Advances and Perspectives in Alkali–Silica Reaction (ASR) Testing: A Critical Review of Reactivity and Mitigation Assessments
Abstract
1. Introduction
2. Aggregate Screening Tests
2.1. Petrographic Analysis
2.2. Chemical Tests
3. ASR Performance Tests
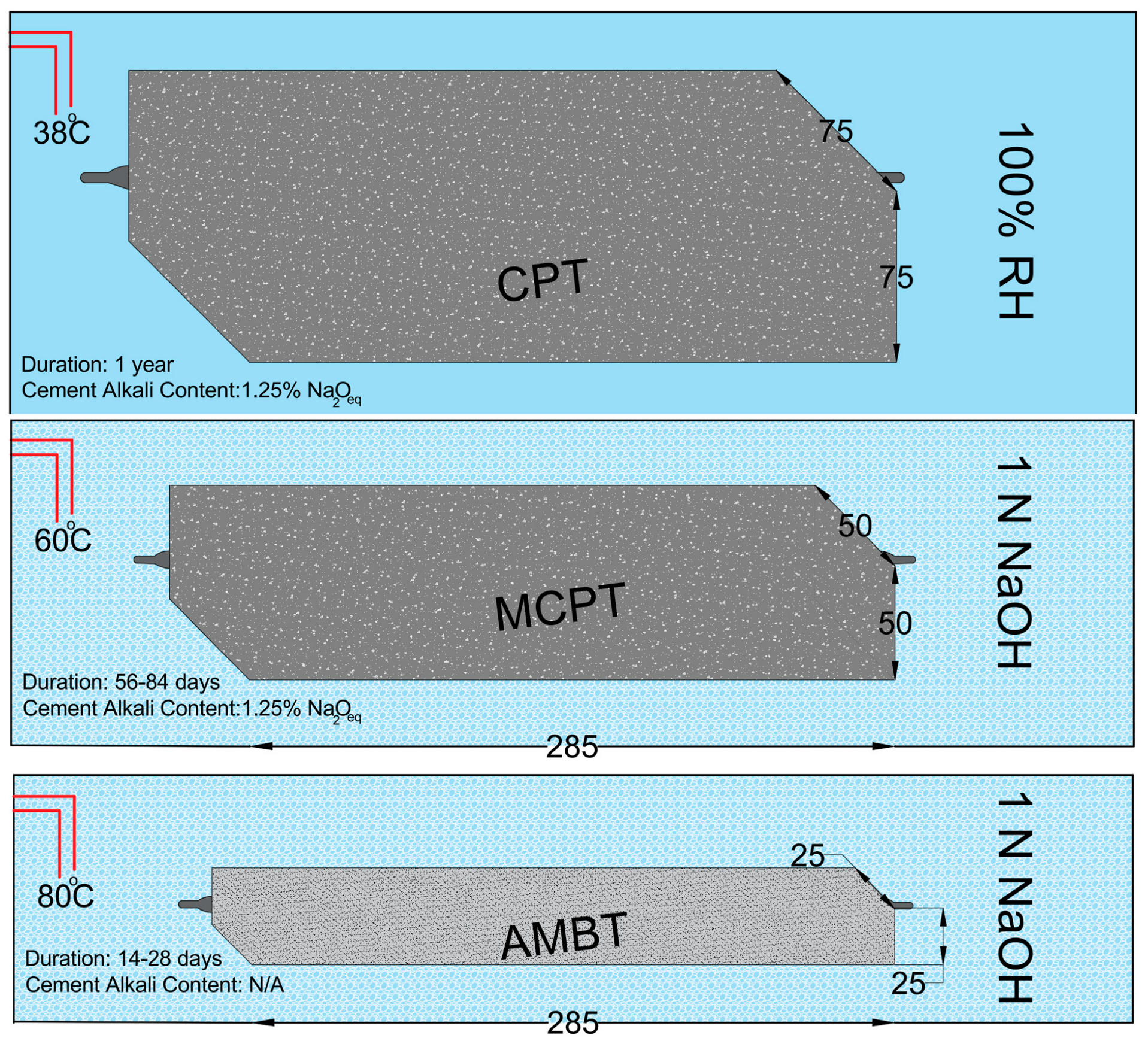
3.1. Concrete Prism Test (CPT)
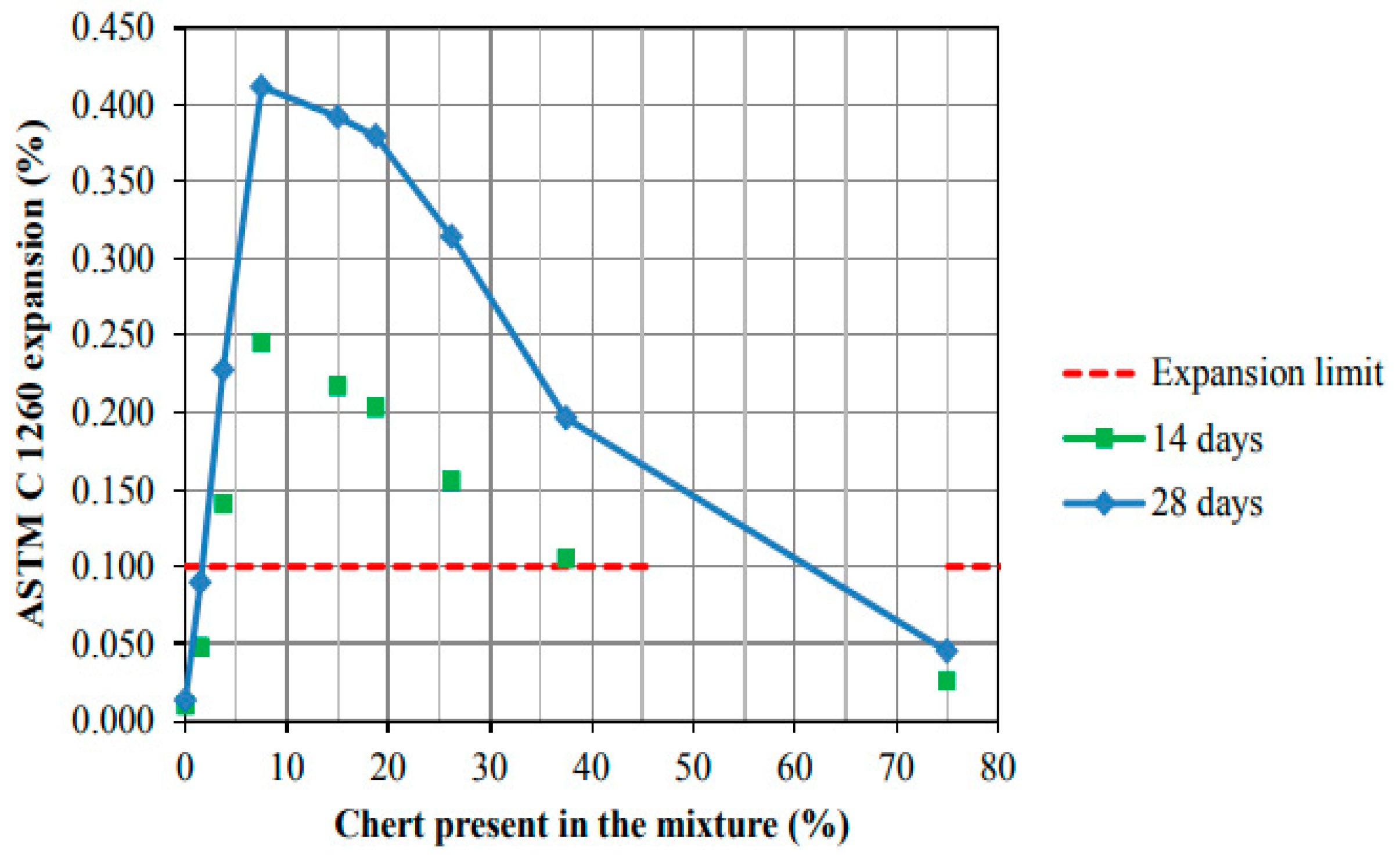
3.2. Accelerated Mortar Bar Test (AMBT)
3.3. Miniature Concrete Prism Test (MCPT)
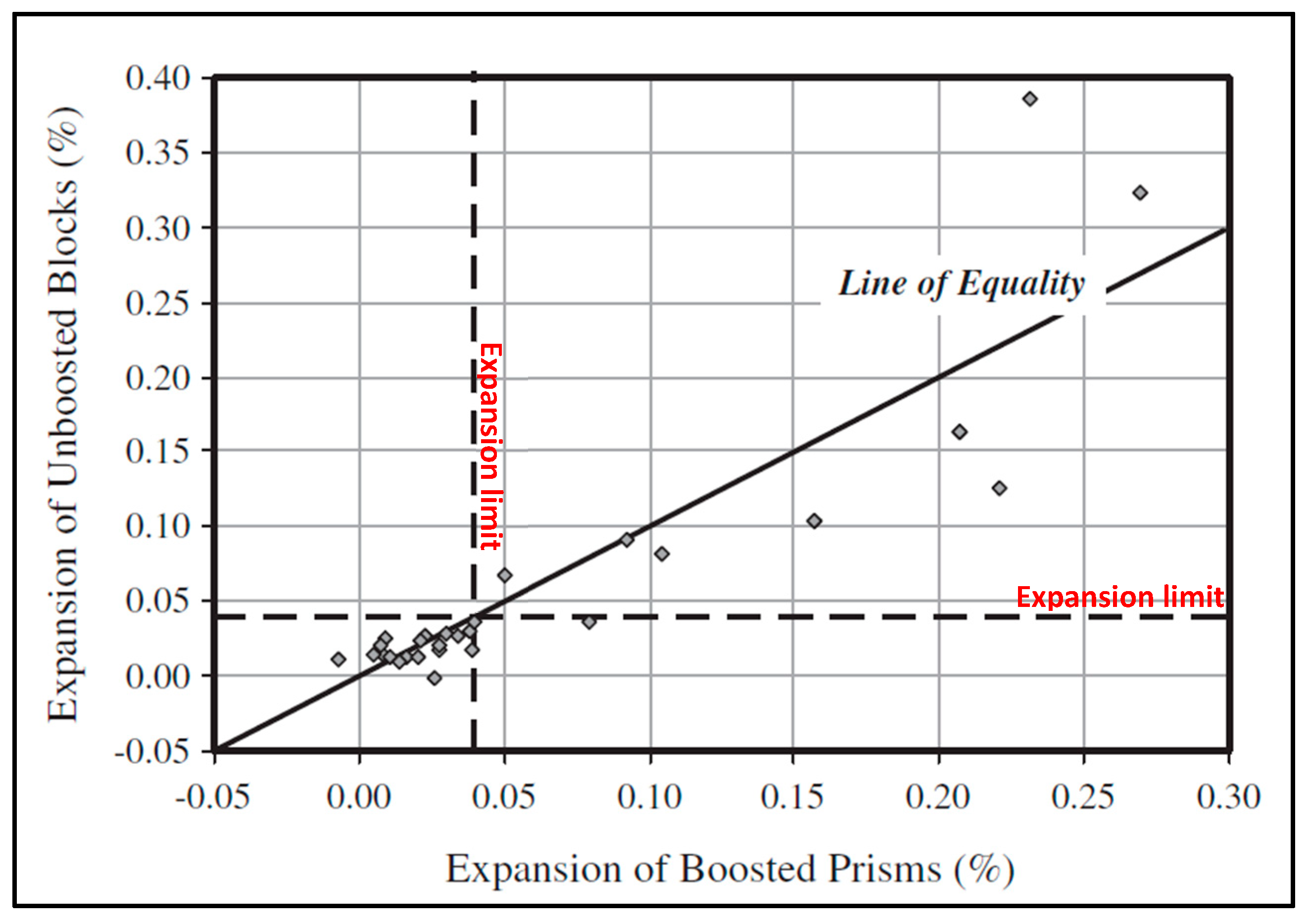
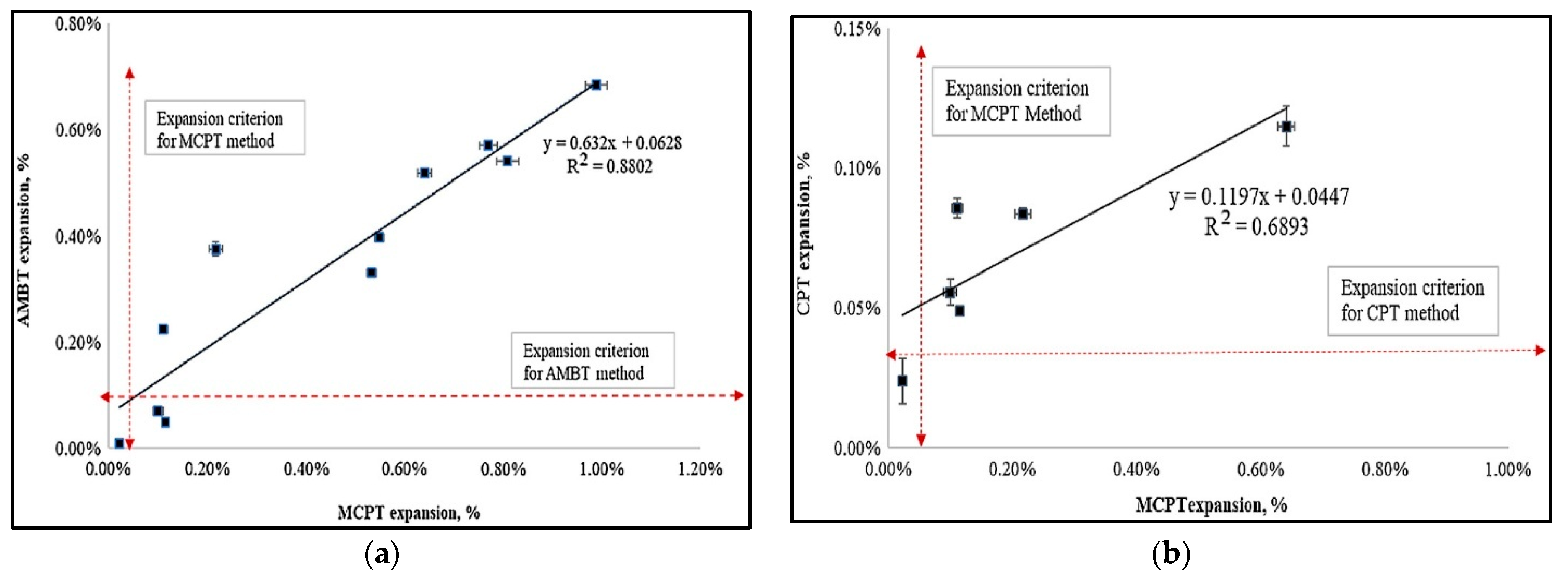
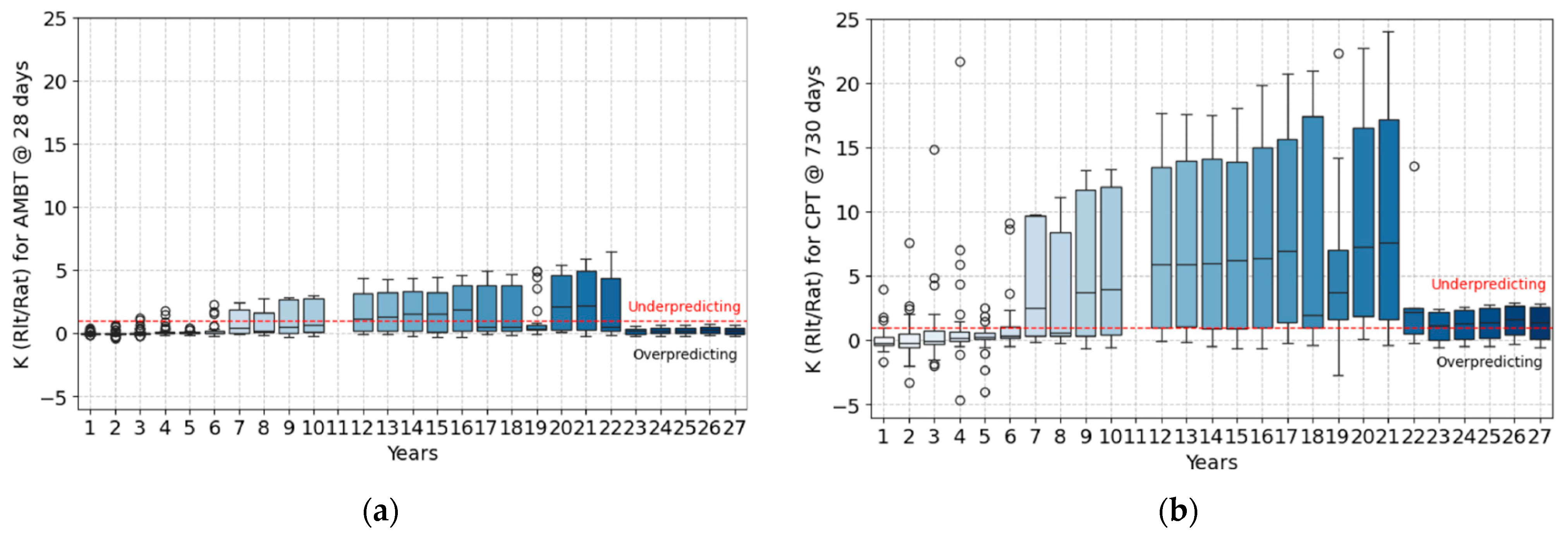
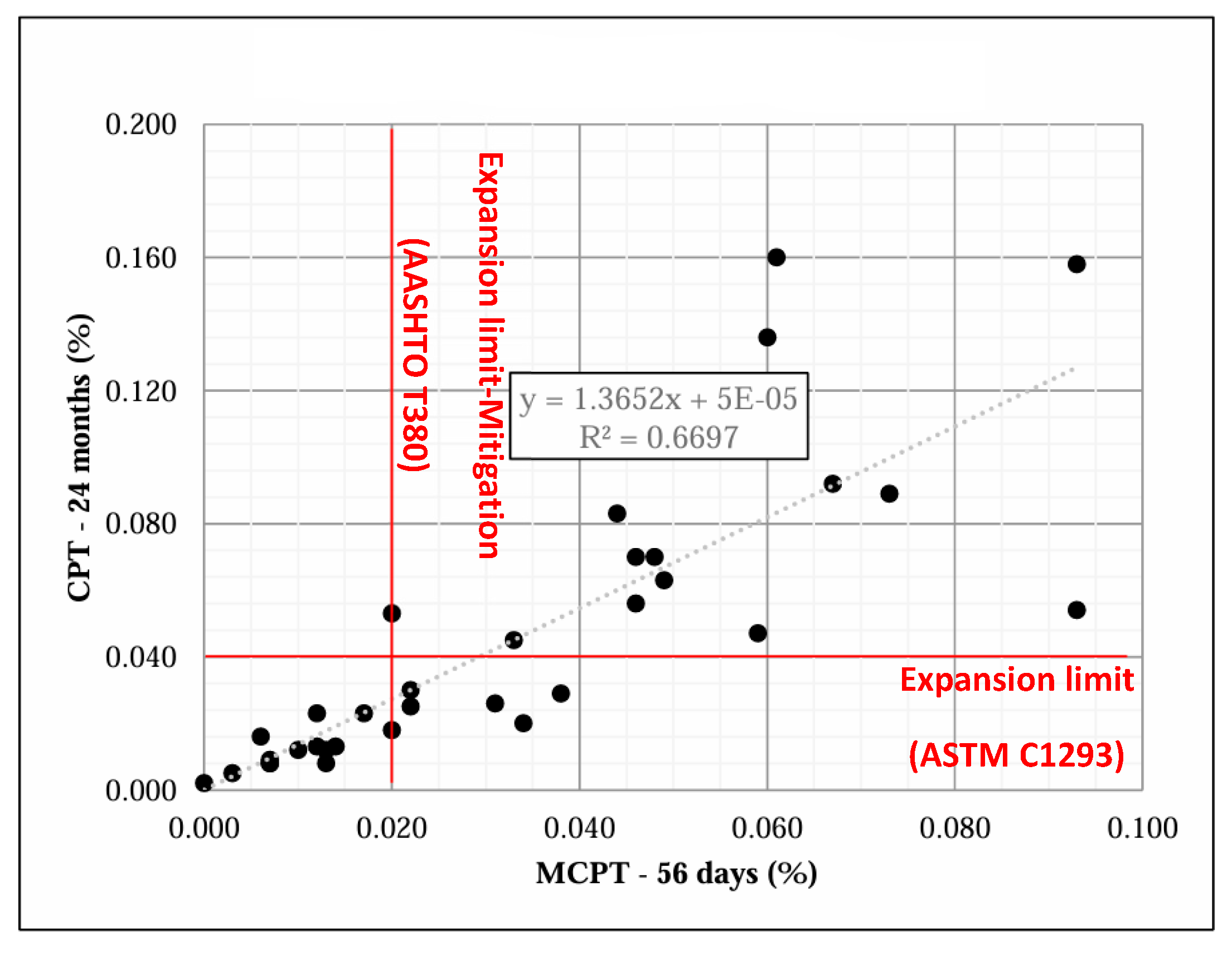
3.4. The Correlation of ASR Performance Tests
3.5. The Correlation of ASR Mitigation Performance Tests
| 14-Day Limit of AMBT | 28-Day Limit of AMBT | Ref. |
|---|---|---|
| AMBT at 14 days can successfully detect reactive aggregates in mixes without SCMs, minimizing false negatives. However, it suffers from false positives. | The 28-day limit can be used for mixes containing SCMs to consider their slow reactivity and provide a more balanced prediction of long-term field performance. | [48] |
| The 14-day limit underestimates the reactivity of moderately reactive aggregates compared to CPT. Therefore, the 28-day limit can be used for aggregates with delayed reactivity. | The 28-day limit correlates better with field exposure block for SCMs mixes and can reduce the underestimation of reactivity. | [47] |
| The 14-day limit of 0.10% correlates well with field performance for SCMs mixes. | The extension to 28 days overestimates the required SCM dosage to mitigate ASR, with 1.5 times higher dosage on average. | [79] |
| Reliable for screening highly reactive aggregates but limited accuracy for slow-reacting aggregates. | The extension to 28 days shows improved classification of slow-reacting aggregates and aligns well with CPT results. | [80] |
| Poor correlation between the 14-day limit and field performance for certain aggregates (e.g., quartzite). | The 28-day limit shows better consistency in capturing slow-reacting aggregate behavior. | [81] |
| The 14-day AMBT limit of 0.06% instead of 0.10% is recommended for minimal false results. | The 28-day limit of 0.13% instead of 0.10% is equivalent to the 14-day 0.06% limit but provides more conservative results. | [82] |
| Several inaccuracies in AMBT at 14 days due to severe test conditions and rapid alkali exposure. | The 28-day limit improves accuracy in capturing delayed expansions, especially with low-reactivity aggregates. | [83] |
| The 14-day limit is typically used for rapid screening of ASR. | The 28-day limit at 0.28% instead of 0.10% provides better accuracy for identifying aggregates with delayed reactivity. | [84] |
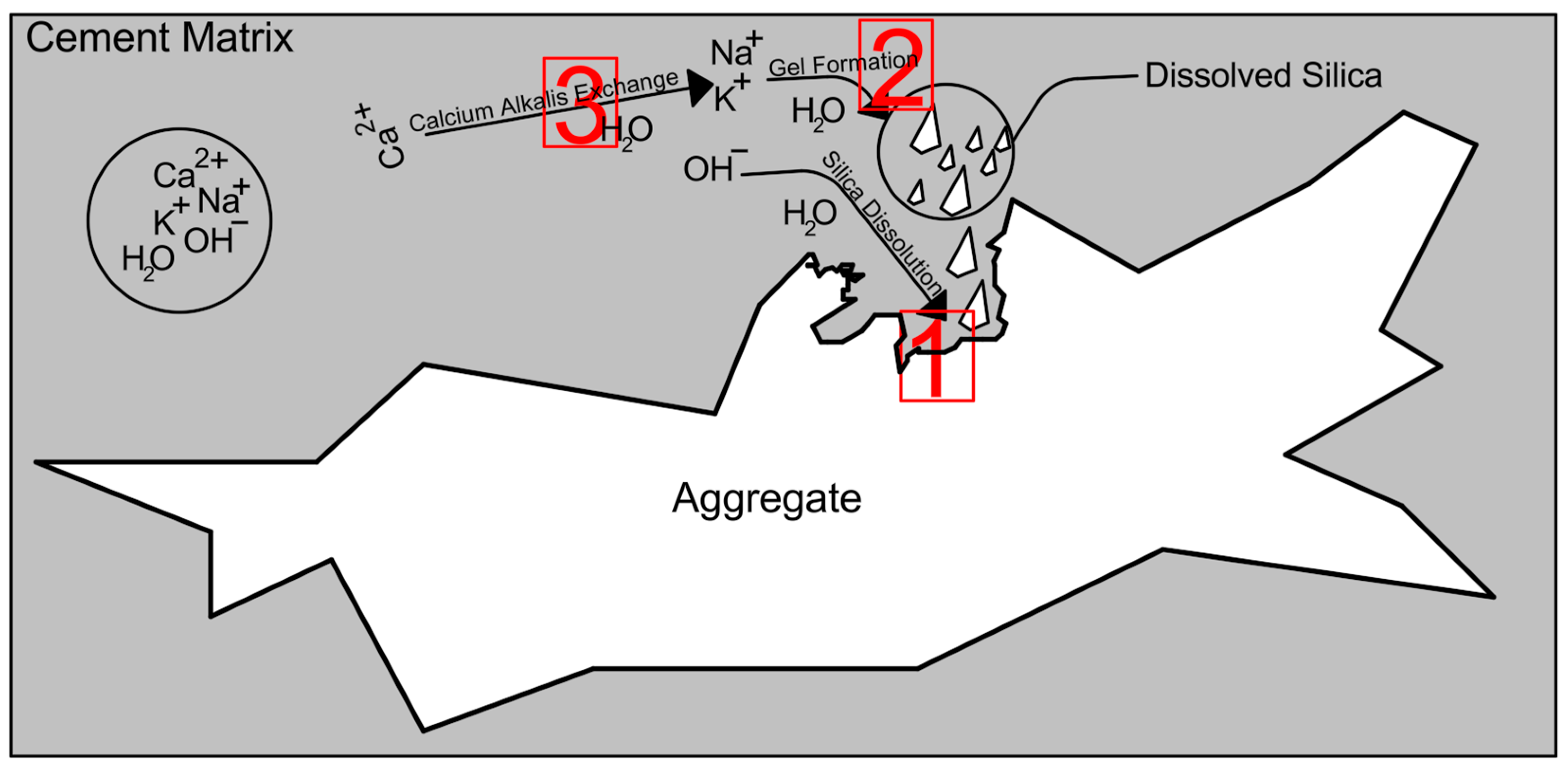
3.6. Indirect Assessments of ASR Mitigation Efficiency
3.6.1. R3 and Modified R3 Tests
3.6.2. Bulk Resistivity
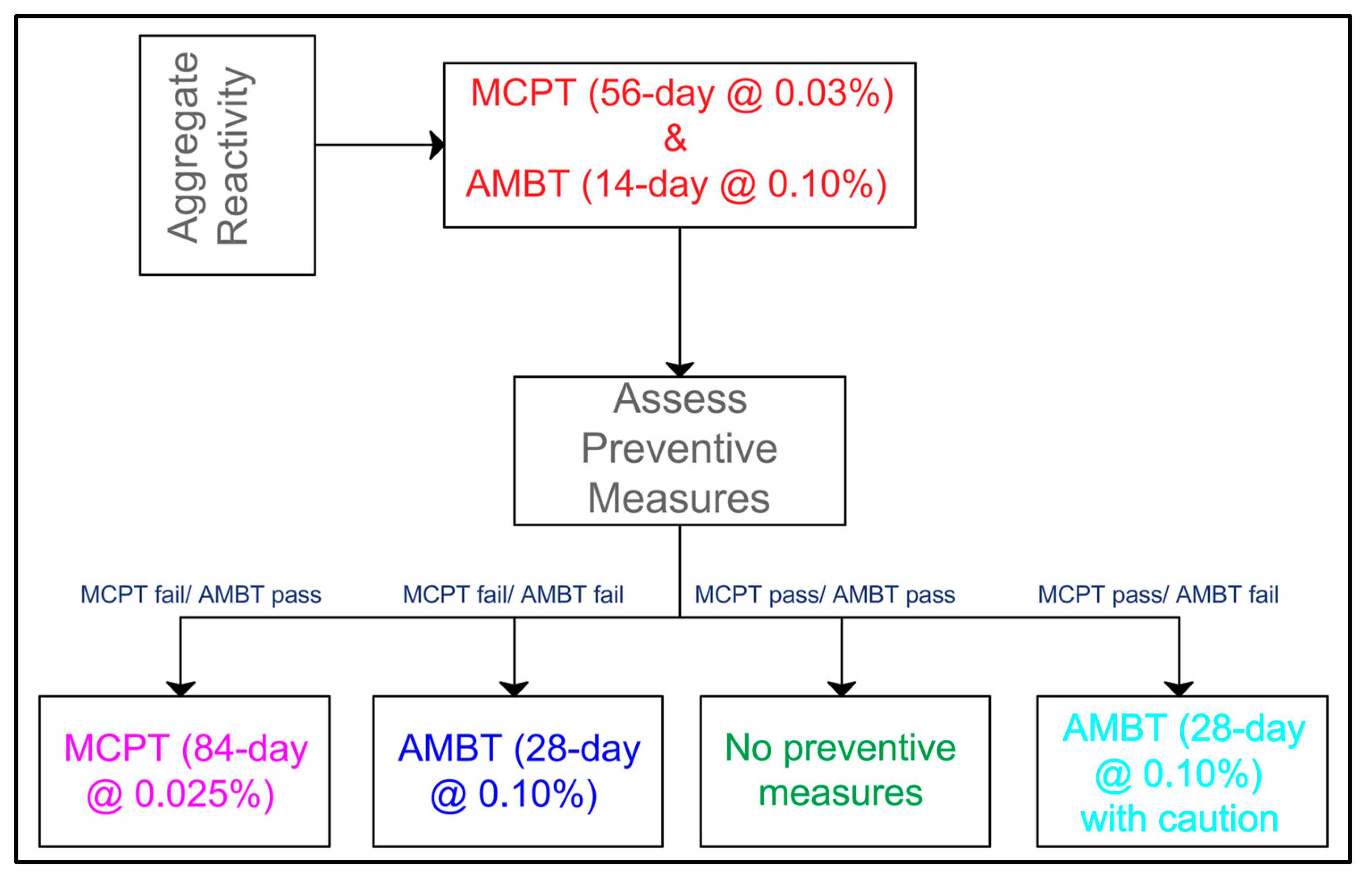
4. Future Perspectives
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ASR | Alkali–silica reaction |
| AMBT | Accelerated Mortar Bar Test |
| CPT | Concrete Prism Test |
| MCPT | Miniature Concrete Prism Test |
| ACPT | Accelerated Concrete Prism Test |
| OPC | Ordinary Portland cement |
| PLC | Portland limestone cement |
| NP | Natural pozzolan |
| SCM | Supplementary cementitious material |
| FA | Fly ash |
| SF | Silica fume |
| S | Slag |
| R3 test | The rapid, relevant, reliable test |
| UR2 | Ultra-rapid reactivity test for real time |
| FRP | Fiber-reinforced-polymers |
| SWSCC | Seawater sea-sand concrete |
References
- Watson, R.; Abbassi, B.; Abu-Hamatteh, Z.S. Life cycle analysis of concrete and asphalt used in road pavements. Environ. Eng. Res. 2020, 25, 52–61. [Google Scholar]
- Li, Z.; Zhou, X.; Ma, H.; Hou, D. Advanced Concrete Technology; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Chiew, S.M.; Ibrahim, I.S.; Ariffin, M.A.M.; Lee, H.-S.; Singh, J.K. Development and properties of light-transmitting concrete (LTC)–A review. J. Clean. Prod. 2021, 284, 124780. [Google Scholar] [CrossRef]
- Snyder, K.A.; Lew, H.S. Alkali-Silica Reaction Degradation of Nuclear Power Plant Concrete Structures: A Scoping Study; Document ID-NISTIR 2013; National Institute of Standards and Technology (NIST), US Department of Commerce: Gaithersburg, MD, USA, 2013; p. 7937. [Google Scholar]
- Martínez, A.; Miller, S.A. A review of drivers for implementing geopolymers in construction: Codes and constructability. Resour. Conserv. Recycl. 2023, 199, 107238. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Zheng, J.; Zhou, J. Study on the Inhibition Effect of Fly Ash on Alkali–Silica Reaction and Its Influence on Building Energy Performance. Buildings 2025, 15, 392. [Google Scholar] [CrossRef]
- Stanton, T.E. Expansion of concrete through reaction between cement and aggregate. Trans. Am. Soc. Civ. Eng. 1942, 107, 54–84. [Google Scholar] [CrossRef]
- Abd-Elssamd, A.; Ma, Z.J. Alkali Silica Reactivity (ASR) Risk Assessment and Mitigation in Tennessee; Tennessee Department of Transportation: Nashville, TN, USA, 2021. [Google Scholar]
- Alsaif, A. Utilization of ceramic waste as partially cement substitute—A review. Constr. Build. Mater. 2021, 300, 124009. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D. Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Olajide, O.D.; Nokken, M.R.; Sanchez, L.F. Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap. Materials 2023, 17, 10. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-silica reaction (ASR) in concrete structures: Mechanisms, effects and evaluation test methods adopted in the United States. Case Stud. Constr. Mater. 2021, 15, e00563. [Google Scholar] [CrossRef]
- Mosaberpanah, M.A.; Umar, S. Utilizing rice husk ash as supplement to cementitious materials on performance of ultra high performance concrete:—A review. Mater. Today Sustain. 2020, 7, 100030. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; El-Sayed, A.A.; Aboraya, A.M.; Fathy, I.N.; Abouelnour, M.A.; Tayeh, B.A.; Nabil, I.M. Investigating the effects of granite, marble, granodiorite, and ceramic waste powders on the physical, mechanical, and radiation shielding performance of sustainable concrete. Ann. Nucl. Energy 2025, 216, 111274. [Google Scholar] [CrossRef]
- Althoey, F.; Ansari, W.S.; Sufian, M.; Deifalla, A.F. Advancements in low-carbon concrete as a construction material for the sustainable built environment. Dev. Built Environ. 2023, 16, 100284. [Google Scholar] [CrossRef]
- ASTM C1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- ASTM C1293; Standard Test Method for Determination of Length Change of Concrete Due to Alkali–Silica Reaction. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM C1567; Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method). Annual Book of ASTM Standards, Volume 04.02, Concrete and Aggregates. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Mindess, S. Developments in the Formulation and Reinforcement of Concrete; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- ASTM C1778; Standard Guide for Reducing the Risk of Deleterious Alkali–Aggregate Reaction in Concrete. Annual Book of ASTM Standards, Volume 04.02. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM C295; Standard Guide for Petrographic Examination of Aggregates for Concrete. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Thomas, M.D.; Fournier, B.; Folliard, K.J. Report on Determining the Reactivity of Concrete Aggregates and Selecting Appropriate Measures for Preventing Deleterious Expansion in New Concrete Construction; Federal Highway Administration: Washington, DC, USA, 2008. [Google Scholar]
- Antolik, A.; Dąbrowski, M.; Jóźwiak-Niedźwiedzka, D. Petrographic Evaluation of Aggregate from Igneous Rocks: Alkali–Silica Reaction Potential. Minerals 2023, 13, 1004. [Google Scholar] [CrossRef]
- Federal Highway Administration. Alkali-Aggregate Reactivity (AAR) Workshops for Engineers and Practitioners Reference Manual; Federal Highway Administration: Washington, DC, USA, 2013. [Google Scholar]
- Jozwiak-Niedzwiedzka, D.; Jaskulski, R.; Glinicki, M.A. Application of Image Analysis to Identify Quartz Grains in Heavy Aggregates Susceptible to ASR in Radiation Shielding Concrete. Materials 2016, 9, 224. [Google Scholar] [CrossRef]
- Mishra, D.; Emad, K.; Shahjalal, C.; Ebenezer, F. Implementing AASHTO TP 110 for Alkali-Silica Reaction Potential Evaluation of Idaho Aggregates; Idaho Transportation Department: Boise, ID, USA, 2020. [Google Scholar]
- ASTM C289; Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method). ASTM International: West Conshohocken, PA, USA, 2007. [CrossRef]
- Munir, M.J.; Abbas, S.; Qazi, A.U.; Nehdi, M.L.; Kazmi, S.M.S. Role of test method in detection of alkali–silica reactivity of concrete aggregates. Proc. Inst. Civ. Eng. Constr. Mater. 2018, 171, 203–221. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Balachandran, C.; Arnold, T.S. New chemical reactivity index to assess alkali–silica reactivity. J. Mater. Civ. Eng. 2021, 33, 04021037. [Google Scholar] [CrossRef]
- Kawabata, Y.; Dunant, C.; Yamada, K.; Scrivener, K. Impact of temperature on expansive behavior of concrete with a highly reactive andesite due to the alkali–silica reaction. Cem. Concr. Res. 2019, 125, 105888. [Google Scholar] [CrossRef]
- Rao, B.; Dai, H.; Gao, L.; Xie, H.; Gao, G.; Peng, K.; Zhang, M.; He, F.; Pan, Y. Surprisingly highly reactive silica that dissolves rapidly in dilute alkali (NaOH) solution even at ambient temperatures (25 °C). J. Clean. Prod. 2022, 341, 130779. [Google Scholar] [CrossRef]
- Thomas, M.D.; Fournier, B.; Folliard, K.J.; Ideker, J.H.; Resendez, Y. The Use of Lithium to Prevent or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures; Turner-Fairbank Highway Research Center: McLean, VA, USA, 2007. [Google Scholar]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between mechanical properties of concrete and alkali-silica reaction (ASR); a review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Pan, J.; Feng, Y.T.; Wang, J.T.; Sun, Q.C.; Zhang, C.H.; Owen, D.R.J. Modeling of alkali-silica reaction in concrete: A review. Front. Struct. Civ. Eng. 2012, 6, 1–18. [Google Scholar] [CrossRef]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of calcium on dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef]
- Doğruyol, M. Determination of ASR in concrete using characterization methods. Buildings 2024, 14, 657. [Google Scholar] [CrossRef]
- Vayghan, A.G.; Rajabipour, F.F.; Arndt, C. The influence of ASR gels composition on their swelling properties. In Proceedings of the 15th International Conference on Alkali-Aggregate Reaction, Sao Paulo, Brazil, 3–7 July 2016. [Google Scholar]
- Lei, J.; Law, W.W.; Yang, E.-H. Effect of calcium hydroxide on the alkali-silica reaction of alkali-activated slag mortars activated by sodium hydroxide. Constr. Build. Mater. 2021, 272, 121868. [Google Scholar] [CrossRef]
- Lindgård, J.; Andiç-Çakır, Ö.; Fernandes, I.; Rønning, T.F.; Thomas, M.D.A. Alkali–silica reactions (ASR): Literature review on parameters influencing laboratory performance testing. Cem. Concr. Res. 2012, 42, 223–243. [Google Scholar] [CrossRef]
- Cao, J.; Gowripalan, N.; Sirivivatnanon, V.; South, W. Accelerated test for assessing the potential risk of alkali-silica reaction in concrete using an autoclave. Constr. Build. Mater. 2021, 271, 121871. [Google Scholar] [CrossRef]
- Wu, W.; Kang, S.; Gong, Q.; Yao, H.; Zhang, K.; Yang, H.; Li, H.; Wang, D. Influences of aggregate gradation on alkali-silica reaction of seawater and sea sand concrete. Constr. Build. Mater. 2024, 427, 136268. [Google Scholar] [CrossRef]
- ASTM C157; Standard Test Method for Length Change of Hardened Hydraulic Cement Mortar and Concrete. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- De Souza, D.J.; Sanchez, L.F. Evaluating the efficiency of SCMs to avoid or mitigate ASR-induced expansion and deterioration through a multi-level assessment. Cem. Concr. Res. 2023, 173, 107262. [Google Scholar] [CrossRef]
- Thomas, M.; Fournier, B.; Folliard, K.; Ideker, J.; Shehata, M. Test methods for evaluating preventive measures for controlling expansion due to alkali–silica reaction in concrete. Cem. Concr. Res. 2006, 36, 1842–1856. [Google Scholar] [CrossRef]
- Ideker, J.H.; East, B.L.; Folliard, K.J.; Thomas, M.D.; Fournier, B. The current state of the accelerated concrete prism test. Cem. Concr. Res. 2010, 40, 550–555. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T. Alkali-silica reaction (ASR) in the alkali-activated cement (AAC) system: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119105. [Google Scholar] [CrossRef]
- Drimalas, T.; Folliard, K.J.; Parashar, A.; Thomas, M.D.A.; Ghanizadeh, A.; Hossack, A.M.; Fournier, B. Alkali-Silica Reactivity Potential and Mitigation: Test Methods and State of Practice; The National Academies Press: Washington, DC, USA, 2023. [Google Scholar]
- Bergmann, A.; Sanchez, L.F. Assessing the reliability of laboratory test procedures for predicting concrete field performance against alkali-aggregate reaction (AAR). CEMENT 2025, 19, 100133. [Google Scholar] [CrossRef]
- Golmakani, F.; Hooton, R.D. Impact of pore solution concentration on the accelerated mortar bar alkali-silica reactivity test. Cem. Concr. Res. 2019, 121, 72–80. [Google Scholar] [CrossRef]
- AASHTO T303; Standard Method of Test for Accelerated Detection of Potentially Deleterious Expansion of Mortar Bars Due to Alkali-Silica Reaction. American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2022.
- Drimalas, T.; Ideker, J.; Arrietta, G.; Folliard, K.J.; Fournier, B.; Thomas, M. Evaluating combination of aggregates in the accelerated mortar bar test. In Proceedings of the 14th International Conference on Alkali-Aggregate Reactivity in Concrete, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Mukhopadhyay, A.K.; Liu, K.-W. ASR Testing: A New Approach to Aggregate Classification and Mix Design Verification: Technical Report; Department of Transportation Research and Technology Implementation Office: Austin, TX, USA, 2014. [Google Scholar]
- Latifee, E.R.; Rangaraju, P.R. Miniature concrete prism test: Rapid test method for evaluating alkali-silica reactivity of aggregates. J. Mater. Civ. Eng. 2015, 27, 04014215. [Google Scholar] [CrossRef]
- AASHTO T380; Standard Method of Test for Potential Alkali Reactivity of Aggregates and Effectiveness of ASR Mitigation Measures (Miniature Concrete Prism Test, MCPT). American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2012.
- Konduru, H.; Rangaraju, P.; Amer, O. Reliability of miniature concrete prism test in assessing alkali-silica reactivity of moderately reactive aggregates. Transp. Res. Rec. 2020, 2674, 23–29. [Google Scholar] [CrossRef]
- Tanesi, J.; Drimalas, T.; Chopperla, K.S.T.; Beyene, M.; Ideker, J.H.; Kim, H.; Montanari, L.; Ardani, A. Divergence between performance in the field and laboratory test results for alkali-silica reaction. Transp. Res. Rec. 2020, 2674, 120–134. [Google Scholar] [CrossRef]
- Ranger, M.; Hasholt, M.T.; Lindgård, J.; Barbosa, R.A. Laboratory and field investigations of alkali-silica reaction prevention by supplementary cementitious materials: Influence of the free alkali loading. Constr. Build. Mater. 2024, 442, 137599. [Google Scholar] [CrossRef]
- Ideker, J.H.; Bentivegna, A.F.; Folliard, K.J.; Juenger, M.C.G. Do current laboratory test methods accurately predict alkali-silica reactivity? ACI Mater. J. 2012, 109, 395–402. [Google Scholar]
- Nguyen, T.N.; Sanchez, L.F.; Li, J.; Fournier, B.; Sirivivatnanon, V. Correlating alkali-silica reaction (ASR) induced expansion from short-term laboratory testings to long-term field performance: A semi-empirical model. Cem. Concr. Compos. 2022, 134, 104817. [Google Scholar] [CrossRef]
- Lindgård, J.; Thomas, M.D.A.; Sellevold, E.J.; Pedersen, B.; Andiç-Çakır, Ö.; Justnes, H.; Rønning, T.F. Alkali–silica reaction (ASR)—Performance testing: Influence of specimen pre-treatment, exposure conditions and prism size on alkali leaching and prism expansion. Cem. Concr. Res. 2013, 53, 68–90. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.; Lindgård, J.; De Weerdt, K. Determining the free alkali metal content in concrete–Case study of an ASR-affected dam. Cem. Concr. Res. 2018, 105, 111–125. [Google Scholar] [CrossRef]
- Lindgård, J.; Østnor, T.; Fournier, B.; Lindgård, Ø.; Danner, T.; Plusquellec, G.; De Weerdt, K. Determining alkali leaching during accelerated ASR performance testing and in field exposed cubes using cold water extraction (CWE) and μXRF. MATEC Web Conf. 2018, 199, 03004. [Google Scholar] [CrossRef]
- Rangaraju, P.; Afshinnia, K.; Enugula, S.S.R.; Latifee, E.R. Evaluation of alkali-silica reaction potential of marginal aggregates using miniature concrete prism test (MCPT). In Proceedings of the 15th International Conference on Alkali-Reaction in Concrete, Sao Paulo, Brazil, 3–7 July 2016. [Google Scholar]
- Fanijo, E.O.; Chowdhury, S.; Kassem, E.; Mishra, D. Evaluating Alkali-Silica Reactivity of Aggregates Using the New Miniature Concrete Prism Test and Other Standard Test Methods. J. Mater. Civ. Eng. 2022, 34, 04022163. [Google Scholar] [CrossRef]
- Kasaniya, M.; Thomas, M.D. Role of the alkalis of supplementary cementing materials in controlling pore solution chemistry and alkali-silica reaction. Cem. Concr. Res. 2022, 162, 107007. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D. The role of alkali content of Portland cement on the expansion of concrete prisms containing reactive aggregates and supplementary cementing materials. Cem. Concr. Res. 2010, 40, 569–574. [Google Scholar] [CrossRef]
- Wei, S.; Zheng, K.; Zhou, J.; Prateek, G.; Yuan, Q. The combined effect of alkalis and aluminum in pore solution on alkali-silica reaction. Cem. Concr. Res. 2022, 154, 106723. [Google Scholar] [CrossRef]
- Kian, A. Alkali-Silica Reactivity of Mortars Containing Natural Pozzolan; University of Nevada: Las Vegas, NV, USA, 2019. [Google Scholar]
- Shehata, M.H.; Thomas, M.D. Alkali release characteristics of blended cements. Cem. Concr. Res. 2006, 36, 1166–1175. [Google Scholar] [CrossRef]
- Al-Jabari, M.; Al-Rashed, R.; Ayers, M.E. Mitigation of alkali silica reactions in concrete using multi-crystalline intermixed waterproofing materials. Cement 2023, 12, 100065. [Google Scholar] [CrossRef]
- Tapas, M.J.; Sofia, L.; Vessalas, K.; Thomas, P.; Sirivivatnanon, V.; Scrivener, K. Efficacy of SCMs to mitigate ASR in systems with higher alkali contents assessed by pore solution method. Cem. Concr. Res. 2021, 142, 106353. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.; Sarker, P.K.; Shaikh, F.A.; Pramanik, A. The ASR mechanism of reactive aggregates in concrete and its mitigation by fly ash: A critical review. Constr. Build. Mater. 2018, 171, 743–758. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Math, S.; Wingard, D.; Rangaraju, P.R. Assessing potential reactivity of aggregates in presence of potassium acetate deicer: Revised mortar bar test method. Transp. Res. Rec. 2011, 2232, 10–24. [Google Scholar] [CrossRef]
- Thomas, M.D.; Fournier, B.; Folliard, K.J. Alkali-Aggregate Reactivity (AAR) Facts Book; Federal Highway Administration Office of Pavement Technology: Washington, DC, USA, 2013. [Google Scholar]
- Alaejos, P.; Lanza, V.; Bermúdez, M.; Velasco, A. Effectiveness of the accelerated mortar bar test to detect rapid reactive aggregates (including their pessimum content) and slowly reactive aggregates. Cem. Concr. Res. 2014, 58, 13–19. [Google Scholar] [CrossRef]
- CRD C662; Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials, Lithium Nitrate Admixture and Aggregate (Accelerated Mortar-Bar Method). U.S. Army Corps of Engineers: Washington, DC, USA, 2010.
- Coutinho, Y.; Montefalco, L.; Carneiro, A. Influence of aggregate crushing on the results of accelerated alkali-silica reactivity tests. Constr. Build. Mater. 2022, 325, 126737. [Google Scholar] [CrossRef]
- Thomas, M.D.; Fournier, B.; Folliard, K.J.; Shehata, M.H.; Ideker, J.H.; Rogers, C. Performance limits for evaluating supplementary cementing materials using accelerated mortar bar test. ACI Mater. J. 2007, 104, 115. [Google Scholar]
- Sirivivatnanon, V.; Thomas, P.; Tapas, M.J.; Nguyen, T.N. Reliability of AMBT and CPT in testing the effectiveness of SCM to mitigate alkali–silica reaction of field concrete. Constr. Build. Mater. 2023, 369, 130510. [Google Scholar] [CrossRef]
- Lu, D.; Fournier, B.; Grattan-Bellew, P. Evaluation of accelerated test methods for determining alkali-silica reactivity of concrete aggregates. Cem. Concr. Compos. 2006, 28, 546–554. [Google Scholar] [CrossRef]
- Lenke, L.R.; Malvar, L.J. Alkali silica reaction criteria for accelerated mortar bar tests based on field performance data. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Joshua Tapas, M.; Thomas, P.; Vessalas, K.; Sirivivatnanon, V. Mechanisms of alkali-silica reaction mitigation in AMBT conditions: Comparative study of traditional supplementary cementitious materials. J. Mater. Civ. Eng. 2022, 34, 04021460. [Google Scholar] [CrossRef]
- Islam, M.S. Comparison of ASR mitigation methodologies. Int. J. Concr. Struct. Mater. 2014, 8, 315–326. [Google Scholar] [CrossRef]
- Arrieta Martinez, G. Experimental Studies of the Behavior of Pessimum Aggregates in Different Test Procedures Used to Evaluate the Alkali Reactivity of Aggregates in Concrete. Master’s Thesis, The University of Texas at Austin, Austin, TX, USA, 2012. Available online: http://hdl.handle.net/2152/ETD-UT-2012-05-5463 (accessed on 19 May 2025).
- Fournier, B.; Rogers, C.; MacDonald, C.-A. Multilaboratory study of the concrete prism and accelerated mortar bar expansion tests with Spratt aggregate. In Proceedings of the 14th International Conference on Alkali Aggregate Reaction, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- AS 1141.60.1; Methods for Sampling and Testing Aggregates Method 60.1: Potential Alkali-Silica Reactivity-Accelerated Mortar Bar Method. Standards Australia Limited: Sydney, Australia, 2014.
- Bavasso, I.; Costa, U.; Mangialardi, T.; Paolini, A.E. Assessment of alkali–silica reactivity of aggregates by concrete expansion tests in alkaline solutions at 38 °C. Materials 2020, 13, 288. [Google Scholar] [CrossRef]
- des Laboratoires d’Essais, R.I. RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures: State-of-the-Art Report of the RILEM Technical Committee 219-ACS; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Golmakani, F.; Hooton, R. Comparison of laboratory performance tests used to assess alkali-silica reactivity. In Resilient Infrastructure; Curran Associates Inc.: Red Hook, NY, USA, 2016; pp. 1167–1173. [Google Scholar]
- Gautam, B.P.; Panesar, D.K. The effect of elevated conditioning temperature on the ASR expansion, cracking and properties of reactive Spratt aggregate concrete. Constr. Build. Mater. 2017, 140, 310–320. [Google Scholar] [CrossRef]
- Folliard, K.J.; Ideker, J.H.; Thomas, M.D.A.; Fournier, B. Assessing aggregate reactivity using the accelerated concrete prism tests. In Aggregates: Asphalt Concrete, Portland Cement Concrete, Bases, and Fines; Twelfth Annual Symposium International Center for Aggregates Research (ICAR); University of Texas at Austin; Texas A&M University System; Aggregates Foundation for Technology, Research & Education (AFTRE); National Stone, Sand & Gravel Association (NSSGA); Florida Rock Industries: Austin, TX, USA, 2004. [Google Scholar]
- Liu, Z.; Milla, J.; Saunders, W. Evaluation of the Miniature Concrete Prism Test (MCPT) for Use in LADOTD; Final Report No. FHWA/LA.24/700; Louisiana Transportation Research Center (LTRC): Baton Rouge, LA, USA, 2024. [Google Scholar]
- AASHTO R80; Determining the Reactivity of Concrete Aggregates and Selecting Appropriate Measures for Preventing Deleterious Expansion in New Concrete Construction. American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2017.
- Ideker, J.H.; Drimalas, T.; Folliard, K.J.; Ghanizadeh, A.; Parashar, A.; Chopperla, K.S.T.; Snyder, A.; Thomas, M.D.A. Preventing Alkali-Silica Reaction in Concrete. CE/Papers 2023, 6, 1101–1109. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Influence of alkali silica reaction on the chemistry of pore solutions in mortars with and without lithium ions. In Brittle Matrix Composites 10; Elsevier: Amsterdam, The Netherlands, 2012; pp. 11–20. [Google Scholar]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Ben Haha, M.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; De Weerdt, K.; Dhandapani, Y.; et al. Reactivity tests for supplementary cementitious materials: RILEM TC 267-TRM phase 1. Mater. Struct. 2018, 51, 151. [Google Scholar] [CrossRef]
- ASTM C1897; Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water Measurements. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Ramanathan, S.; Croly, M.; Suraneni, P. Comparison of the effects that supplementary cementitious materials replacement levels have on cementitious paste properties. Cem. Concr. Compos. 2020, 112, 103678. [Google Scholar] [CrossRef]
- Suraneni, P. Recent developments in reactivity testing of supplementary cementitious materials. RILEM Tech. Lett. 2021, 6, 131–139. [Google Scholar] [CrossRef]
- Sivakumar, P.P.; Matthys, S.; De Belie, N.; Gruyaert, E. Reactivity assessment of modified ferro silicate slag by R3 method. Appl. Sci. 2021, 11, 366. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Al-Shmaisani, S. Assessing Supplementary Cementitious Materials for Use in Concrete: Long-Term and Rapid Testing. Ph.D. Dissertation, The University of Texas at Austin, Austin, TX, USA, 2020. [Google Scholar] [CrossRef]
- Suraneni, P.; Hajibabaee, A.; Ramanathan, S.; Wang, Y.; Weiss, J. New insights from reactivity testing of supplementary cementitious materials. Cem. Concr. Compos. 2019, 103, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Ramanathan, S.; Chopperla, K.S.T.; Ideker, J.H.; Suraneni, P. Estimation of non-traditional supplementary cementitious materials potential to prevent alkali-silica reaction using pozzolanic reactivity and bulk resistivity. Cem. Concr. Compos. 2022, 133, 104723. [Google Scholar] [CrossRef]
- Min, Y.; Kabir, H.; Kothari, C.; Iqbal, M.F.; Garg, N. UR2: Ultra-rapid reactivity test for real-time, low-cost quality control of calcined clays. Cem. Concr. Res. 2025, 191, 107806. [Google Scholar] [CrossRef]
- Araújo, L.B.; Targino, D.L.L.; Babadopulos, L.F.A.L.; Fabbri, A.; Cabral, A.E.B.; Chehade, R.; Costa, H.N. Impact of Curing Temperature and Steel Slag Aggregates on High-Strength Self-Compacting Alkali-Activated Concrete. Buildings 2025, 15, 457. [Google Scholar] [CrossRef]
- Ramezani, A.M.; Khajehdezfuly, A.; Poorveis, D. Structural Lightweight Concrete Containing Basalt Stone Powder. Buildings 2024, 14, 1904. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Electrical resistivity of concrete for durability evaluation: A review. Adv. Mater. Sci. Eng. 2017, 2017, 8453095. [Google Scholar] [CrossRef]
- Chopperla, K.S.T.; Drimalas, T.; Beyene, M.; Tanesi, J.; Folliard, K.; Ardani, A.; Ideker, J.H. Combining reliable performance testing and binder properties to determine preventive measures for alkali-silica reaction. Cem. Concr. Res. 2022, 151, 106641. [Google Scholar] [CrossRef]
- Chopperla, K.S.T.; Ideker, J.H. Using electrical resistivity to determine the efficiency of supplementary cementitious materials to prevent alkali-silica reaction in concrete. Cem. Concr. Compos. 2022, 125, 104282. [Google Scholar] [CrossRef]
- Ghiasvand, E.; Mohammadi, H.; Rezaei, Z.; Ayyoubi, M.; Dehghani, S. Evaluation of the durability of concretes containing alkali-activated slag exposed to the alkali-silica reaction by measuring electrical resistivity. Constr. Build. Mater. 2023, 367, 130094. [Google Scholar] [CrossRef]
- Khajehnouri, Y.; Rivard, P.; Chouteau, M.; Bérubé, C.L. Validation of complex electrical properties of concrete affected by accelerated alkali-silica reaction. Cem. Concr. Compos. 2020, 113, 103660. [Google Scholar] [CrossRef]
- Chopperla, K.S.T.; Smith, J.A.; Ideker, J.H. The efficacy of portland-limestone cements with supplementary cementitious materials to prevent alkali-silica reaction. Cement 2022, 8, 100031. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Kim, T.; Castel, A. Mitigation of alkali-silica reaction by limestone calcined clay cement (LC3). Cem. Concr. Res. 2020, 137, 106176. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A. Cement Replacement Materials; Springer Geochemistry/Mineralogy; Springer: Berlin/Heidelberg, Germany, 2014; Volume 10, pp. 978–983. [Google Scholar]
- Tagnit-Hamou, A.; Petrov, N.; Luke, K. Properties of concrete containing diatomaceous earth. ACI Mater. J. 2003, 100, 73–78. [Google Scholar]
- Wallace, A.R.; Frank, D.G.; Founie, A. Freshwater Diatomite Deposits in the Western United States; U.S. Geological Survey Fact Sheet 2006–3044, Version 1.0; U.S. Geological Survey: Reston, VA, USA, 2006. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhao, Q.; Chang, J.; Ding, S.; Liu, X.; Poon, C.S. Long-term investigation of alkali-silica reaction behaviors in seawater sea-sand concrete. Cem. Concr. Compos. 2024, 151, 105611. [Google Scholar] [CrossRef]





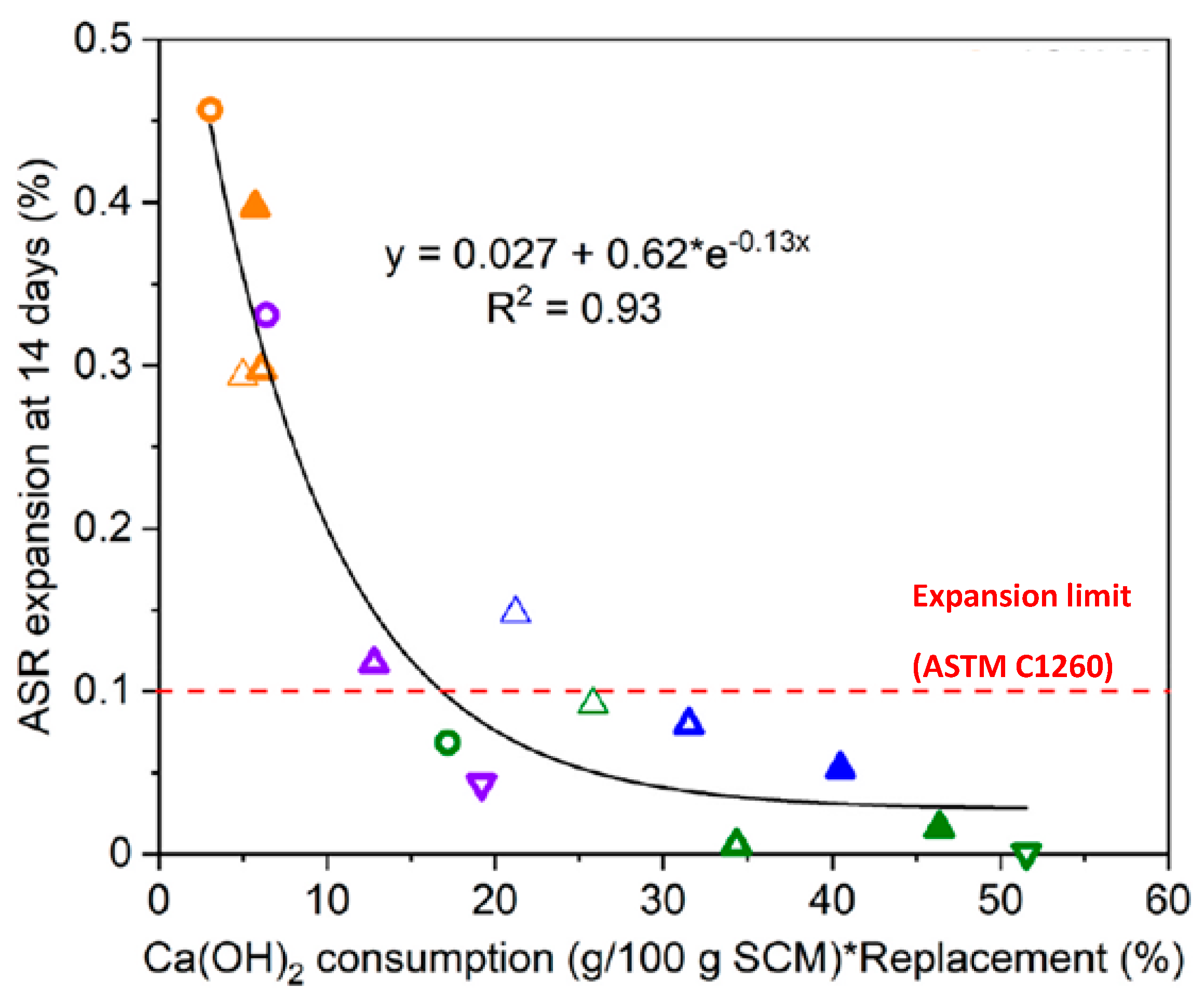
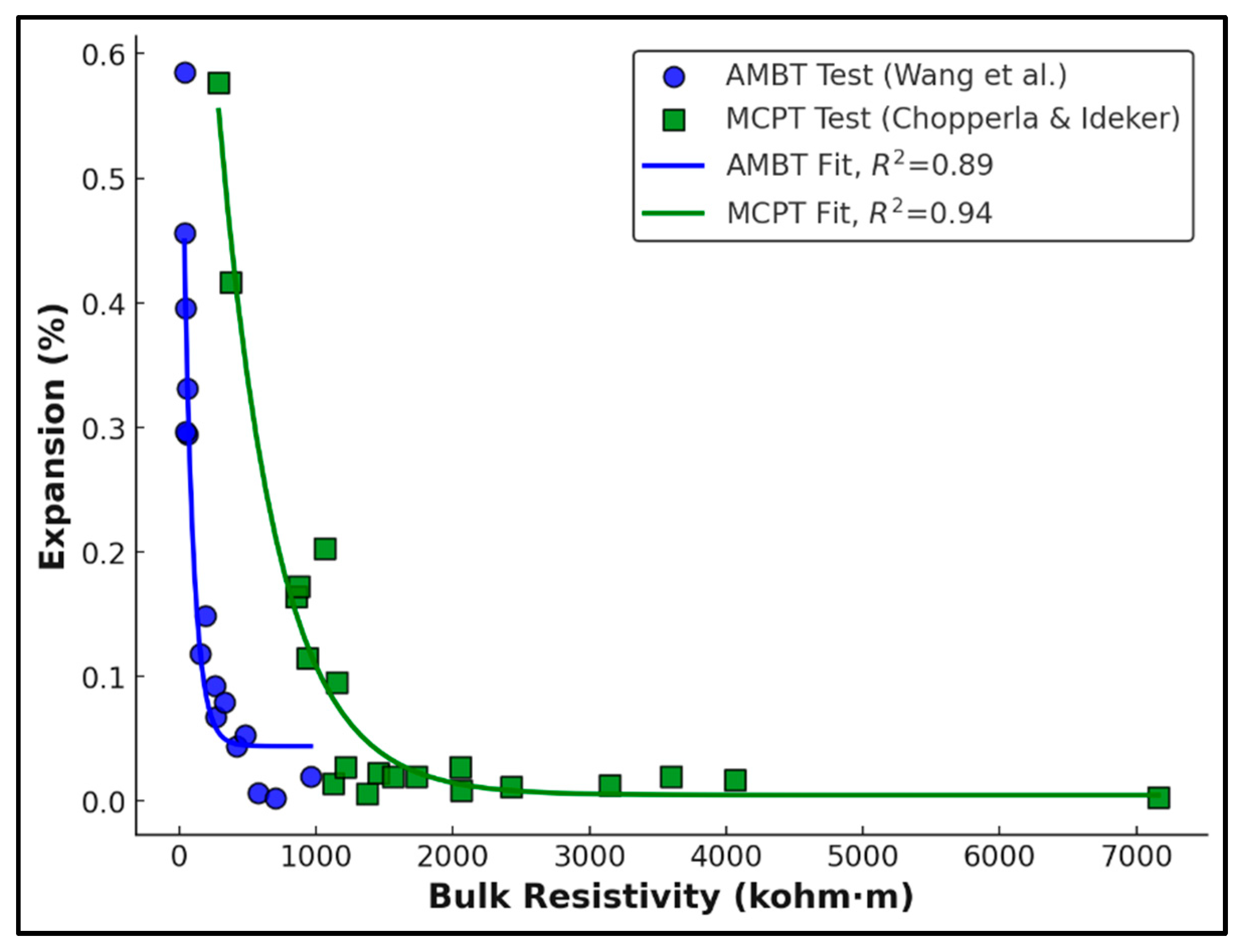
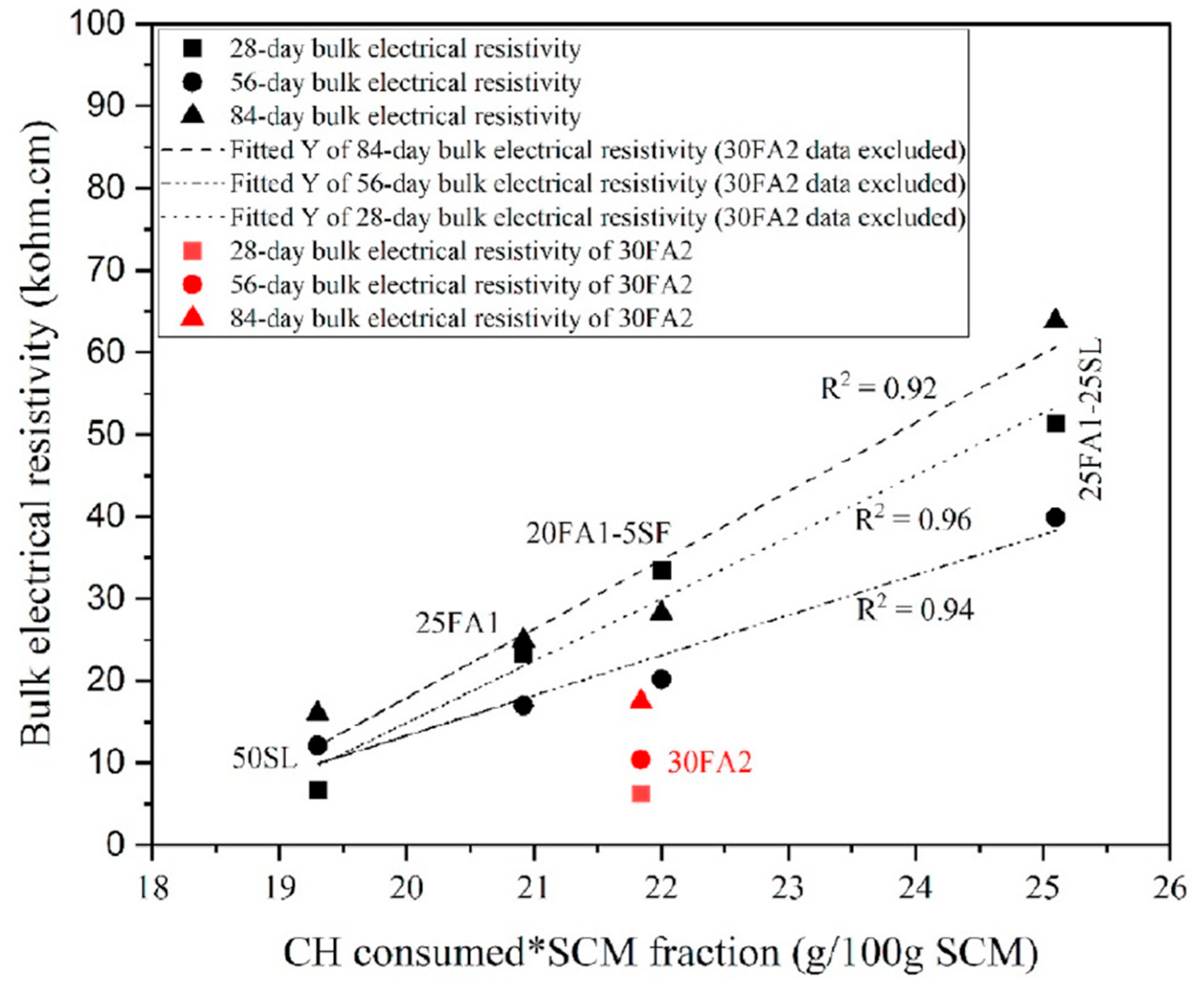
| Aggregate Type | Total Samples | CPT vs. Field (Disagreement) | AMBT vs. Field (Disagreement) |
|---|---|---|---|
| Fine Aggregates | 7 | None | 1 (F1) |
| Coarse Aggregates | 17 | 5 (C1, C5, C6, C7, C10) | 10 (C1, C2, C3, C4, C5, C6, C7, C8, C9, C10) |
| Aggregate Mineralogy | CPT Classification | MCPT Classification | AMBT Classification |
|---|---|---|---|
| Dolomite | 🟩 M (0.033%) | 🟩 M (0.042%) | 🟩 M (0.105%) |
| Chert (fine) | 🟩 M (0.050%) | 🟩 M (0.046%) | 🟩 M (0.235%) |
| Argillite | 🟩 M (0.083%) | 🟩 M (0.083%) | 🟩 M (0.120%) |
| Limestone | 🟩 M (0.046%) | 🟩 M (0.055%) | 🟥 N (0.070%) |
| Limestone | 🟩 M (0.065%) | 🟩 M (0.054%) | 🟥 N (0.054%) |
| Mixes with SCMs | Expansion Results | |||
|---|---|---|---|---|
| AMBT (0.01% at 14 Days) | CPT (ASTM C1293) (0.04% at 2 Years) | CPT (AS 1141.60.1) (0.03% at 2 Years) | Field Expansion (0.05% at 20 Years) | |
| 50% S 1 | 🟩 N (0.059) | 🟩 N (0.029) * | 🟩 N (0.029) * | 🟩 N |
| 18% FA 2 | 🟥 R (0.111) | 🟩 N (0.037) | 🟥 R (0.037) | 🟥 R |
| 25% S | 🟥 R (0.187) | 🟥 R (0.045) | 🟥 R (0.045) | 🟥 R |
| 25% S and 3.8% SF | 🟩 N (0.041) | 🟩 N (0.029) * | 🟩 N (0.029) * | 🟩 N |
| Aggregate Type | Mixes with SCMs | Expansion Results | |||
|---|---|---|---|---|---|
| CPT (ASTM C1293) (0.04% at 2-Years) | MCPT (AASHTO T380) (0.03% at 56 Days) | MCPT (AASHTO T380) (0.03% at 84 Days) | Field Expansion (0.05% at 20-Years) | ||
| Spratt | 40% FA-Class C | 🟩 N 0.14 | 🟥 R 0.029 | 🟥 R 0.038 | 🟥 R 0.05 |
| 20% FA-Class C | 🟩 N 0.26 | 🟥 R 0.052 | 🟥 R 0.063 | 🟥 R 0.16 | |
| Placitas | 40% FA-Class C | 🟩 N 0.013 | 🟥 R 0.029 | 🟥 R 0.038 | 🟥 R 0.32 |
| 20% FA-Class C | 🟥 R 0.043 | 🟥 R 0.061 | 🟥 R 0.08 | 🟥 R 0.45 | |
| 100% lithium nitrate | 🟥 R 0.046 | 🟥 R 0.091 | 🟥 R 0.137 | 🟥 R 0.33 | |
| Wright | 40% FA-Class F | 🟩 N 0.005 | 🟥 R 0.021 | 🟥 R 0.044 | 🟥 R 0.14 |
| 20% FA-Class C | 🟩 N 0.033 | 🟥 R 0.077 | 🟥 R 0.113 | 🟥 R 0.42 | |
| 40% S | 🟩 N 0.025 | 🟥 R 0.052 | 🟥 R 0.052 | 🟥 R 0.34 | |
| 35% S and 5% SF | 🟩 N 0.023 | 🟩 N 0.015 | 🟩 N 0.017 | 🟥 R 0.20 | |
| 35% C and 5% SF | 🟩 N 0.017 | 🟥 R 0.025 | 🟥 R 0.033 | 🟥 R 0.13 | |
| Jobe | 20% Class F | 🟩 N 0.02 | 🟥 R 0.033 | 🟥 R 0.071 | 🟩 N 0.02 |
| 50% S | 🟩 N 0.033 | 🟥 R 0.063 | 🟥 R 0.115 | 🟩 N 0.02 | |
| 100% lithium nitrate | 🟩 N 0.038 | 🟥 R 0.261 | 🟥 R 0.447 | 🟥 R 0.12 | |
| Test Method | Aggregate Reactivity Testing | Preventative Measures Testing |
|---|---|---|
| AMBT (ASTM C1260 /1567 or AASHTO T303) | The 14-day at 0.10% limit is appropriate for aggregate reactivity and it correlated well with MCPT and CPT. | The 28-day at 0.10% limit is more appropriate for preventive measures since it correlated well with field results and MCPT (84-day). |
| CPT (ASTM C1293) | It is the best indicator for aggregate reactivity since it showed the best correlation with field results. It benchmarks well with AMBT (14 days) and MCPT (56 days). | The 2-year at 0.04% limit should not be used for preventive measures as it underestimates the required dosage for mitigating ASR, and it lacks the sensitivity for different alkali loadings. |
| MCPT (AASHTO T380) | Excellent test for aggregate reactivity at 56 days of 0.03% limit since it strongly correlated with CPT and AMBT. The slow reactivity category between 8 and 12 weeks should be removed. The adoption of 0.03% as a reactivity limit should be adequate. | It was recommended to adopt the expansion limit of 0.025% at 84 days since it correlated well with the field results. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, O.; Al Hatailah, H.; Nanni, A. Advances and Perspectives in Alkali–Silica Reaction (ASR) Testing: A Critical Review of Reactivity and Mitigation Assessments. Designs 2025, 9, 71. https://doi.org/10.3390/designs9030071
Omar O, Al Hatailah H, Nanni A. Advances and Perspectives in Alkali–Silica Reaction (ASR) Testing: A Critical Review of Reactivity and Mitigation Assessments. Designs. 2025; 9(3):71. https://doi.org/10.3390/designs9030071
Chicago/Turabian StyleOmar, Osama, Hussain Al Hatailah, and Antonio Nanni. 2025. "Advances and Perspectives in Alkali–Silica Reaction (ASR) Testing: A Critical Review of Reactivity and Mitigation Assessments" Designs 9, no. 3: 71. https://doi.org/10.3390/designs9030071
APA StyleOmar, O., Al Hatailah, H., & Nanni, A. (2025). Advances and Perspectives in Alkali–Silica Reaction (ASR) Testing: A Critical Review of Reactivity and Mitigation Assessments. Designs, 9(3), 71. https://doi.org/10.3390/designs9030071






