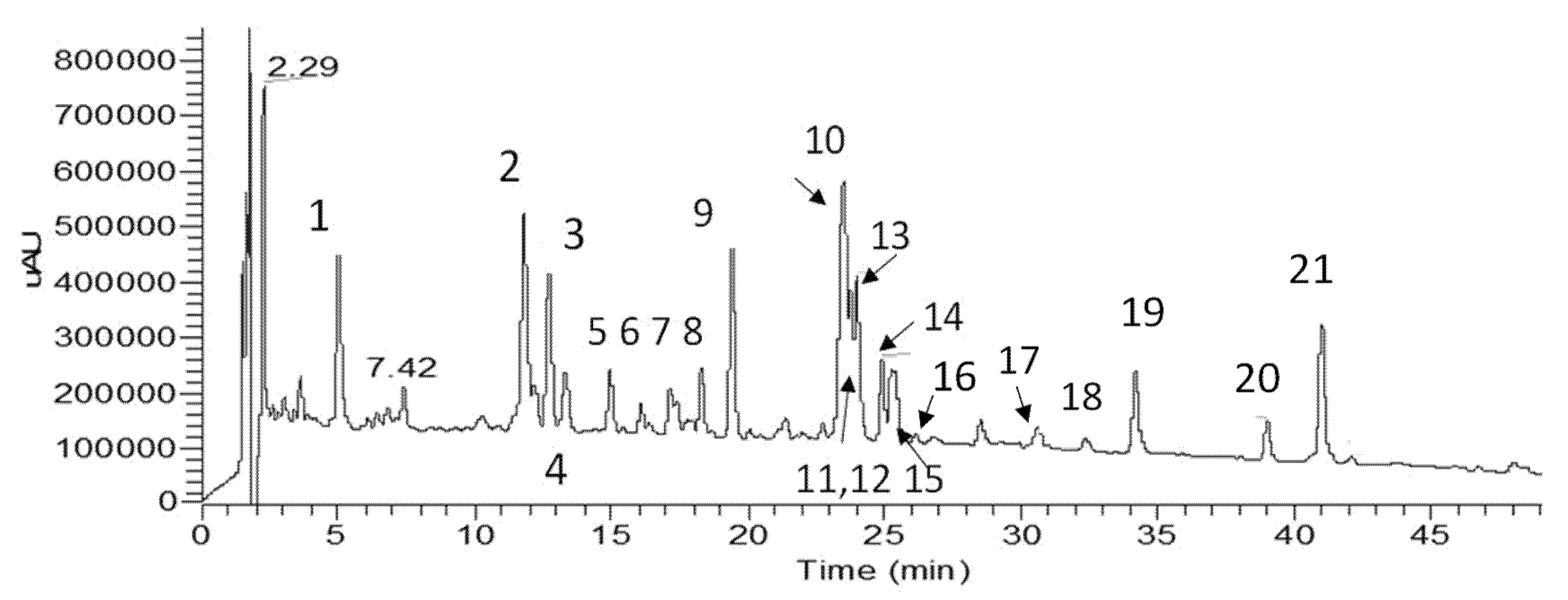
3.1. Extraction, Isolation and Identification of the Constituents
| Rt (min) | UV (nm) | m/z (−) Negative Mode | Identification | Mode of Identification | |
|---|---|---|---|---|---|
| 1 | 5.04 | 297, 326 | 191 [quinic acid-H]−, 353 [M-H]− | chlorogenic acid | UV/MS, std |
| 2 | 11.83 | 259, 274, 357 | 317 [A-H]−, 479 [M-H]− | quercetagenin-3-O-glucoside | NMR, UV/MS |
| 3 | 12.73 | 255, 269, 349 | 327 [M-120-H]−, 357 [M-90-H]−, 447 [M-H]− | isoorientin | NMR, UV/MS |
| 4 | 13.40 | 255, 268, 342 | 327 [M-120-H]−, 357 [M-90-H]−, 447 [M-H]− | orientin | UV/MS |
| 5 | 14.96 | 281, 343 | 301 [A-H]−, 463 [M-H]− | 6-hydroxyluteolin-7-O-glucoside | NMR, UV/MS |
| 6 | 16.11 | 255, 369 | 301 [A-H]−, 463 [M-H]− | quercetin-7-O-glucoside | NMR, UV/MS |
| 7 | 17.20 | 276, 337 | 301 [A-H]−, 463 [Μ-H]− | 6/8-hydroxyluteolin-4′-O-glucoside | UV/MS, tentatively |
| 8 | 17.45 | 269, 336 | 311 [M-120-H]−, 341 [M-90-H]−, 431 [M-H]− | isovitexin (lack of shoulder at 302 nm) | NMR, UV/MS |
| 9 | 18.32 | 259, 276sh, 356 | 331 [A-H]−, 493 [M-H]− | patuletin-3-O-glucoside | UV/MS, [18] |
| 10 | 19.45 | 254, 266sh, 347 | 285 [A-H]−, 447 [M-H]− | luteolin-7-O-glucoside | NMR, UV/MS |
| 11–12 | 23.55 | 245, 300, 328 | 179 [caffeic acid-H]−, 191 [quinic acid-H]−, 353 [M-caffeoyl group-H]−, 515 [Μ-H]- | 3,5-O-dicaffeoylquinic acid + 1,5-O-dicaffeoylquinic acid | UV/MS, std, lab isolate [17] |
| 13 | 23.80 | 268, 336 | 285 [A-H]−, 447 [M-H]− | luteolin-4′-O-glucoside | NMR, UV/MS |
| 14 | 24.02 | 267, 335 | 269 [A-H]−, 431 [M-H]− | apigenin-7-O-glucoside | UV/MS, std |
| 15 | 24.93 | 267, 338 | 269 [A-H]−, 445 [M-H]− | apigenin-7-O-glucuronide | UV/MS lab isolate [19] |
| 16 | 25.20 | 298, 327 | 161, 179, 381, 543 [M-H]− | derivative of caffeic acid | UV/MS |
| 17 | 30.72 | 308 | 269, 639 [M-H]− | tri-p-coumaroyl derivative of spermine/thermospermine | NMR, UV/MS |
| 18 | 32.56 | 254, 368 | 301 [M-H]− | quercetin | NMR, UV/MS, std |
| 19 | 34.22 | 253, 266, 348 | 285 [M-H]− | luteolin | NMR, UV/MS |
| 20 | 39.02 | 295, 326 | 705 [M-H]− | polyamine derivative—not identified | UV/MS |
| 21 | 41.02 | 267, 337 | 269 [M-H]− | apigenin | NMR, UV/MS |
| Mr | Rt (min) | UV (nm) | m/z (−) Negative Mode | Identification | Mode of Identification |
|---|---|---|---|---|---|
| Mr-1 | 5.16 | 296, 326 | 191 [quinic acid-H]−, 353 [M-H]− | chlorogenic acid | UV/MS, std |
| Mr-2 | 5.40 | 279, 301 | 134 [A-CO2-CH3-H]−, 149 [A-CO2-H]−, 193 [A-H]−, 355 [M-H]−, 711 [2M-H]− | cis-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS, [20] |
| Mr-3 | 11.08 | 295, 318 | 135 [A-CO2-CH2-H]−, 149 [A-CO2-H]−, 193 [A-H]−, 355 [M-H]−, 711 [2M-H]− | trans-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS, [20] |
| Mr-4 | 11.90 | 259, 354 | 317 [A-H]−, 479 [M-H]− | quercetagenin-3-O-glucoside | NMR/UV/MS |
| Mr-5 | 15.98 | 255, 370 | 301 [A-H]−, 463 [M-H]− | quercetin-7-O-hexoside | UV/MS, [12,20] |
| Mr-6 | 17.90 | 258, 369 | 331 [A-H]−, 493 [M-H]− | patuletin-7-O-glucoside | UV/MS, [12] |
| Mr-7 | 18.47 | 259, 356 | 331 [A-H]−, 493 [M-H]− | patuletin-3-O-glucoside | UV/MS, [18] |
| Mr-8 | 19.59 | 255, 347 | 285 [A-H]−, 447 [M-H]− | luteolin-7-O-glucoside | NMR/UV/MS, std |
| Mr-9 | 21.07 | - | 711 | not identified | |
| Mr-10 | 22.61 | 254, 370 | 315 [A-H]−, 477 [M-H]− | isorhamnetin-7-O-hexoside | UV/MS, [12] |
| Mr-11 | 22.94 | 254, 352 | 314 [A-H]−, 477 [M-H]− | isorhamnetin-3-O-glucoside | UV/MS, std |
| Mr-12 | 23.34 | 298, 327 | 179 [caffeic acid-H]−, 191 [quinic acid-H]−, 353 [M-caffeoyl-H]−, 515 [Μ-H]− | 3,5-O-dicaffeoylquinic acid | UV/MS, std |
| Mr-13 | 23.93 | 267, 336 | 268 [A-2H]−, 431 [M-H]− | apigenin-7-O-glucoside | NMR/UV/MS, std |
| Mr-14 | 25.10 | 252, 266, 347 | 299 [A-H]−, 446 [Μ-CH3-H]−, 461 [Μ-H]− | chrysoeriol-7-O-glucoside | UV/MS, [12] |
| Mr-15 | 25.36 | 298, 327 | 179 [caffeic acid-H]−, 191 [quinic acid-H]−, 353 [Μ-caffeoyl-H]−, 515 [Μ-H]− | 4,5-O-dicaffeoylquinic acid | UV/MS, std |
| Mr-16 | 30.63 | 267, 329 | 269 [A-H]−, 473 [M-H]− | apigenin-4′-acetyl-hexoside (tentatively) | UV/MS |
| Mr-17 | 31.93 | 267, 336 | 269 [A-H]−, 473 [M-H]− | apigenin-7-acetyl-hexoside | UV/MS, [21] |
| Mr-18 | 35.88 | 267, 336 | 269 [A-H]−, 473 [M-H]− | apigenin-7-acetyl hexoside isomer | UV/MS, [21] |
| Mr-19 | 37.20 | 267, 336 | 269 [A-H]−, 515 [M-H]− | apigenin-7-O-(6″-malonyl)-glucoside | UV/MS, [21] |
3.2. Quantitative Data
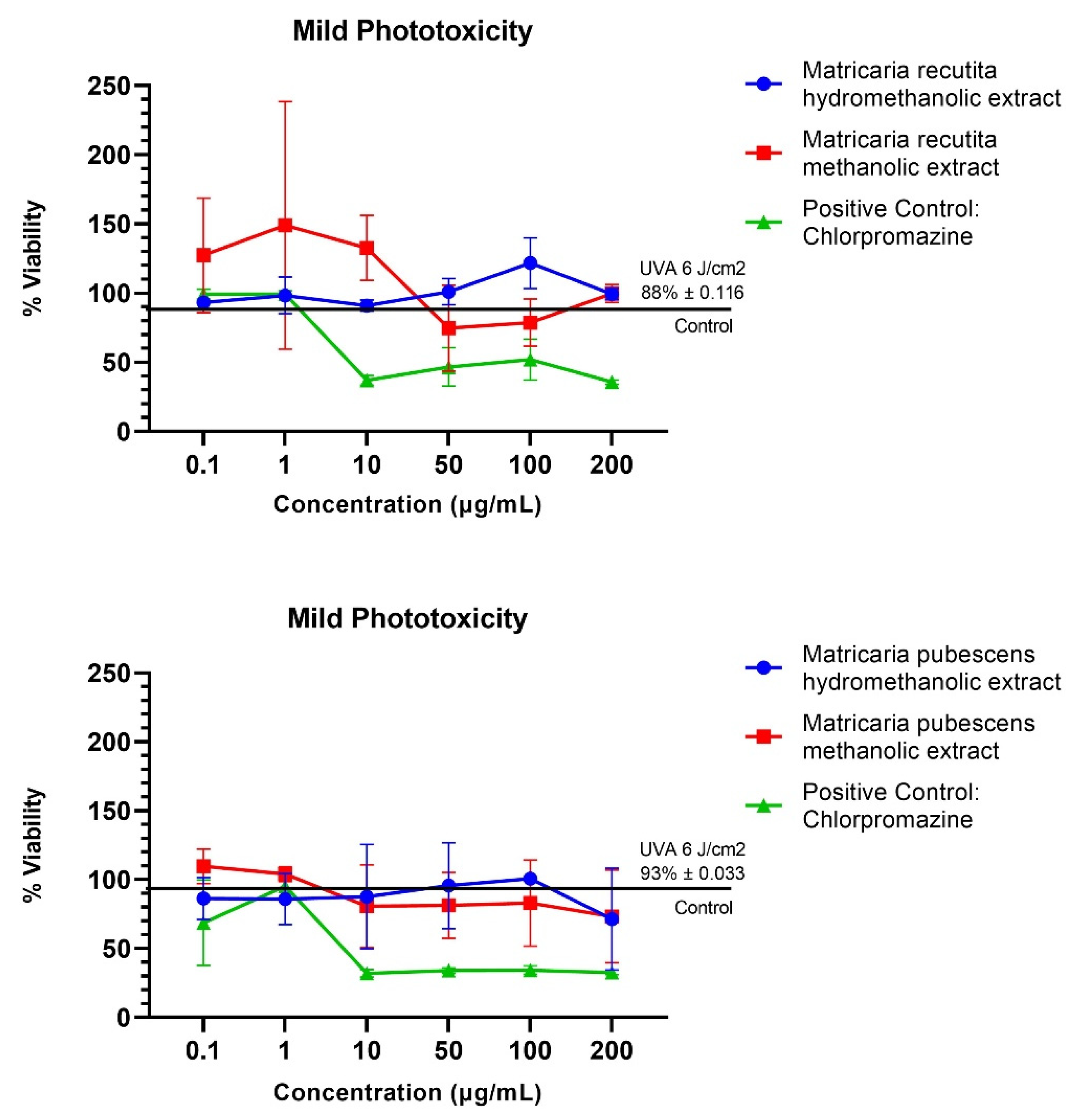
3.3. In Vitro Protective Activity on BALbC 3T3 Mouse Skin Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cherif, H.S.; Ferrah, R.; Bennacer, A.; Tail, G.; Saidi, F. Traditional use of Matricaria pubescens (Desf.) Schultz in two regions of Southern Algeria and contribution to study the antioxidant activity. Indian J. Trad. Knowl. 2017, 16, 562–567. [Google Scholar]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef]
- Gherboudj, O.; Benkiki, N.; Seguin, E.; Tillequin, F.; Kabouche, Z. Components of Matricaria pubescens from Algerian septentrional Sahara. Chem. Nat. Comp. 2012, 48, 470–471. [Google Scholar] [CrossRef]
- EMA/HMPC/55843/2011. European Union Herbal Monograph on Matricaria recutita L., Fflos. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiKjIuBrM_2AhWhyYsBHcoeDb8QFnoECAgQAQ&url=https%3A%2F%2Fwww.ema.europa.eu%2Fdocuments%2Fherbal-monograph%2Ffinal-european-union-herbal-monograph-matricaria-recutita-l-flos-first-version_en.pdf&usg=AOvVaw2PiRhwaUwdNzyCBuaJGM9u (accessed on 3 December 2021).
- Ferreira, E.B.; Vasques, C.I.; Jesus, C.A.C.; Reis, P.E.D. Topical effects of Chamomilla recutita in skin damage: A literature review. Pharmacologyonline 2015, 3, 123–130. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Chamomile, German and Chamomile, Roman. In Herbal Medicines, 3rd ed.; Pharmaceutical Press: London, UK, 2007; pp. 152–158. [Google Scholar]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; p. 262. [Google Scholar]
- Tsivelika, N.; Sarrou, E.; Gusheva, K.; Pankou, C.; Koutsos, T.; Chatzopoulou, P.; Mavromatis, A. Phenotypic variation of wild Chamomile (Matricaria recutita L.) populations and their evaluation for medicinally important essential oil. Biochem. Syst. Ecol. 2018, 80, 21–28. [Google Scholar] [CrossRef]
- Saroglou, V.; Karioti, A.; Heilmann, J.; Kypriotakis, Z.; Skaltsa, H. Sesquiterpene Lactones from Anthemis melanolepis. Helv. Chim. Acta 2007, 90, 171–175. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F. Sesquiterpene lactones and other terpenes from Geigeria species. Phytochemistry 1989, 28, 3105–3120. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzová, V.; Mrnka, L.; Hajšlová, J. Influence of Harvest Date and Postharvest Treatment on Carotenoid and Flavonoid Composition in French Marigold Flowers. J. Agric. Food Chem. 2020, 68, 7880–7889. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, X.; He, W.H.; Xu, H.G.; Yuan, F.; Gao, Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef]
- Greger, H. Laubblatt-Flavonoide und Systematik bei Matricaria und Tripleurospermum (Asteraceae-Anthemideae). Plant Syst. Evol. 1975, 124, 35–55. [Google Scholar] [CrossRef]
- Karioti, A.; Bolognesi, L.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC-DAD and HPLC-ESI-MS. J. Pharm Biomed. Anal. 2010, 53, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Ibrahim, R.K. Ombuin 3-sulphate from Flaveria chloraefolia. Phytochemistry 1988, 27, 2362–2363. [Google Scholar] [CrossRef]
- Govari, S.; Paloukopoulou, C.; Soulioti, A.; Tasi, G.; Karioti, A. Origanum dictamnus, an important Greek species with long ethnomedicinal history as anti-inflammatory agent, is rich in phenolic content as confirmed by HPLC-PDA-MS and phytochemical analyses. In Proceedings of the 19th International Congress of International Society for Ethnopharmacology (ISE), Dresden, Germany, 12–14 June 2019. [Google Scholar]
- Avula, B.; Wang, Y.H.; Wang, M.; Avonto, C.; Zhao, J.; Smillie, T.J.; Rua, D.; Khan, I.A. Quantitative determination of phenolic compounds by UHPLC-UV–MS and use of partial least-square discriminant analysis to differentiate chemo-types of Chamomile/Chrysanthemum flower heads. J. Pharm. Biomed. Anal. 2014, 88, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. LC-PDA-ESI/MS Identification of the Phenolic Components of Three Compositae Spices: Chamomile, Tarragon, and Mexican Arnica. Nat. Prod. Commun. 2012, 7, 749–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avonto, C.; Rua, D.; Lasonkar, P.B.; Chittiboyina, A.G.; Khan, I.A. Identification of a compound isolated from German chamomile (Matricaria chamomilla) with dermal sensitization potential. Toxicol. Appl. Pharm. 2017, 318, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, L.; Sanmugam, A.; Manoharan, S. Fabrication of dual layered biocompatible herbal biopatch from biological waste for skin—Tissue regenerative applications. Int. J. Biol. Macromol. 2021, 183, 1106–1118. [Google Scholar] [CrossRef]
- Nematollahi, P.; Mohammadi Aref, N.; Zahmatkesh Meimandi, F.; Rozei, S.L.; Zareé, H.; Mirlohi, S.M.; Rafiee, S.; Mohsenikia, M.; Soleymani, A.; Ashkani-Esfahani, S.; et al. Matricaria chamomilla Extract Improves Diabetic Wound Healing in Rat Models. Trauma Mon. 2019, 24, e14318. [Google Scholar] [CrossRef]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.; Pavesi, V.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Youn, J.; Kim, K.; da Joo, H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.H.; Bahuguna, A.; Kim, H.H.; Kim, D.I.; Kim, H.J.; Yu, J.M.; Jung, H.G.; Jang, J.Y.; Kwak, J.H.; Park, G.H.; et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B 2017, 174, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, Y.T.; Kuo, H.C.; Chiou, W.F.; Lee, M.H. Compounds isolated from Eriobotrya deflexa leaves protect against ultraviolet radiation B-induced photoaging in human fibroblasts. J. Photochem. Photobiol. B 2017, 175, 244–253. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharm. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Diren Sigirci, B.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, D.; Yang, Y.; Yu, M.; Han, Z.Z.; Wei, M.; Zhang, H.W.; Jia, H.M.; Zou, Z.M. Anti-inflammatory chemical constituents of Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020, 11, 6340–6351. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, E.; Félix-Silva, J.; Xavier-Santos, J.B.; Fernandes, J.M.; Guerra, G.; de Araújo, A.A.; Araújo, D.; de Santis Ferreira, L.; da Silva, A.A., Jr.; Fernandes-Pedrosa, M.F.; et al. Local anti-inflammatory activity: Topical formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata leaf aqueous extract. Biomed. Pharmacother. 2019, 113, 108721. [Google Scholar] [CrossRef]
- Corrêa, W.R.; Serain, A.F.; Aranha Netto, L.; Marinho, J.V.N.; Arena, A.C.; de Santana Aquino, D.F.; Kuraoka-Oliveira, Â.M.; Júnior, A.J.; Bernal, L.P.T.; Kassuya, C.A.L.; et al. Anti-Inflammatory and Antioxidant Properties of the Extract, Tiliroside, and Patuletin 3-O-β-D-Glucopyranoside from Pfaffia townsendii (Amaranthaceae). Evid. Based Complement. Altern. Med. 2018, 2018, 6057579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, K.; Kasahara, Y. Effects of Flavonoids from French Marigold (Florets of Tagetes patula L.) on Acute Inflammation Model. Int. J. Inflam. 2013, 2013, 309493. [Google Scholar] [PubMed] [Green Version]
- Dilshara, M.G.; Lee, K.T.; Jayasooriya, R.G.; Kang, C.H.; Park, S.R.; Choi, Y.H.; Choi, I.W.; Hyun, J.W.; Chang, W.Y.; Kim, Y.S.; et al. Downregulation of NO and PGE2 in LPS-stimulated BV2 microglial cells by trans-isoferulic acid via suppression of PI3K/Akt-dependent NF-kappaB and activation of Nrf2-mediated HO-1. Int. Immunopharmacol. 2014, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Shi, Y.; Wang, J.; Shen, X.; Zhang, J. Anticancer agents derived from natural cinnamic acids. J. Anticancer. Agents Med. Chem. 2015, 15, 980–987. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.; Granato, D.; Azevedo, L. Antioxidant/pro-oxidant and antiproliferative activities of phenolic-rich foods and extracts: A cell-based point of view. Adv. Food Nutr. Res. 2021, 98, 253–280. [Google Scholar]


| Name | MrM | MrHM | MpM | MpHM |
|---|---|---|---|---|
| apigenin | 0.20 ± 0.01 | <0.001 | 0.24 ± 0.01 | 0.10 ± 0.01 |
| Apigenin-7-O-glucoside | 1.20 ± 0.03 | 0.59 ± 0.01 | 0.50 ± 0.01 | 0.36 ± 0.01 |
| Apigenin-7-O-glucuronide | - | - | 0.24 ± 0.01 | 0.34 ± 0.01 |
| Luteolin | - | - | 0.60 ± 0.01 | 0.31 ± 0.01 |
| 6-hydroxyluteolin-glucoside | - | - | 0.60 ± 0.01 | 0.64 ± 0.04 |
| Luteolin-7-O-glucoside | - | - | 1.13 ± 0.02 | 0.73 ± 0.03 |
| Patuletin-3-O-glucoside | 1.91 ± 0.06 | 1.89 ± 0.03 | 0.49 ± 0.02 | 0.38 ± 0.01 |
| Quercetin-7-O-glucoside | 0.47 ± 0.01 | 0.34 ± 0.01 | 0.21 ± 0.02 | 0.52 ± 0.01 |
| Patuletin-7-O-glucoside | 0.46 ± 0.02 | 0.33 ± 0.02 | - | - |
| Isorhamnetin-3-O-glucoside | 0.81 ± 0.04 | 0.60 ± 0.01 | - | - |
| Total flavonoids | 5.06 ± 0.01 | 3.75 ± 0.05 | 4.00 ± 0.06 | 3.38 ± 0.08 |
| 3,5- +1,5- dicaffeoylquinic acids | 0.90 ± 0.03 | 1.13 ± 0.02 | 1.78 ± 0.01 | 1.67 ± 0.02 |
| 4,5-dicaffeoylquinic acid | 0.68 ± 0.01 | 1.02 ± 0.01 | 0.65 ± 0.03 | 1.11 ± 0.03 |
| cis-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | 0.49 ± 0.01 | 0.41 ± 0.02 | - | - |
| trans-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | 0.63 ± 0.01 | 0.48 ± 0.01 | - | - |
| Total phenolic acids | 2.70 ± 0.05 | 3.04 ± 0.04 | 2.43 ± 0.04 | 2.79 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatiadou, M.-E.; Kostaki, M.; Kabouche, Z.; Chatzopoulou, P.; Rallis, M.C.; Karioti, A. HPLC–NMR-Based Chemical Profiling of Matricaria pubescens (Desf.) Schultz and Matricaria recutita and Their Protective Effects on UVA-Exposed Fibroblasts. Sci 2022, 4, 14. https://doi.org/10.3390/sci4010014
Ignatiadou M-E, Kostaki M, Kabouche Z, Chatzopoulou P, Rallis MC, Karioti A. HPLC–NMR-Based Chemical Profiling of Matricaria pubescens (Desf.) Schultz and Matricaria recutita and Their Protective Effects on UVA-Exposed Fibroblasts. Sci. 2022; 4(1):14. https://doi.org/10.3390/sci4010014
Chicago/Turabian StyleIgnatiadou, Maria-Elena, Maria Kostaki, Zahia Kabouche, Paschalina Chatzopoulou, Michail Christou Rallis, and Anastasia Karioti. 2022. "HPLC–NMR-Based Chemical Profiling of Matricaria pubescens (Desf.) Schultz and Matricaria recutita and Their Protective Effects on UVA-Exposed Fibroblasts" Sci 4, no. 1: 14. https://doi.org/10.3390/sci4010014
APA StyleIgnatiadou, M.-E., Kostaki, M., Kabouche, Z., Chatzopoulou, P., Rallis, M. C., & Karioti, A. (2022). HPLC–NMR-Based Chemical Profiling of Matricaria pubescens (Desf.) Schultz and Matricaria recutita and Their Protective Effects on UVA-Exposed Fibroblasts. Sci, 4(1), 14. https://doi.org/10.3390/sci4010014






