Abstract
Mechanical forces and mechanical energy are prevalent in living cells. This may be because mechanical forces and mechanical energy preceded chemical energy at life’s origins. Mechanical energy is more readily available in nonliving systems than the various forms of chemical energy used by living systems. Two possible prebiotic environments that might have provided mechanical energy are hot pools that experience wet/dry cycles and mica sheets as they move, open and shut, as heat pumps or in response to water movements.
Keywords:
origin of life; origins of life; mechanical energy; mechanochemistry; work; entropic forces; mica; biotite; Muscovite; wet/dry cycles; clay 1. Introduction
Mechanical forces and mechanical energy are prominent in living systems, at all size scales, from the molecular to the cellular and beyond [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. Much of cells’ chemical energy, such as ATP, is used to generate these forces. Perhaps mechanical energy in living cells is a remnant of mechanical energy that brought life into being, before chemical energy was readily available.
Mechanical forces shift reaction pathways [20]. Mechanical force lowers the transition states for reactions by tilting the energy landscape, and can give different reaction products than reactions without mechanical force [21].
Mechanical energy at life’s origins would resemble synthetic mechanochemistry in the lab, because there were no enzymes to carry out the (bio)chemical reactions. How feasible is synthetic mechanochemistry, in practice? Synthetic organic mechanochemistry has been used to produce many organic molecules, including pyrimidines [22], peptides, nucleosides, optically active products, oxidations, reductions, condensations, nucleophilic reactions, and cascade reactions [23]. The industrial appeal of synthetic organic mechanochemistry is that it reduces the use of solvents.
Many forces pushed molecules around before ‘biochemical’ energy was available. These forces include hydration, dehydration, and surface forces, as well as entropic forces. Entropic forces “can have the counterintuitive effect of apparently introducing ‘order’” [24]. Entropic forces are also known as ‘excluded-volume forces’ or ‘depletion interactions.’ As water molecules become scarce during drying, the entropy increases when monomers polymerize. Monomer polymerization frees up a few water molecules that were constrained to linger near the ends of the monomers before they polymerized. The monomers experience an entropic force of attraction that brings them together.
Entropic forces could have generated mechanical energy (the product of force and distance), without chemical energy, at life’s origins. In living systems, the motions and forces of enzymes usually need energy transduction from an energy source such as ATP. ATP and most of the chemical energy sources now used by living systems were not available at life’s origins. Mechanical forces, entropic forces, redox reactions—and of course, the sun!—were available for providing energy at life’s origins.
The mechanical forces of mechanochemistry are a possible energy source for forming monomers as well as polymers. Some monomers, such as amino acids, may have been present on the early earth, but others, such as nucleotides, were not present and were synthesized in some way/s [25].
2. Materials and Methods
Muscovite mica was obtained from New York Mica Co., New York, NY, USA, and was scanned at 600 dpi with an HP Officejet 4635.
3. Results and Discussion
Research on Previous ‘Prebiotic’ Polymerizations. Proteins and nucleic acids are the main biopolymers involved in cell functions. Lipids are essential for cell functions but are not ‘beads-on-a-string’ polymers. Carbohydrates are also biopolymers, but are used more for energy storage and cell structure. In most research investigating the prebiotic formation of proteins/peptides and nucleic acids/oligomers, the monomers are chemically activated, (e.g., [26,27,28,29,30]), which is not a realistic model for the origins of life.
Much relevant research has used unactivated amino acids and nucleotides. Unactivated amino acids polymerize into peptides when dried but hydrolyze when wet [31], which is also not ideal for the origins of life. A newer, better ‘prebiotic’ way of forming peptides used hot/dry vs. cool/wet cycles [32]. Another improvement was the use of both amino acids and hydroxy acids to form depsipeptides, which have both amide (peptide) and ester bonds. The ester bonds form and break more easily than the peptide bonds. Initially, mostly ester bonds formed. With cyclic wetting and drying, ester residues were replaced by amino acid residues, leading to a heteropolymer that was increasingly rich in amino acids [32].
Clays have been used for many ‘prebiotic’ polymerization experiments, catalyzing or supporting the formation of both peptides and oligonucleotides (e.g., [29,30]). Clays are layered silicate minerals that swell when wet and shrink when dry. Montmorillonite clays are best for these polymerizations. The anionic silicate layers of Montmorillonite clays are held together by hydrated sodium (Na) ions. The hydration of the Na ions causes the clay to shrink and swell in response to drying and wetting.
Two Embodiments of Mechanical Energy at Life’s Origins. Wet/dry cycles and moving mica sheets are two sources of mechanical energy available for powering the many types of chemical reactions that occurred as life was coming into being. Mechanical energy might be capable of forming the monomers and polymers of prebiotic molecules. Polymer formation by mechanochemistry is diagrammed in Figure 1 for the reaction of alanine + di-alanine to form tri-alanine.
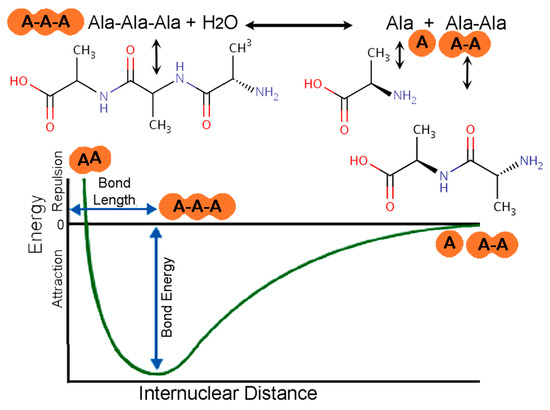
Figure 1.
Energy diagram of the way that mechanochemistry might polymerize molecules, such as Alanine (A), shown here. (Top) tri-alanine, A-A-A, forming, reversibly from, alanine and di-alanine (A-A), with the release of a water molecule. (Bottom) Mechanical force vs. distance curve showing Attractive and Repulsive regimes as molecules are pushed closer together, to the bonding distance. Modified from [33].
“Fresh water” origins are assumed here, because low ionic-strength solutions are needed to form lipid membranes [34,35]. (Deamer’s paper [34] has a wonderful analysis of lipids, membranes, and ‘informed guesses’ about their prebiotic assembly.) There is also new evidence for origins in hot water on land [36,37,38] and evidence for origins in shallow ponds [39]. Whether life began in water on land or in water between mica sheets, the “fresh water” requirement holds for any origins scenario involving water–air interfaces. As Deamer points out, biochemists use dilute buffered aqueous solutions to do their biochemistry experiments, as opposed to ‘salt water’ [40].
3.1. Wet/Dry Cycles
Cyclic wetting and drying on land occur in hot puddles where volcanoes form, such as the Kamchatka peninsula in Russia [41], or in active geothermal fields, such as The Geysers in California [42]. Cyclic wetting and drying occurs on mineral or rock surfaces and has the advantage of concentrating prebiotic molecules during the drying phase, which overcomes the problems of dilution in Darwin’s “warm little ponds”. Prebiotic chemistry was tested in the Kamchatka peninsula, by pouring a sample of white powder into a hot clay-lined pool. The powder contained four amino acids and four chemical bases that compose naturally occurring nucleic acids, plus sodium phosphate, glycerol, and a fatty acid. Foam and a white scum appeared quickly [36,41].
An amazing discovery from the Deamer lab is that unactivated mononucleotides will polymerize during wetting and drying in the presence of lipids [43,44]. Lipids protect the oligonucleotides from the hydrolysis that occurs during drying without lipids.
This lipid-assisted origin of life is proposed for the ancient Dresser Formation in the Pilbara region of Australia. These rocks contain evidence of the earliest life on land, more than half a billion years earlier than previously believed [36,45]. Once an active geothermal field, the Dresser Formation now has 3.48 billion-year-old stromatolite fossils, which appear to have formed on land and not in oceans, as was previously believed [40].
As Damer describes the wet/dry cycles, there was a third stage in the cycles of vesicles forming and breaking—a moist gel stage [36,38,46]. Molecules reassorted and grew in complexity in the moist gel, climbing up an ‘evolutionary ladder’ and ‘booting up’ the functions of life, through ‘programs written in polymers.’ Doyle uses similar language in describing the emerging complexity of life and its processes: “All life and advanced technologies rely on protocol-based architectures” that are ‘robust yet fragile’ [47].
A fractionation of organic molecules would occur during drying in rocky puddles, with the most lipid-rich material forming ‘bathtub rings’ [40] with divalent salts at the earliest stages of drying. With continued drying and concentration of salts, prebiotic mixtures with less and less lipid would dry onto the rocky walls as the density of the salt solution increased, with a moist gel phase at the bottom that would be enriched in nonlipid molecules.
The situation is analogous to isolating lipoproteins from blood plasma, except that lipoproteins are isolated by increasing the salt concentration such that different lipoproteins float to the top. The most lipid-rich lipoproteins rise to the top of the initial solution, and successively more lipid-poor lipoproteins rise to the top as the solution density is adjusted with salt to densities of 1.02 g/mL for Low Density Lipoprotein (LDL) and 1.21 g/mL for High Density Lipoprotein [48]. A similar fractionation likely occurred in drying pools at life’s origins.
Theoretical analyses of the wet/dry cycles have provided additional insights: As polymers dry, polymerization rates increase, because diffusion distances are shorter. As polymers continue drying, polymerization rates decrease, because crowding decreases diffusion rates [49]. Polymerization can be explained thermodynamically by excluded volume effects and molecular crowding [50], where entropic forces would generate mechanical energy.
3.2. Moving Mica Sheets
Mica is old—old enough to be the mineral from which life emerged [51]. Mica has a clay-like silicate structure with potassium (K) ions holding its anionic sheets together. K ions are larger than Na ions, so there is no space for water molecules between unsplit mica sheets. Therefore, mica does not shrink and swell with drying and wetting, providing a more stable environment than clay particles. However, water seeps in at the edges of mica sheets with cycles of heating and cooling (Figure 2C) [20], and water can move farther in between the mica sheets, gradually, even to the point where the mica becomes ‘matted’, with large spaces between sheets.
Mica’s mineral sheets move, open and shut, in response to water flow (Figure 2A) and heating and cooling (Figure 2B). The movements of the mica sheets squeeze and stretch the molecules with enough mechanical force to make and break covalent bonds between them [52]. Spaces between mica sheets form cantilever-type springs, capable of generating a vast array of different mechanical forces, depending on the area and thickness of the mica cantilever.
Longer DNA molecules bind more strongly to mica sheets than shorter DNA molecules, which are more likely to be washed away, as observed by atomic force microscopy (AFM) [53,54]. This stronger binding favors the accumulation of longer nucleic-acid molecules on mica, thus accumulating nucleic acids long enough to carry ‘enough’ information.
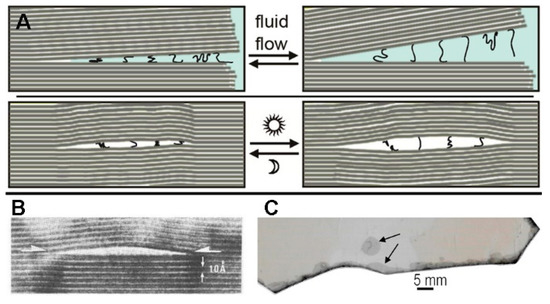
Figure 2.
(A) Diagram of mechanical forces between biotite mica sheets, stretching and compressing polymers, due to: (Upper panels) water flow at the edges of the biotite sheets, and (Lower panels) heat pumps in a biotite bubble. (B) Biotite bubble imaged by HRTEM (high-resolution transmission electron microscopy). The thickness of a single biotite sheet is 1 nm (10 Angstroms) [55]. (C) Top view of a bubble in Muscovite mica (upper arrow) and of sheet separation at the edges of the mica sheet (bottom arrow). Bubbles are common even in ‘high grade’ micas. Biotite is now the preferred mica, for life’s origins between mica sheets [56,57].
Mica’s anionic crystal lattice has a periodicity of 0.5 nm (Figure 3), which is also the periodicity of phosphates on extended nucleic acids and the periodicity of sugar residues of carbohydrates. This shared periodicity makes mica a possible template for polymerizing nucleotides into nucleic acids or sugars into polysaccharides.
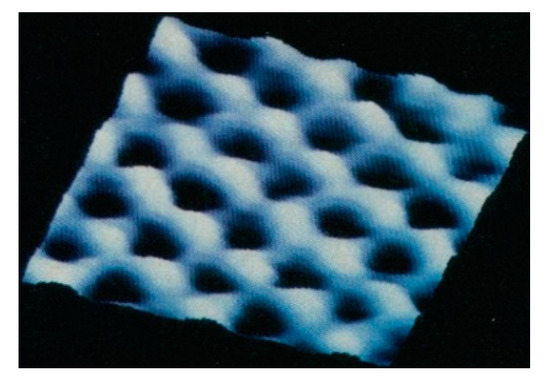
Figure 3.
Crystal lattice of Muscovite mica imaged by atomic force microscopy (AFM), showing locations of recessed hydroxyl (OH) and ionized hydroxyl (O−) groups, as depressions (dark spots) on the mica surface. Image size is 2.6 nm × 2.6 nm. Modified from [58].
DNA and mica are both also anionic, and cations bridge the anionic charges in both DNA and mica. In mica, the 0.5 nm periodicity of anionic negative charges corresponds to the recessed hydroxyl groups in the mica surface, each of which carries half negative charge (i.e., either OH or O− groups). In DNA, the phosphate groups are anionic and interact with cations in living cells. Perhaps DNA, and life, emerged on anionic mica sheets [54]. Amino acids in peptides have a smaller periodicity, such that a tripeptide has a length of ~1 nm.
Mica sheets might shelter emerging life without the need for membranes. Membranes are fragile. They leak, acquire and lose molecules, swell, and rupture. Mica is a robust mineral with nearly endless spaces between its sheets.
In living cells, membraneless organelles such as nucleoli contain RNA and protein. Although ribosomes are smaller than membraneless organelles in living cells, ribosomes have some of the most ancient RNAs and proteins. Ribosomes were present in the Last Universal Common Ancestor of life (LUCA) [59]. When life was coming into being, in the pre-LUCA stages, ribosomes and their precursors may have been the first ‘membraneless organelles’ [60]. Membraneless organelles form by liquid–liquid phase separation [61]. Phase-separated membraneless organelles at life’s origins are now proposed by various groups [62,63,64,65,66]. A delightful news article for [66] is titled “In the Beginning was the Phase Separation” [67]
Life might have started in mica without membranes, but membranes and lipids are compatible with mica surfaces, as seen through atomic force microscopy (AFM) of lipids and membranes on mica [68,69,70]. Therefore, mica could be the site for life’s origins, whether lipids were needed at the earliest stages of proto-life, or only at later stages in life’s emergence.
4. Conclusions
Mechanical energy in living systems provides energy in a form that is common in nonliving systems. Why do living systems convert chemical energy to mechanical energy, instead of using chemical energy directly? Perhaps this is because mechanical energy was an original energy source at the origins of life. Entropic forces provide mechanical energy during drying or molecular crowding.
Mechanical work can be done without chemical energy, ion gradients, or proton gradients, which now provide energy for most of the processes in living systems. The creation of ion and proton gradients need an energy source, and these gradients need a continuous supply of energy to be maintained. Mechanical energy is a readily available energy source that may have brought life into being, and it is now found throughout living systems.
Funding
This research received no external funding.
Acknowledgments
Thank you to Bruce Damer and the other participants at the Origins of Life Gordon Research Conference in January 2018 for helpful discussions. Thank you to Katherine Lieban for writing advice and to Fyl Pincus for the stimulating presentations he facilitated at UCSB about entropic forces. Thank you to the reviewers for their suggestions an recommended references.
Conflicts of Interest
The author declares no conflict of interest.
References
- Garcia-Manyes, S.; Beedle, A.E. Steering chemical reactions with force. Nat. Rev. Chem. 2017, 1, 0083. [Google Scholar] [CrossRef]
- Friedland, J.C.; Lee, M.H.; Boettiger, D. Mechanically activated integrin switch controls α5β1 function. Science 2009, 323, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Simoni, A.; Wolfgang, W.; Topping, M.P.; Kavlie, R.G.; Stanewsky, R.; Albert, J.T. A mechanosensory pathway to the Drosophila circadian clock. Science 2014, 343, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Simons, B.D. Getting Your Gut into Shape. Science 2013, 342, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Harridge, S.D. See how we run. Science 2014, 344, 1235. [Google Scholar] [CrossRef]
- Gillen, C.M. The Hidden Mechanics of Exercise; Harvard University Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Dumont, S.; Salmon, E.D.; Mitchison, T.J. Deformations within moving kinetochores reveal different sites of active and passive force generation. Science 2012, 337, 355–358. [Google Scholar] [CrossRef]
- Lafaurie-Janvore, J.; Maiuri, P.; Wang, I.; Pinot, M.; Manneville, J.B.; Betz, T.; Balland, M.; Piel, M. ESCRT-III assembly and cytokinetic abscission are induced by tension release in the intercellular bridge. Science 2013, 339, 1625–1629. [Google Scholar] [CrossRef]
- Buckley, C.D.; Tan, J.; Anderson, K.L.; Hanein, D.; Volkmann, N.; Weis, W.I.; Nelson, W.J.; Dunn, A.R. The minimal cadherin-catenin complex binds to actin filaments under force. Science 2014, 346, 1254211. [Google Scholar] [CrossRef]
- Junker, J.P.; Ziegler, F.; Rief, M. Ligand-dependent equilibrium fluctuations of single calmodulin molecules. Science 2009, 323, 633–637. [Google Scholar] [CrossRef]
- del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching single talin rod molecules activates vinculin binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef]
- Natkanski, E.; Lee, W.-Y.; Mistry, B.; Casal, A.; Molloy, J.E.; Tolar, P. B cells use mechanical energy to discriminate antigen affinities. Science 2013, 340, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Hickenboth, C.R.; Moore, J.S.; White, S.R.; Sottos, N.R.; Baudry, J.; Wilson, S.R. Biasing reaction pathways with mechanical force. Nature 2007, 446, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Whittier, S.K.; Hengge, A.C.; Loria, J.P. Conformational motions regulate phosphoryl transfer in related protein tyrosine phosphatases. Science 2013, 341, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Christof, J.; Gebhardt, M.; Rief, M. Force signaling in biology. Science 2009, 324, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. The origin of cellular life. Bioessays 2000, 22, 1160–1170. [Google Scholar] [CrossRef]
- Bustamante, C.; Chemla, Y.R.; Forde, N.R.; Izhaky, D. Mechanical processes in biochemistry. Annu. Rev. Biochem. 2004, 73, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M. Cell and tissue mechanics: The new cell biology frontier. Am. Soc. Cell Biol. 2017, 28, 1815–1818. [Google Scholar] [CrossRef]
- Tseng, C.-Y.; Wang, A.; Zocchi, G. Mechano-chemistry of the enzyme guanylate kinase. EPL 2010, 91, 18005. [Google Scholar] [CrossRef]
- Evans, E. Probing the relation between force—Lifetime—And chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 105–128. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. Altering product selectivity by mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef]
- Raj, T.; Sharma, H.; Mayank; Singh, A.; Aree, T.; Kaur, N.; Singh, N.; Jang, D.O. “Solvent-Less” Mechanochemical Approach to the Synthesis of Pyrimidine Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 1468–1475. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Kondev, J.; Theriot, J. Physical Biology of the Cell; Garland Science: New York, NY, USA, 2008. [Google Scholar]
- Matthew, W.; Powner, J.D.S. Potentially Prebiotic Synthesis of Pyrimidine β-D-Ribonucleotides by Photoanomerization/Hydrolysis of α-d-Cytidine-2′-Phosphate. ChemBioChem 2008, 9, 2386–2387. [Google Scholar]
- Joyce, G.F.; Orgel, L.E. Progress toward Understanding the Origin of the RNA World. In The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA; Gesteland, R.F., Cech, T.R., Atkins, J.F., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2006; pp. 23–56. [Google Scholar]
- Chen, I.A.; Hanczyc, M.M.; Sazani, P.L.; Szostak, J.W. Protocells: Genetic Polymers Inside Membrane Vesicles. Cold Spring Harb. Monogr. Arch. 2006, 43, 57–88. [Google Scholar]
- Orgel, L.E. Polymerization on the Rocks: Theoretical Introduction. Orig. Life Evol. Biosph. 1998, 28, 227–234. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hill, A.R.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef]
- Joshi, P.C.; Aldersley, M.F.; Delano, J.W.; Ferris, J.P. Mechanism of Montmorillonite Catalysis in the Formation of RNA Oligomers. J. Am. Chem. Soc. 2009, 131, 13369–13374. [Google Scholar] [CrossRef]
- Lahav, N.; White, D.; Chang, S. Peptide formation in the prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 1978, 201, 67–69. [Google Scholar] [CrossRef]
- Forsythe, J.G.; Petrov, A.S.; Millar, W.C.; Yu, S.-S.; Krishnamurthy, R.; Grover, M.A.; Hud, N.V.; Fernández, F.M. Surveying the sequence diversity of model prebiotic peptides by mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, E7652–E7659. [Google Scholar] [CrossRef]
- Hansma, H.G. The Power of Crowding for the Origins of Life. Orig. Life Evol. Biosph. 2014, 44, 307–311. [Google Scholar] [CrossRef]
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Milshteyn, D.; Damer, B.; Havig, J.; Deamer, D. Amphiphilic compounds assemble into membranous vesicles in hydrothermal hot spring water but not in seawater. Life 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Van Kranendonk, M.J.; Deamer, D.W.; Djokic, T. Life springs. Sci. Am. 2017, 317, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. Membranes and the Origin of Life: A Century of Conjecture. J. Mol. Evol. 2016, 83, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef]
- Ranjan, S.; Todd, Z.R.; Rimmer, P.B.; Sasselov, D.D.; Babbin, A.R. Nitrogen Oxide Concentrations in Natural Waters on Early Earth. Geochem. Geophys. Geosyst. 2019, 20, 2021–2039. [Google Scholar] [CrossRef]
- Deamer, D. Assembling Life: How Can Life Begin on Earth and Other Habitable Planets; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Deamer, D.; Singaram, S.; Rajamani, S.; Kompanichenko, V.; Guggenheim, S. Self-assembly processes in the prebiotic environment. J. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1809–1818. [Google Scholar] [CrossRef]
- Lund, J.W. The USA geothermal country update. Geothermics 2003, 32, 409–418. [Google Scholar] [CrossRef]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-assisted Synthesis of RNA-like Polymers from Mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef]
- Olasagasti, F.; Kim, H.J.; Pourmand, N.; Deamer, D.W. Non-enzymatic transfer of sequence information under plausible prebiotic conditions. Biochimie 2011, 93, 556–561. [Google Scholar] [CrossRef]
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [PubMed]
- Damer, B. A field trip to the Archaean in search of Darwin’s warm little pond. Life 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.; Csete, M. Rules of engagement. Nature 2007, 446, 860. [Google Scholar] [CrossRef] [PubMed]
- Sardet, C.; Hansma, H.G.; Ostwald, R. Characterization of guinea pig plasma lipoproteins: The appearance of new lipoproteins in response to dietary cholesterol. J. Lipid Res. 1972, 13, 624–639. [Google Scholar]
- Ross, D.S.; Deamer, D. Dry/wet cycling and the thermodynamics and kinetics of prebiotic polymer synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef]
- Ross, D.; Deamer, D. Prebiotic Oligomer Assembly: What Was the Energy Source? Astrobiology 2019, 19, 517–521. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral Evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Hansma, H.G. Possible origin of life between mica sheets. J. Theor. Biol. 2010, 266, 175–188. [Google Scholar] [CrossRef]
- Hansma, H.G.; Bezanilla, M.; Zenhausern, F.; Adrian, M.; Sinsheimer, R.L. Atomic Force Microscopy of DNA in Aqueous Solutions. Nucleic Acids Res. 1993, 21, 505–512. [Google Scholar] [CrossRef]
- Hansma, H.G.; Laney, D.E. DNA binding to mica correlates with cationic radius: Assay by atomic force microscopy. Biophys. J. 1996, 70, 1933–1939. [Google Scholar] [CrossRef]
- Banos, J.O.; Amouric, M.; de Fouquet, C.; Baronnet, A. Interlayering and interlayer slip in biotite as seen by HRTEM. Am. Mineral. 1983, 68, 754–758. [Google Scholar]
- Hansma, H. Biotite is a better mica for the origins of life. In Proceedings of the Life3E’2019: Search for Life, from Early Earth to Exoplanets, Quy Nhon, Vietnam, 25–29 March 2019. [Google Scholar]
- Hansma, H.G. Liquid-Liquid Phase Transitions at the Origins of Life? Biophys. J. 2018, 114, 440a. [Google Scholar] [CrossRef][Green Version]
- Drake, B.; Prater, C.; Weisenhorn, A.; Gould, S.; Albrecht, T.; Quate, C.; Cannell, D.; Hansma, H.; Hansma, P. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.; Mohan, S.; Kalahar, B.K.; Williams, L.D. Peeling the onion: Ribosomes are ancient molecular fossils. Mol. Biol. Evol. 2009, 26, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G. Better than Membranes at the Origin of Life? Life 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P. Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 2013, 203, 875–881. [Google Scholar] [CrossRef]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J., II. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef]
- Drobot, B.; Iglesias-Artola, J.M.; Le Vay, K.; Mayr, V.; Kar, M.; Kreysing, M.; Mutschler, H.; Tang, T.-Y.D. Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nat. Commun. 2018, 9, 3643. [Google Scholar] [CrossRef]
- Poudyal, R.R.; Guth-Metzler, R.M.; Veenis, A.J.; Frankel, E.A.; Keating, C.D.; Bevilacqua, P.C. Template-directed RNA polymerization and enhanced ribozyme catalysis inside membraneless compartments formed by coacervates. Nat. Commun. 2019, 10, 490. [Google Scholar] [CrossRef]
- Cakmak, F.P.; Keating, C.D. Combining Catalytic Microparticles with Droplets Formed by Phase Coexistence: Adsorption and Activity of Natural Clays at the Aqueous/Aqueous Interface. Sci. Rep. 2017, 7, 3215. [Google Scholar] [CrossRef]
- Tena-Solsona, M.; Wanzke, C.; Riess, B.; Bausch, A.R.; Boekhoven, J. Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 2018, 9, 2044. [Google Scholar] [CrossRef] [PubMed]
- In the Beginning Was the Phase Separation. Available online: www.sciencedaily.com/releases/2018/05/180523145957.htm (accessed on 7 January 2020).
- Hansma, H.G.; Gould, S.A.C.; Hansma, P.K.; Gaub, H.E.; Longo, M.L.; Zasadzinski, J.A.N. Imaging nanometer scale defects in Langmuir-Blodgett films with the atomic force microscope. Langmuir 1991, 7, 1051–1054. [Google Scholar] [CrossRef]
- Hansma, H.G.; Weisenhorn, A.L.; Edmundson, A.B.; Gaub, H.E.; Hansma, P.K. Atomic force microscopy: Seeing molecules of lipid and immunoglobulin. Clin. Chem. 1991, 37, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G.; Hoh, J. Biomolecular imaging with the atomic force microscope. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 115–139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).