Lamellar Orientation of a Block Copolymer via an Electron-Beam Induced Polarity Switch in a Nitrophenyl Self-Assembled Monolayer or Si Etching Treatments
Abstract
1. Introduction
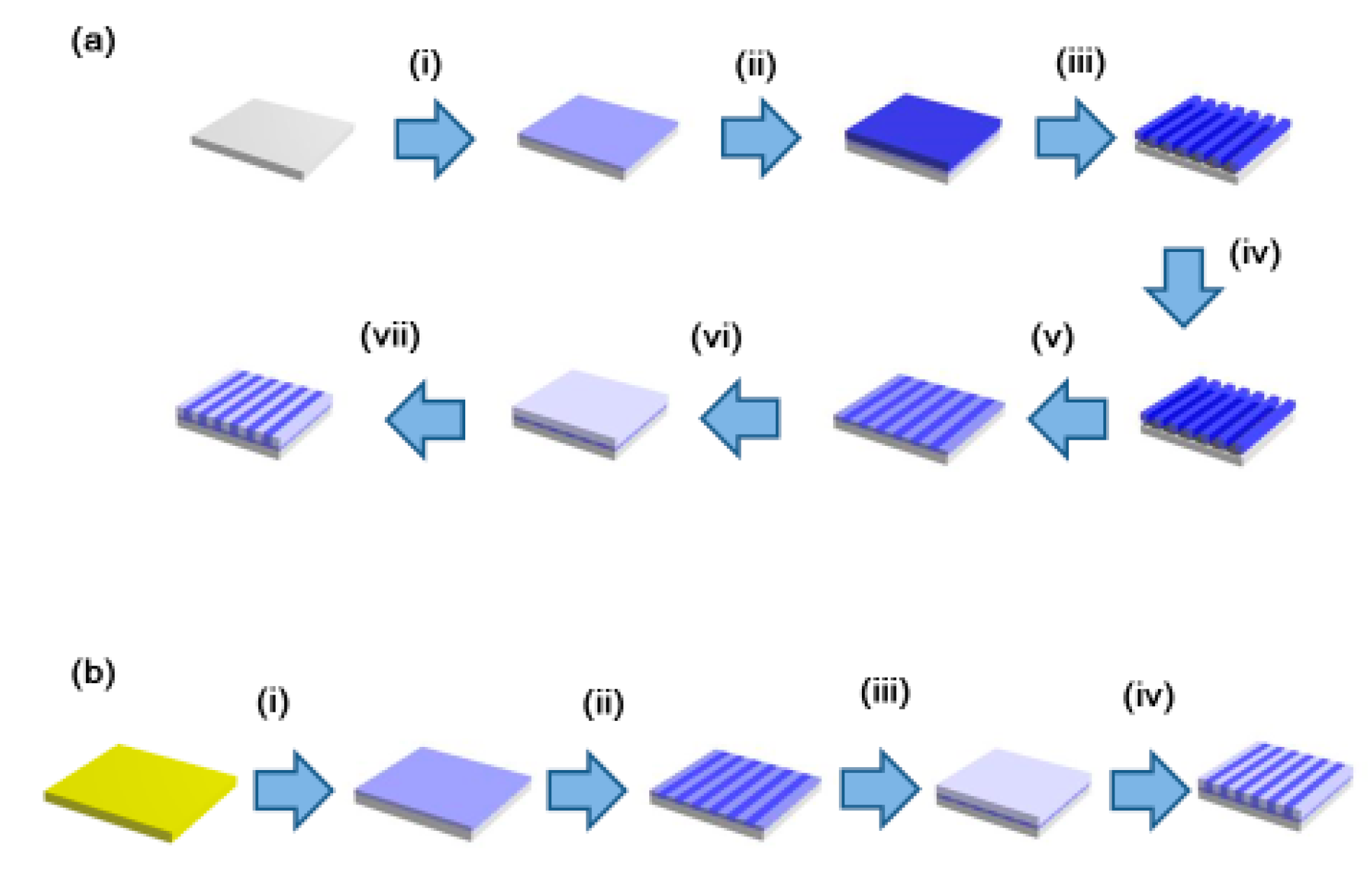
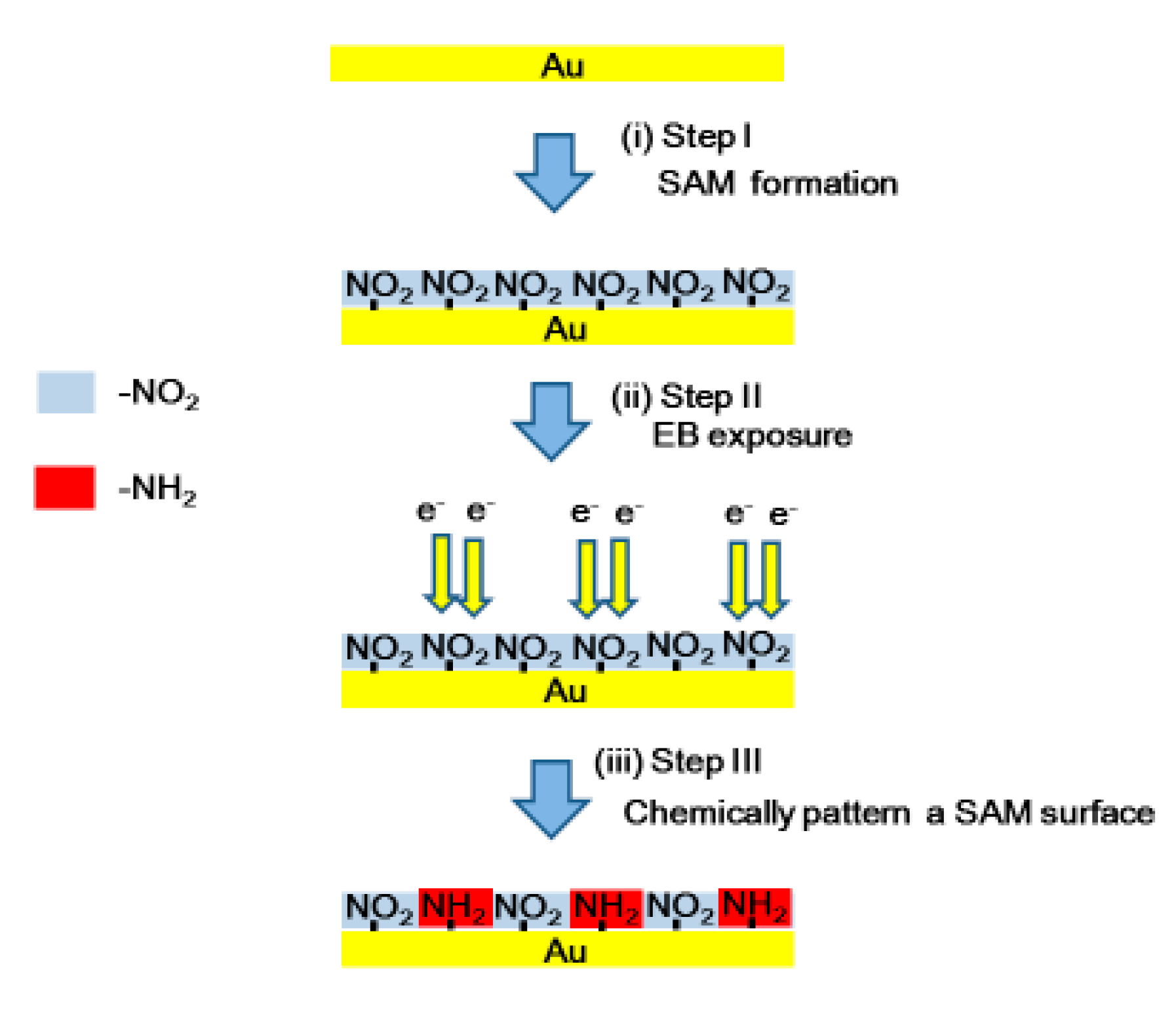
2. Materials and Methods
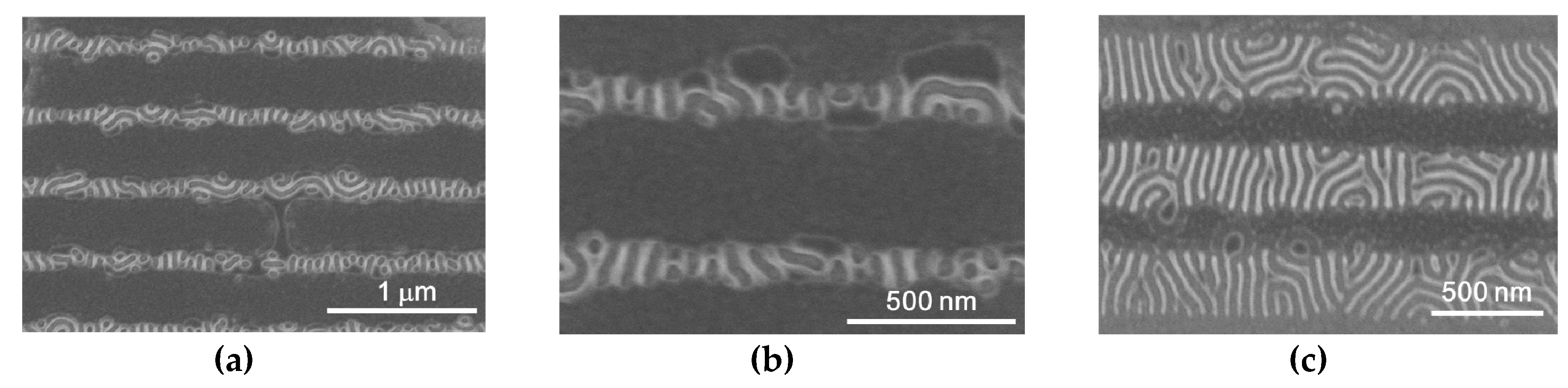
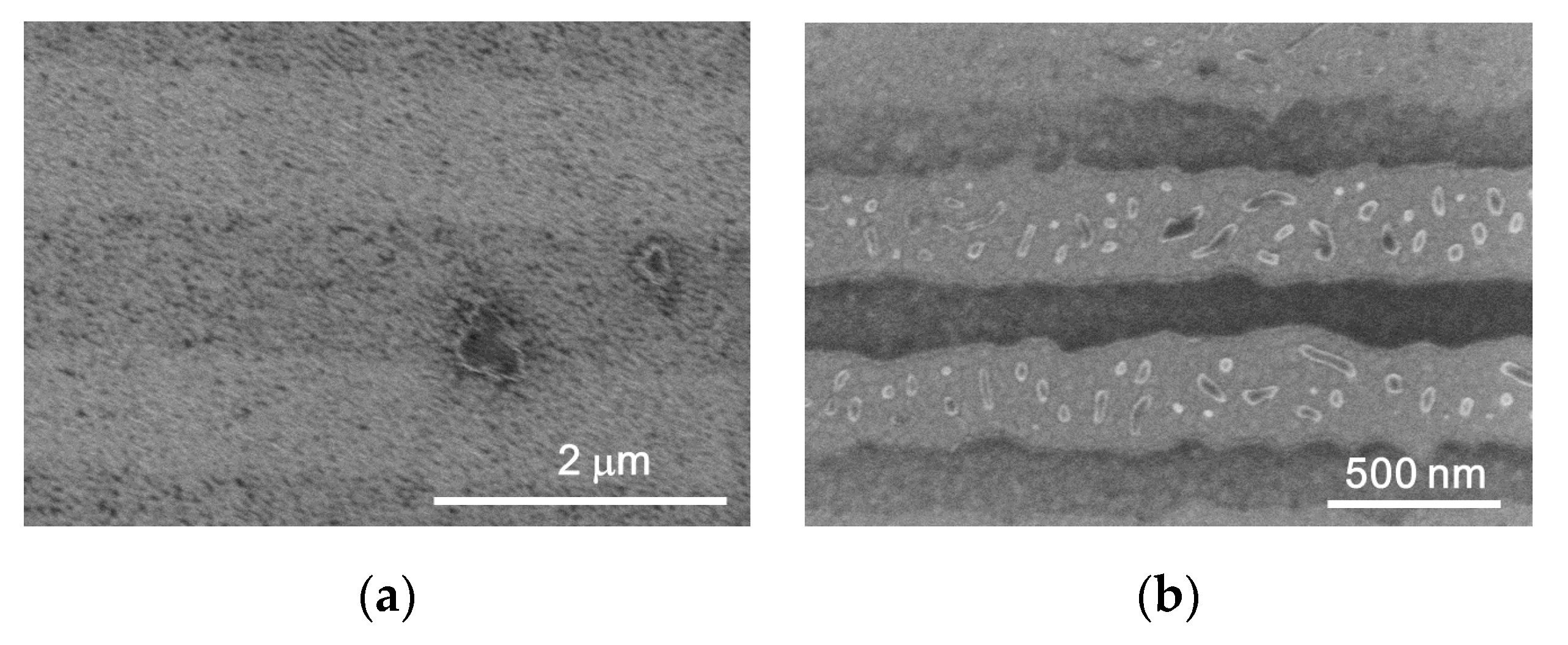
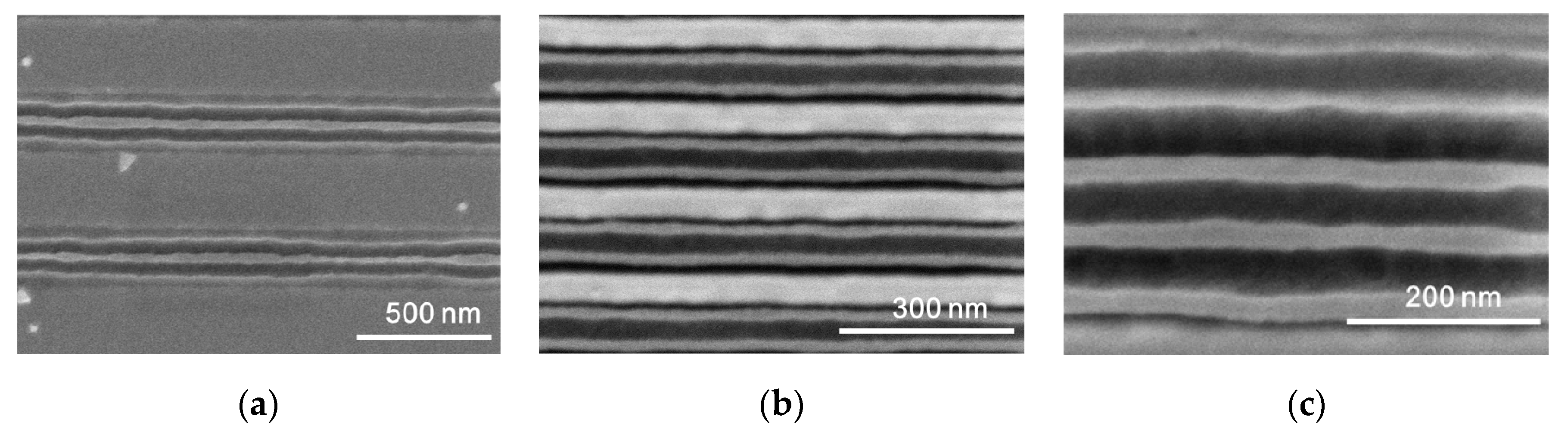
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.O.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; de Pablo, J.J.; Nealey, P.F. Directed Assembly of Block Copolymer Blends into Nonregular Device-Oriented Structure. Science 2005, 308, 1442. [Google Scholar]
- Segalman, R.A.; Yokoyama, H.; Kramer, E.J. Graphoepitaxy of Spherical Domain Block Copolymer Films. Adv. Mater. 2001, 13, 1152. [Google Scholar] [CrossRef]
- Shin, K.; Leach, K.A.; Goldbach, J.T.; Kim, D.H.; Jho, J.Y.; Tuominen, M.; Hawker, C.J.; Russel, T.P. A Simple Route to Metal Nanodots and Nanoporous Metal Films. Nano Lett. 2002, 2, 933. [Google Scholar] [CrossRef]
- Lopes, W.A.; Jaeger, H.M. Hierarchical self-assembly of metal nanostructures on diblock copolymer scaffolds. Nature 2001, 414, 735. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, D.H. Block Copolymer Lithography: Periodic Arrays of ~1011 Holes in 1 Square Centimeter. Science 1997, 276, 1401. [Google Scholar] [CrossRef]
- Templin, M.; Franck, A.; Chesne, A.D.; Leist, H.; Zhang, Y.; Ulrich, R.; Schadler, V.; Wiesner, U. Organically Modified Aluminosilicate Mesostructures from Block Copolymer Phases. Science 1997, 278, 1795. [Google Scholar] [CrossRef]
- International Technology Roadmap for Semiconductors. Available online: http://www.itrs2.net/ (accessed on 31 December 2016).
- Kim, S.H.; Misner, M.J.; Xu, T.; Kimura, M.; Russell, T.P. Highly Oriented and Ordered Arrays from Block Copolymers via Solvent Evaporation. Adv. Mater. 2004, 16, 226–231. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Ross, C.A.; Smith, H.I.; Thomas, E.L. Templated Self-Assembly of Block Copolymers: Top-Down Helps Bottom-Up. Adv. Mater. 2006, 18, 2505–2521. [Google Scholar] [CrossRef]
- Black, C.T. Self-aligned self assembly of multi-nanowire silicon field effect transistors. Appl. Phys. Lett. 2005, 87, 163116. [Google Scholar] [CrossRef]
- Sundrani, D.; Darling, S.B.; Sibener, S.J. Hierarchical Assembly and Compliance of Aligned Nanoscale Polymer Cylinders in Confinement. Langmuir 2004, 20, 5091–5099. [Google Scholar] [CrossRef]
- Rockford, L.; Liu, Y.; Mansky, P.; Russell, T.P.; Yoon, M.; Mochrie, S.G.J. Polymers on Nanoperiodic, Heterogeneous Surfaces. Phys. Rev. Lett. 1999, 82, 2602–2605. [Google Scholar] [CrossRef]
- Kim, S.O.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; de Pablo, J.J.; Nealey, P.F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature 2003, 424, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.; Russel, T.P.; Hawker, C.J.; Mays, J.; Cook, D.C.; Satija, S.K. Interfacial Segregation in Disordered Block Copolymers: Effect of Tunable Surface Potentials. Phys. Rev. Lett. 1997, 79, 237. [Google Scholar] [CrossRef]
- Peters, R.D.; Yang, X.M.; Kim, T.K.; Sohn, B.H.; Nealey, P.F. Using Self-Assembled Monolayers Exposed to X-rays To Control the Wetting Behavior of Thin Films of Diblock Copolymers. Langmuir 2000, 16, 4625. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Shin, K.; Hawker, E.D.C.J.; Russell, T.P. A generalized approach to the modification of solid surfaces. Science 2005, 308, 236. [Google Scholar] [CrossRef]
- Kim, S.H.; Misner, M.J.; Russell, T.P. Solvent-Induced Ordering in Thin Film Diblock Copolymer/Homopolymer Mixtures. Adv. Mater. 2004, 16, 2119–2123. [Google Scholar] [CrossRef]
- Sivaniah, E.; Hayashi, Y.; Iino, M.; Hashimoto, T.; Fukunaga, K. Observation of Perpendicular Orientation in Symmetric Diblock Copolymer Thin Films on Rough Substrates. Macromolecules 2003, 36, 5894–5896. [Google Scholar] [CrossRef]
- Yager, K.G.; Berry, B.C.; Page, K.; Patton, D.; Karim, A.; Amis, E.J. Disordered nanoparticle interfaces for directed self-assembly. Soft Matter 2009, 5, 622–628. [Google Scholar] [CrossRef]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.P.; Hawker, C. Controlling Polymer-Surface Interactions with Random Copolymer Brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Suh, H.S.; Kang, H.; Liu, C.-C.; Nealey, P.F.; Char, K. Orientation of Block Copolymer Resists on Interlayer Dielectrics with Tunable Surface Energy. Macromolecules 2010, 43, 461–466. [Google Scholar] [CrossRef]
- Cheng, J.; Lawson, R.A.; Yeh, W.-M.; Tolbert, L.M.; Henderson, C.L. Photodefinable Substrate Guiding Layers for Block Copolymer Directed Self-Assembly (DSA) Patterning. Proc. SPIE 2011, 7972, 79722I. [Google Scholar]
- Han, E.; Leolukman, M.; Kim, M.; Gopalan, P. Resist Free Patterning of Nonpreferential Buffer Layers for Block Copolymer Lithography. ACS Nano 2010, 4, 6527–6534. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Lӧwenhielm, J.B.P.; Spiessberger, C.; Given-Beck, S.A.; Russell, T.P.; Hawker, C.J. Facile Routes to Patterned Surface Neutralization Layers for Block Copolymer Lithography. Adv. Mater. 2007, 19, 4552. [Google Scholar] [CrossRef]
- Han, E.; In, I.; Park, S.-M.; La, Y.-H.; Wang, Y.; Nealey, P.F.; Gopalan, P. Photopatternable Imaging Layers for Controlling Block Copolymer Microdomain Orientation. Adv. Mater. 2007, 19, 4448–4452. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, D.O.; Kim, B.H.; Yoon, D.K.; Kim, K.; Lee, S.Y.; Oh, S.-H.; Choi, S.-W.; Jeon, S.C.; Kim, S.O. Block copolymer multiple patterning integrated with conventional ArF lithography. Soft Matter 2010, 6, 120–125. [Google Scholar] [CrossRef]
- Shin, D.O.; Kim, B.H.; Kang, J.-H.; Jeong, S.-J.; Park, S.H.; Lee, Y.-H.; Kim, S.O. One-Dimensional Nanoassembly of Block Copolymers Tailored by Chemically Patterned Surfaces. Macromolecules 2009, 42, 1189–1193. [Google Scholar] [CrossRef]
- Liu, G.; Thomas, C.S.; Craig, G.S.W.; Nealey, P.F. Integration of Density Multiplication in the Formation of Device-Oriented Structures by Directed Assembly of Block Copolymer–Homopolymer Blends. Adv. Funct. Mater. 2010, 20, 1251–1257. [Google Scholar] [CrossRef]
- Daga, V.K.; Schwarz, E.L.; Chandler, C.M.; Lee, J.-K.; Lin, Y.; Ober, C.K.; Watkins, J.J. Photoinduced Ordering of Block Copolymers. Nano Lett. 2011, 11, 1153–1160. [Google Scholar] [CrossRef]
- Yao, L.; Watkins, J.J. Photoinduced Disorder in Strongly Segregated Block Copolymer Composite Films for Hierarchical Pattern Formation. ACS Nano 2013, 7, 1513–1523. [Google Scholar] [CrossRef]
- Morikawa, Y.; Kondo, T.; Nagano, S.; Seki, T. Photoinduced 3D Ordering and Patterning of Microphase-Separated Nanostructure in Polystyrene-Based Block Copolymer. Chem. Mater. 2007, 19, 1540–1542. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.-Y.; Zhao, W.; Li, L.; Wei, X.; Balazs, A.C.; Matyjaszewski, K.; Russel, T.P. Photocontrol over the Disorder-to-Order Transition in Thin Films of Polystyrene-block-poly(methyl methacrylate) Block Copolymers Containing Photodimerizable Anthracene Functionality. J. Am. Chem. Soc. 2011, 133, 17217–17224. [Google Scholar] [CrossRef] [PubMed]
- Onses, M.S.; Song, C.; Williamson, L.; Suntanto, E.; Ferreira, P.M.; Alleyne, A.G.; Nealey, P.F.; Ahn, H.; Rogers, J.A. Hierarchical patterns of three-dimensional block-copolymer films formed by electrohydrodynamic jet printing and self-assembly. Nat. Nanotechnol. 2013, 8, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.; Kramer, E.J.; Whalheim, S.; Krausch, G. Thin Diblock Copolymer Films on Chemically Heterogeneous Surfaces. Macromolecules 1997, 30, 6610. [Google Scholar] [CrossRef]
- Heier, J.; Genzer, J.; Kramer, E.J.; Bates, F.S.; Whalheim, S.; Krausch, G. Transfer of a chemical substrate pattern into an island-forming diblock copolymer film. J. Chem. Phys. 1999, 111, 11101. [Google Scholar] [CrossRef]
- Heier, J.; Kramer, E.J.; Groenewold, J.; Fredrickson, G.H. Kinetics of Individual Block Copolymer Island Formation and Disappearance near an Absorbing Boundary. Macromolecules 2000, 33, 6060. [Google Scholar] [CrossRef]
- Nisato, G.; Erimi, B.; Douglas, J.F.; Karim, A. Excitation of Surface Deformation Modes of a Phase-Separating Polymer Blend on a Patterned Substrate. Macromolecules 1999, 32, 2356. [Google Scholar] [CrossRef]
- Karim, A.; Douglas, J.F.; Lee, B.P.; Rogers, S.C.G.J.A.; Jackman, R.J.; Amis, E.J.; Whitesides, G.M. Phase separation of ultrathin polymer-blend films on patterned substrates. Phys. Rev. E 1998, 57, R6273. [Google Scholar] [CrossRef]
- Boltau, M.; Walheim, S.; Mlynek, J.; Krausch, G.; Steiner, U. Steiner, Surface-induced structure formation of polymer blends on patterned substrates. Nature 1998, 391, 877. [Google Scholar] [CrossRef]
- Kim, T.K.; Yang, X.M.; Peters, R.D.; Sohn, B.H.; Nealey, P.F. Chemical Modification of Self-Assembled Monolayers by Exposure to Soft X-rays in Air. J. Phys. Chem. B 2000, 104, 7403. [Google Scholar] [CrossRef]
- Ruiz, R.; Kang, H.; Detcheverry, F.A.; Dobisz, E.; Kercher, S.S.; Albrecht, T.R.; de Pablo, J.J.; Nealey, P.F. Density multiplication and improved lithography by directed block copolymer assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Rettner, C.T.; Sanders, D.P.; Kim, H.-C.; Hinsberg, W.D. Dense Self-Assembly on Sparse Chemical Patterns: Rectifying and Multiplying Lithographic Patterns Using Block Copolymers. Adv. Mater. 2008, 20, 3155–3158. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Truong, D.P.S.H.D.; Harrer, S.; Friz, A.; Holmes, S.; Colburn, M. Simple and Versatile Methods To Integrate Directed Self-Assembly with Optical Lithography Using a Polarity-Switched Photoresist. ACS Nano 2010, 4, 4815–4823. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.J.; Rettner, C.T.; Bates, C.M.; Blachut, G.; Carlson, M.C.; Durand, W.J.; Ellison, C.J.; Sanders, D.P.; Cheng, J.Y.; Willson, C.G. Directed Self-Assembly of Silicon-Containing Block Copolymer Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Cushen, J.; Wan, L.; Blachut, G.; Maher, M.J.; Albrecht, T.R.; Ellison, C.J.; Willson, C.G.; Ruiz, R. Double-Patterned Sidewall Directed Self-Assembly and Pattern Transfer of Sub-10 nm PTMSS-b-PMOST. ACS Appl. Mater. Interfaces 2015, 7, 13476–13483. [Google Scholar] [CrossRef]
- Leigh, S.J.; Prieto, J.L.; Bowen, J.; Lewis, S.; Robinson, A.P.G.; Iqbal, P.; Preece, J.A. Controlling gold nanoparticle assembly on electron beam-reduced nitrophenyl self-assembled monolayers via electron dose. Colloids Surf. A 2013, 433, 181–190. [Google Scholar] [CrossRef]
- Mendes, P.M.; Preece, J.A. Precision chemical engineering: Integrating nanolithography and nanoassembly. Curr. Opin. Colloids Surf. Sci. 2004, 9, 236–248. [Google Scholar] [CrossRef]
- Gӧlzhӓuser, A.; Eck, W.; Geyer, W.; Stadler, V.; Weimann, T.; Hinze, P.; Grunze, M. Chemical Nanolithography with Electron Beams. Adv. Mater. 2001, 13, 806–809. [Google Scholar]
- Mendes, P.M.; Jacke, S.; Critchley, K.; Plaza, J.; Chen, Y.; Nikitin, K.; Palmer, R.E.; Preece, J.A.; Evans, S.D.; Fitzmaurice, D. Gold Nanoparticle Patterning of Silicon Wafers Using Chemical e-Beam Lithography. Langmuir 2004, 20, 3766–3768. [Google Scholar] [CrossRef]
- Mendes, P.M.; Belloni, M.; Ashworth, M.; Hardy, C.; Nikitin, K.; Fitzmaurice, D.; Critchley, K.; Evans, S.D.; Preece, J.A. A Novel Example of X-Ray-Radiation-Induced Chemical Reduction of an Aromatic Nitro-Group-Containing Thin Film on SiO2 to an Aromatic Amine Film. Chem. Phys. Chem. 2003, 4, 884–889. [Google Scholar] [CrossRef]
- Eck, W.; Stadler, V.; Geyer, W.; Zharnikov, M.; Gӧlzhӓuser, A.; Grunze, M. Generation of Surface Amino Groups on Aromatic Self-Assembled Monolayers by Low Energy Electron Beams—A First Step Towards Chemical Lithography. Adv. Mater. 2000, 12, 805–808. [Google Scholar] [CrossRef]
- Iqbal, P.; Critchley, K.; Attwood, D.; Tunnicliffe, D.; Evans, S.D.; Preece, J.A. Chemical Manipulation by X-rays of Functionalized Thiolate Self-Assembled Monolayers on Au. Langmuir 2008, 24, 13969. [Google Scholar] [CrossRef] [PubMed]
- Geyer, E.; Stadler, V.; Eck, W.; Zharnikov, M.; Gӧlzhӓuser, A.; Grunze, M. Electron-induced crosslinking of aromatic self-assembled monolayers: Negative resists for nanolithography. Appl. Phys. Lett. 1999, 75, 2401–2403. [Google Scholar] [CrossRef]
- Hyun, J.; Kim, J.; Craig, S.L.; Chilkoti, A. Chilkoti, Enzymatic Nanolithography of a Self-Assembled Oligonucleotide Monolayer on Gold. JACS 2004, 126, 4770–4771. [Google Scholar] [CrossRef] [PubMed]
- Aizaki, N. Proximity effect dependence on substrate material. J. Vac. Sci. Technol. 1979, 16, 1726–1733. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, H.; Dawson, G.; Kozawa, T.; Robinson, A.P.G. Lamellar Orientation of a Block Copolymer via an Electron-Beam Induced Polarity Switch in a Nitrophenyl Self-Assembled Monolayer or Si Etching Treatments. Quantum Beam Sci. 2020, 4, 19. https://doi.org/10.3390/qubs4020019
Yamamoto H, Dawson G, Kozawa T, Robinson APG. Lamellar Orientation of a Block Copolymer via an Electron-Beam Induced Polarity Switch in a Nitrophenyl Self-Assembled Monolayer or Si Etching Treatments. Quantum Beam Science. 2020; 4(2):19. https://doi.org/10.3390/qubs4020019
Chicago/Turabian StyleYamamoto, Hiroki, Guy Dawson, Takahiro Kozawa, and Alex P. G. Robinson. 2020. "Lamellar Orientation of a Block Copolymer via an Electron-Beam Induced Polarity Switch in a Nitrophenyl Self-Assembled Monolayer or Si Etching Treatments" Quantum Beam Science 4, no. 2: 19. https://doi.org/10.3390/qubs4020019
APA StyleYamamoto, H., Dawson, G., Kozawa, T., & Robinson, A. P. G. (2020). Lamellar Orientation of a Block Copolymer via an Electron-Beam Induced Polarity Switch in a Nitrophenyl Self-Assembled Monolayer or Si Etching Treatments. Quantum Beam Science, 4(2), 19. https://doi.org/10.3390/qubs4020019





