Evolution of Realistic Organic Mixtures for the Origins of Life through Wet–Dry Cycling
Abstract
:1. Introduction
2. Materials and Methods
3. Results
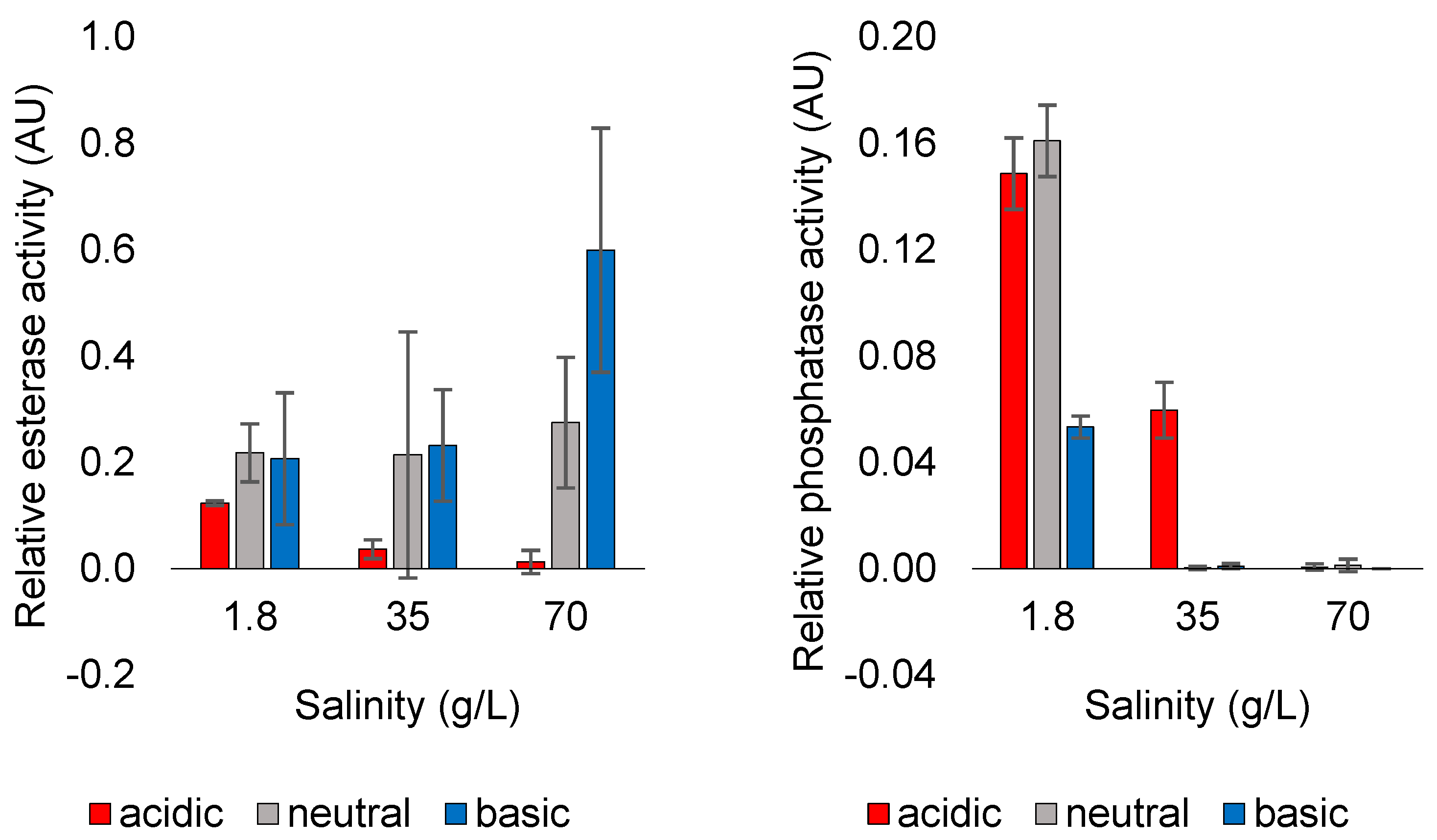
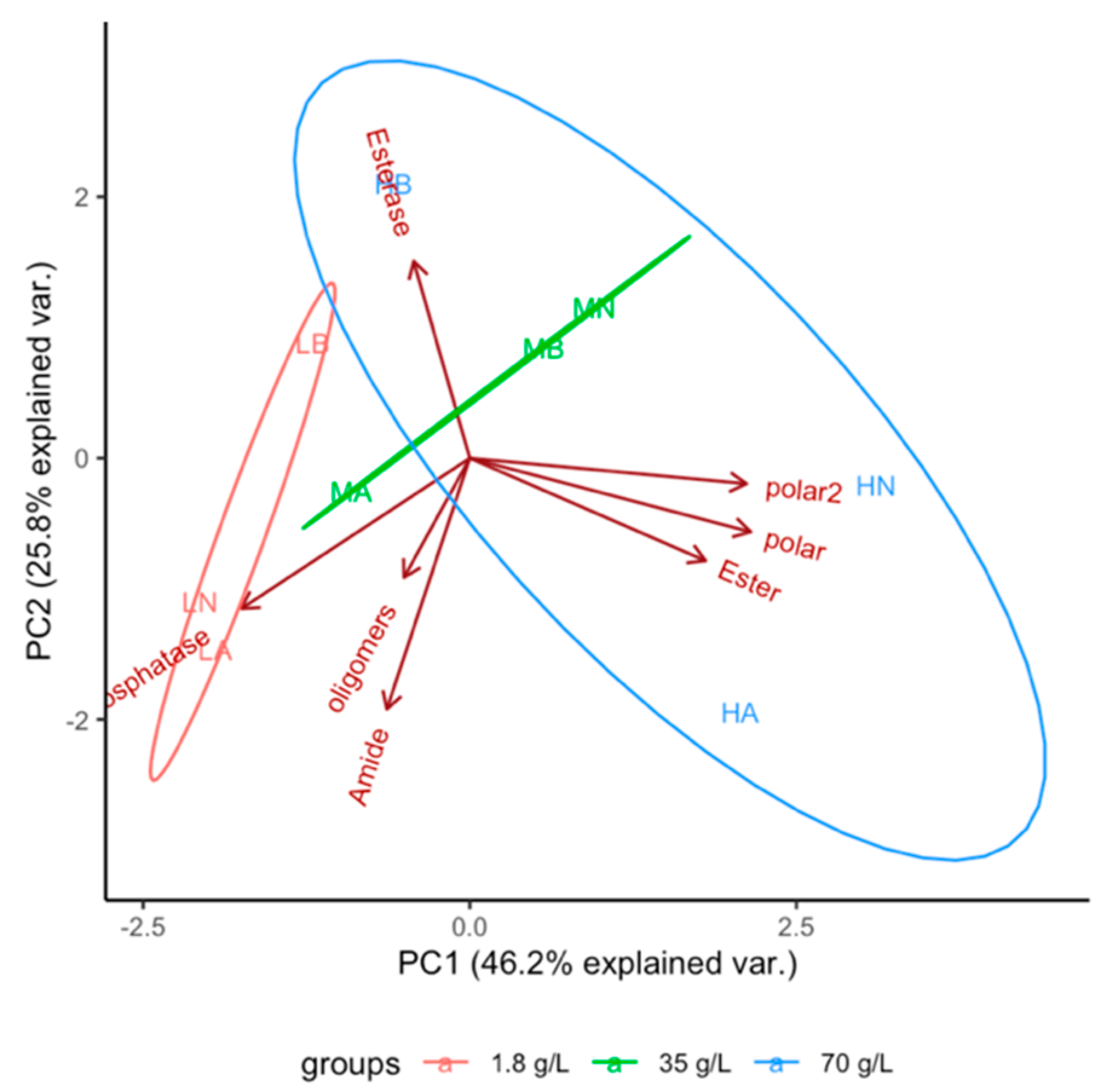
3.1. Evolution of Function
3.2. Effect of Dissolved Solutes in Starting and Rehydration Solution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, S.L.; Urey, H.C. Organic Compound Synthesis on the Primitive Earth. Science 1959, 130, 7. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous Production, Exogenous Delivery and Impact-Shock Synthesis of Organic Molecules—An Inventory for the Origins of Life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Perez, A.; Thompson, G.; Pasek, M. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, J.D. The Origin of Life—Out of the Blue. Angew. Chem. Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Islam, S.; Powner, M.W. Prebiotic Systems Chemistry: Complexity Overcoming Clutter. Chem 2017, 2, 470–501. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.H.; Hyde, A.S.; Simkus, D.N.; Libby, E.; Maurer, S.E.; Graham, H.V.; Kempes, C.P.; Sherwood Lollaret, B.; Chou, L.; Ellington, A.D. The Grayness of the Origin of Life. Life 2021, 11, 498. [Google Scholar] [CrossRef]
- McCollom, T.M. Miller-Urey and Beyond: What Have We Learned About Prebiotic Organic Synthesis Reactions in the Past 60 Years? Annu. Rev. Earth Planet. Sci. 2013, 41, 207–229. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [Green Version]
- Wollrab, E.; Scherer, S.; Aubriet, F.; Carré, V.; Carlomagno, T.; Codutti, L.; Ott, A. Chemical Analysis of a “Miller-Type” Complex Prebiotic Broth. Orig. Life Evol. Biosph. 2016, 46, 149–169. [Google Scholar] [CrossRef]
- Scherer, S.; Wollrab, E.; Codutti, L.; Carlomagno, T.; da Costa, S.G.; Volkmer, A.; Bronja, A.; Schmitz, O.J.; Ott, A. Chemical Analysis of a “Miller-Type” Complex Prebiotic Broth. Orig. Life Evol. Biosph. 2017, 47, 381–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branciamore, S.; Gallori, E.; Szathmáry, E.; Czárán, T. The Origin of Life: Chemical Evolution of a Metabolic System in a Mineral Honeycomb? J. Mol. Evol. 2009, 69, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, E.C.; Tuck, A.F.; Vaida, V. Ocean & Atmosphere Interactions in the Emergence of Complexity in Simple Chemical Systems. Acc. Chem. Res. 2012, 45, 2106–2113. [Google Scholar] [PubMed]
- Tena-Solsona, M.; Wanzke, C.; Riess, B.; Bausch, A.R.; Boekhoven, J. Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 2018, 9, 2044. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Berg, M.; Krismer, M.; Saghafi, S.T.; Cosby, J.; Sankari, T.; Vetsigian, K.; Cleaves, H.J., II; Baum, D.A. Chemical Ecosystem Selection on Mineral Surfaces Reveals Long-Term Dynamics Consistent with the Spontaneous Emergence of Mutual Catalysis. Life 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef] [Green Version]
- Gillams, R.; Jia, T. Mineral Surface-Templated Self-Assembling Systems: Case Studies from Nanoscience and Surface Science towards Origins of Life Research. Life 2018, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Kaneko, T.; Saito, T.; Oshima, T. Amino Acid Formation in Gas Mixtures by High Energy Particle Irradiation. Orig. Life Evol. Biosph. 1998, 28, 155–165. [Google Scholar] [CrossRef]
- Scattergood, T.W.; McKay, C.P.; Borucki, W.J.; Giver, L.P.; van Ghyseghem, H.; Parris, J.E.; Miller, S.L. Production of organic compounds in plasmas: A comparison among electric sparks, laser-induced plasmas, and UV light. Icarus 1989, 81, 413–428. [Google Scholar] [CrossRef]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic chemistry and atmospheric warming of early Earth by an active young Sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA World: The Fate of Nucleobases in Warm Little Ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfio, C.; Valer, L.; Scintilla, S.; Shah, S.; Evans, D.J.; Jin, L.; Szostak, J.V.; Sasselov, D.D.; Sutherland, J.D.; Mansy, S.S. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nat. Chem. 2017, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Bizzarri, B.M.; Botta, L.; Šponer, J.; Šponer, J.E.; Georgelin, T.; Jaber, M.; Rigaud, B.; Kapralov, M.; Tmoshenko, G.N.; et al. Proton irradiation: A key to the challenge of N-glycosidic bond formation in a prebiotic context. Sci. Rep. 2017, 7, 14709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.W.J.; Fahrenbach, A.C. Radicals in prebiotic chemistry. Pure Appl. Chem. 2020, 92, 1971–1986. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Rushdi, A.I.; Deamer, D.W. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv. Space Res. 2007, 40, 1649–1656. [Google Scholar] [CrossRef]
- Toppozini, L.; Dies, H.; Deamer, D.W.; Rheinstädter, M.C. Adenosine Monophosphate Forms Ordered Arrays in Multilamellar Lipid Matrices: Insights into Assembly of Nucleic Acid for Primitive Life. PLoS ONE 2013, 8, e62810. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-S.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V.; Schork, F.J.; Grover, M.A. Kinetics of prebiotic depsipeptide formation from the ester–amide exchange reaction. Phys. Chem. Chem. Phys. 2016, 18, 28441–28450. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Haynes, J.W.; Martin, C.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar]
- Yu, S.-S.; Solano, M.D.; Blanchard, M.K.; Soper-Hopper, M.T.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V.; Schork, F.J.; Grover, M.A. Elongation of Model Prebiotic Proto-Peptides by Continuous Monomer Feeding. Macromolecules 2017, 50, 9286–9294. [Google Scholar] [CrossRef]
- Campbell, T.D.; Febrian, R.; McCarthy, J.T.; Kleinschmidt, H.E.; Forsythe, J.G.; Bracher, P.J. Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 2019, 10, 4508. [Google Scholar] [CrossRef]
- Chandru, K.; Mamajanov, I.; Cleaves, H.J.; Jia, T.Z. Polyesters as a Model System for Building Primitive Biologies from Non-Biological Prebiotic Chemistry. Life 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of Phospholipids and Membranes in Prebiotic Conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D.W. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.M.; Flores, E.; Baum, M.M.; VanderVelde, D.G.; Russell, M.J. Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl. Acad. Sci. USA 2019, 116, 4828–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M.A.; Zylstra, A.; Castro, C.; Turchyn, A.V.; Griffin, J.L.; Ralser, M. Conditional iron and pH-dependent activity of a non-enzymatic glycolysis and pentose phosphate pathway. Sci. Adv. 2016, 2, e1501235. [Google Scholar] [CrossRef] [Green Version]
- Maurer, S.E.; Tølbøl Sørensen, K.; Iqbal, Z.; Nicholas, J.; Quirion, K.; Gioia, M.; Monnard, P.-A.; Hanczyc, M.M. Vesicle Self-Assembly of Monoalkyl Amphiphiles under the Effects of High Ionic Strength, Extreme pH, and High Temperature Environments. Langmuir 2018, 34, 15560–15568. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Polymerization of beta-amino acids in aqueous solution. Orig. Life Evol. Biosph. 1998, 28, 47–60. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Prebiotic organic synthesis under hydrothermal conditions: An overview. Adv. Space Res. 2004, 33, 88–94. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 degrees C. Orig. Life Evol. Biosph. 2001, 31, 103–118. [Google Scholar] [CrossRef]
- Ying, J.; Chen, P.; Wu, Y.; Yang, X.; Yan, K.; Xu, P.; Zhao, Y. Effect of high hydrostatic pressure on prebiotic peptide synthesis. Chin. Chem. Lett. 2019, 30, 367–370. [Google Scholar] [CrossRef]
- Surman, A.J.; Rodriguez-Garcia, M.; Abul-Haija, Y.M.; Cooper, G.J.T.; Gromski, P.S.; Turk-MacLeod, R.; Mullin, M.; Mathis, C.; Walker, S.I.; Cronin, L. Environmental control programs the emergence of distinct functional ensembles from unconstrained chemical reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 5387–5392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, K.; Pedreira-Segade, U.; Fox, P.; Shelley, J.T.; Steele, A.; Trail, D. Reimagining Origins of Life Research: Innovation and Synthesis via Experimentation, Instrumentation, and Data Analytics. Bull. Am. Astron. Soc. 2021, 43, 215. [Google Scholar]
- Vincent, L.; Colón-Santos, S.; Cleaves, H.J., II; Baum, D.A.; Maurer, S.E. The Prebiotic Kitchen: A Guide to Composing Prebiotic Soup Recipes to Test Origins of Life Hypotheses. Life 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.W.; Onwo, W.M.; Cronin, J.R. Alkyl phosphonic acids and sulfonic acids in the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4109–4115. [Google Scholar] [CrossRef]
- Knauth, L.P. Temperature and salinity history of Precambrian ocean: Implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 53–69. [Google Scholar] [CrossRef]
- Thompson, A.R. A Colorimetric Method for the Determination of Esters. Aust. J. Chem. 1950, 3, 128–135. [Google Scholar] [CrossRef]
- Doran, D.; Abul-Haija, Y.M.; Cronin, L. Emergence of Function and Selection from Recursively Programmed Polymerisation Reactions in Mineral Environments. Angew. Chemie Int. Ed. 2019, 58, 11253–11256. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, K.; Hillman, B.; Rajaei, V.; Seng, K.; Maurer, S. Evolution of Realistic Organic Mixtures for the Origins of Life through Wet–Dry Cycling. Sci 2022, 4, 22. https://doi.org/10.3390/sci4020022
Foster K, Hillman B, Rajaei V, Seng K, Maurer S. Evolution of Realistic Organic Mixtures for the Origins of Life through Wet–Dry Cycling. Sci. 2022; 4(2):22. https://doi.org/10.3390/sci4020022
Chicago/Turabian StyleFoster, Kiernan, Brooke Hillman, Vahab Rajaei, Kimsorn Seng, and Sarah Maurer. 2022. "Evolution of Realistic Organic Mixtures for the Origins of Life through Wet–Dry Cycling" Sci 4, no. 2: 22. https://doi.org/10.3390/sci4020022
APA StyleFoster, K., Hillman, B., Rajaei, V., Seng, K., & Maurer, S. (2022). Evolution of Realistic Organic Mixtures for the Origins of Life through Wet–Dry Cycling. Sci, 4(2), 22. https://doi.org/10.3390/sci4020022






