The Retinal Complications of C3 Dense Deposit Disease: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Included Reports
3.2. Patient Population
3.3. Eye Disease
3.4. Reported Symptoms
3.5. Retinal Pathologies: Prevalence and Location
3.6. Treatment
4. Discussion
4.1. Ocular Complications
4.2. Management and Treatments
4.3. The Role of Complement Factor H (CFH)
4.4. Strengths
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. PRISMA SCr Checklist
| Section | Item | PRISMA-ScR Checklist Item | Reported on Page # |
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 2 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 3 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 3–4 |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 3 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 3 |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 3–4 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 4 |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | n/a |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 4 |
| RESULTS | |||
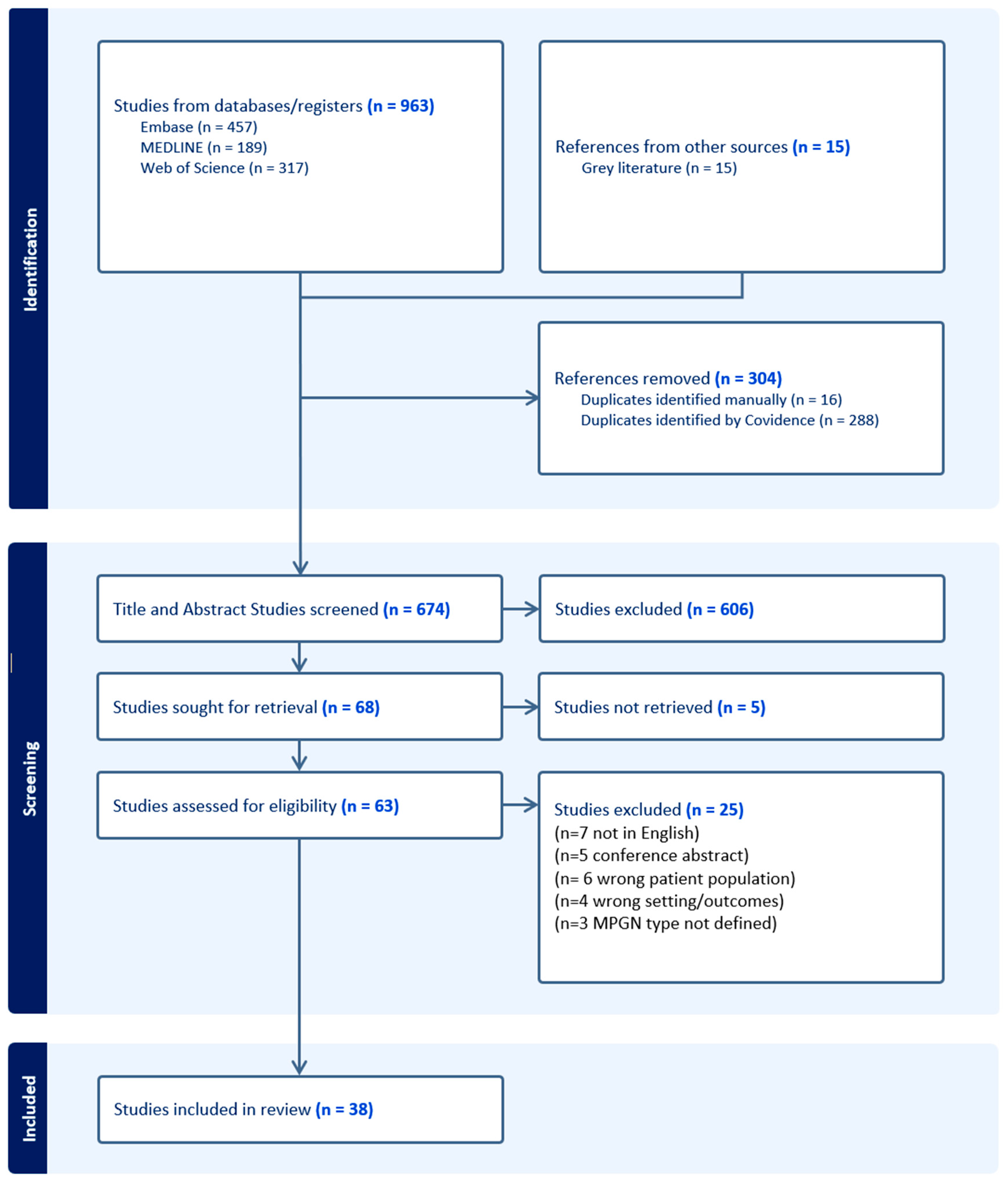
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 4 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 4–9 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | n/a |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 4–9 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 4–9 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 9–10 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 11 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 11 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 15 |
References
- Nasr, S.H.; Valeri, A.M.; Appel, G.B.; Sherwinter, J.; Stokes, M.B.; Said, S.M.; Markowitz, G.S.; D’Agati, V.D. Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 22–32. [Google Scholar] [CrossRef]
- Amir Hamzah, N.A.; Wan Zaki, W.M.D.; Wan Abdul Halim, W.H.; Mustafar, R.; Saad, A.H. Evaluating the potential of retinal photography in chronic kidney disease detection: A review. PeerJ 2024, 12, e17786. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Lima, L.H.; Arevalo, J.F.; Amaro, M.H.; Lozano, V.; Ghannam, A.B.; Chan, E.W. Retinal findings in membranoproliferative glomerulonephritis. Am. J. Ophthalmol. Case Rep. 2017, 7, 83–90. [Google Scholar] [CrossRef]
- Cunningham, A.; Kotagiri, A. A long history of dense deposit disease. BMC Ophthalmol. 2018, 18, 228. [Google Scholar] [CrossRef]
- Smith, R.J.; Alexander, J.; Barlow, P.N.; Botto, M.; Cassavant, T.L.; Cook, H.T.; Hageman, G.S.; Jokiranta, T.S.; Kimberling, W.J.; Lambris, J.D.; et al. New approaches to the treatment of dense deposit disease. J. Am. Soc. Nephrol. 2007, 18, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Barbour, T.D.; Pickering, M.C.; Terence Cook, H. Dense deposit disease and C3 glomerulopathy. Semin. Nephrol. 2013, 33, 493–507. [Google Scholar] [CrossRef]
- Duvall-Young, J.; MacDonald, M.K.; McKechnie, N.M. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: A histopathological report. Br. J. Ophthalmol. 1989, 73, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Cebeci, Z.; Bayraktar, S.; Oray, M.; Kir, N. Multimodal imaging of membranoproliferative glomerulonephritis type II. Saudi. J. Ophthalmol. 2016, 30, 260–263. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Fervenza, F.C.; Sethi, S.; Pulido, J.S. Manifestations of Complement-Mediated and Immune Complex-Mediated Membranoproliferative Glomerulonephritis: A Comparative Consecutive Series. Ophthalmology 2016, 123, 1588–1594. [Google Scholar] [CrossRef]
- de-Pablo-Gomez-de-Liano, L.; Canas Zamarra, I.; Fernandez-Vigo, J.I.; Fernandez Vidal, M.; Navarro-Perea, C.; Cavero Escribano, T. Retinal manifestations in patients with complement-mediated membranoproliferative glomerulonephritis. Arch. Soc. Esp. Oftalmol. 2019, 94, 95–99. [Google Scholar] [CrossRef]
- Farah, S.E.; Fazelat, A.; Frei, G. Treatment of subretinal neovascular membrane in a patient with membranoproliferative glomerulonephritis type II. Ophthalmic Surg Lasers Imaging 2009, 40, 416–418. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Abazi, Z.; Magarasevic, L.; Sukalo, S.; Kosanovic-Jakovic, N.; Risovic, D. Ocular complications after kidney transplantation: A case report and review of literature. Int. Urol. Nephrol. 2014, .46, 665–668. [Google Scholar] [CrossRef]
- Adhi, M.; Read, S.P.; Liu, J.J.; Fujimoto, J.G.; Duker, J.S. High-speed ultrahigh-resolution OCT of Bruch’s membrane in membranoproliferative glomerulonephritis type 2. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.A.; Grierson, D.J.; Walker, S. Bilateral macular sub-retinal fluid and retinal pigment epithelial detachment associated with type 2 membrano-proliferative glomerulonephritis. Clin. Exp. Optom. 2008, 91, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Batioglu, F.; Muftuoglu, O.; Atmaca, L. Optical coherence tomography of fundus abnormalities associated with type II membranoproliferative glomerulonephritis. Retina 2003, 23, 261–262. [Google Scholar] [CrossRef]
- Chalam, K.V.; Li, J.P.; Tripathi, R.C. Occurrence of atypical acute bilateral multifocal choroidal neovascularization in membranoproliferative glomerulonephritis type II. Ann. Ophthalmol. 2000, 32, 320–324. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Bandello, F.; Introini, U. Progressive Retinochoroidal Atrophy in Dense Deposit Disease. Ophthalmol. Retin. 2021, 5, 663. [Google Scholar] [CrossRef]
- Colville, D.; Guymer, R.; Sinclair, R.A.; Savige, J. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II (“dense deposit disease”). Am. J. Kidney Dis. 2003, 42, e3.1–e3.4. [Google Scholar] [CrossRef]
- Dimaggio, A.; Loperfido, A.; Scatizzi, A. Retinal Lesions Specific for Membranoproliferative Glomerulonephritis Type-Ii—Description of 2 Cases. Nephron 1993, 63, 365–366. [Google Scholar]
- Empeslidis, T.; Imrani, U.; Vardarinos, A.; Menassa, N.; Banerjee, S. Spontaneous Resolution of Retinal Pigment Epithelial Detachments and Visual Improvement in Patient with MPGN II: A Case Report. Case Rep. Ophthalmol. Med. 2012, 2012, 864198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerth, C.; Zawadzki, R.J.; Licht, C.; Werner, J.S.; Heon, E. A microstructural retinal analysis of membrano-proliferative glomerulonephritis type II. Br. J. Ophthalmol. 2008, 92, 1150–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.Y.; Wu, C.K.; Liu, P.Y. Assistive technology in smart cities: A case of street crossing for the visually-impaired. Technol. Soc. 2022, 68, 101805. [Google Scholar] [CrossRef]
- Kim, D.D.; Mieler, W.F.; Wolf, M.D. Posterior segment changes in membranoproliferative glomerulonephritis. Am. J. Ophthalmol. 1992, 114, 593–599. [Google Scholar] [CrossRef]
- Kim, R.Y.; Faktorovich, E.G.; Kuo, C.Y.; Olson, J.L. Retinal function abnormalities in membranoproliferative glomerulonephritis type II. Am. J. Ophthalmol. 1997, 123, 619–628. [Google Scholar] [CrossRef]
- Mehmet, K.; Hikmet, D.; Sevdegül, M.; Adem, T.; Süleyman, M.; Elif, B. Spectral Domain Optical Coherence Tomography Findings in a Pediatric Case with Type II Membranoproliferative Glomerulonephritis. Retin. Vitr. J. 2014, 23, 246–248. [Google Scholar]
- Lent-Schochet, D.; Yiu, G. Drusen in dense deposit disease: Not just age-related macular degeneration. Lancet 2020, 395, 1726. [Google Scholar] [CrossRef]
- Leys, A.; Vanrenterghem, Y.; Vandamme, B.; Snyers, B.; Pirson, Y. Fundus Changes in Membranoproliferative Glomerulonephritis Type-Ii—a Fluorescein Angiographic Study of 23 Patients. Graef. Arch. Clin. Exp. 1991, 229, 406–410. [Google Scholar] [CrossRef]
- Leys, A.; VanDamme, B.; Verberckmoes, R. Ocular complications of type 2 membranoproliferative glomerulonephritis. Nephrol. Dial. Transpl. 1996, 11, 211–214. [Google Scholar] [CrossRef]
- Leys, A.; Vanrenterghem, Y.; Van Damme, B.; Snyers, B.; Pirson, Y.; Leys, M. Sequential observation of fundus changes in patients with long standing membranoproliferative glomerulonephritis type II (MPGN type II). Eur. J. Ophthalmol. 1991, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Leys, A.; Proesmans, W.; Vandammelombaerts, R.; Vandamme, B. Specific Eye Fundus Lesions in Type-Ii Membranoproliferative Glomerulonephritis. Pediatr. Nephrol. 1991, 5, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Leys, A.; Michielsen, B.; Leys, M.; Vanrenterghem, Y.; Missotten, L.; Vandamme, B. Subretinal Neovascular Membranes Associated with Chronic Membranoproliferative Glomerulonephritis Type-Ii. Graef. Arch. Clin. Exp. 1990, 228, 499–504. [Google Scholar] [CrossRef]
- Mammo, D.A.; Roizenblatt, R. Drusen in Dense Deposit Disease. Ophthalmology 2021, 128, 1221. [Google Scholar] [CrossRef]
- McAvoy, C.E.; Silvestri, G. Retinal changes associated with type 2 glomerulonephritis. Eye 2005, 19, 985–989. [Google Scholar] [CrossRef]
- McCullagh, D.; Silvestri, G.; Maxwell, A.P. Treatment of choroidal neovascularisation secondary to membranoproliferative glomerulonephritis type II with intravitreal ranibizumab. BMJ Case Rep. 2014, 2014, bcr2013010247. [Google Scholar] [CrossRef]
- Liang-Peng, C.; Olvera-Barrios, A.; Schwartz, R.; Grimaldi, G.; Egan, C.; Tufail, A. Novel Outer Retinal Columnar Abnormalities (ORCA) and Non-Vasogenic Cystoid Macular Edema in Dense Deposit Disease. Retin. Cases Brief Rep. 2025, 19, 54–59. [Google Scholar] [CrossRef]
- Polk, T.D.; Kimura, A.E.; Park, D.; Gass, J.D.M. Subretinal fluid associated with membranoproliferative glomerulonephritis type 2. Arch. Ophthalmol. 1997, 115, 927–928. [Google Scholar] [CrossRef]
- Ritter, M.; Bolz, M.; Haidinger, M.; Deák, G.; Sacu, S.; Säemann, M.; Schmidt-Erfurth, U. Functional and morphological macular abnormalities in membranoproliferative glomerulonephritis type II. Bri. J. Ophthalmol. 2010, 94, 1112–1114. [Google Scholar] [CrossRef]
- Savige, J.; Amos, L.; Ierino, F.; Mack, H.G.; Symons, R.C.A.; Hughes, P.; Nicholls, K.; Colville, D. Retinal disease in the C3 glomerulopathies and the risk of impaired vision. Ophthalmic Genet. 2016, 37, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.; McCillvenny, S. Microperimetric evaluation of macula in retinopathy of membranoproliferative glomerulonephritis type II: A case report. Eur. J. Ophthalmol. 2006, 16, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Karatza, E.C.; Shields, C.L.; Cohen, S.B. Drusen like deposits and sclerochoroidal calcification in a patient with glomerulonephritis. Retina 2004, 24, 304–306. [Google Scholar] [CrossRef]
- Tanner, A.; Chan, H.W.; Stears, A.; Moosajee, M. Bilateral macular drusen in acquired partial lipodystrophy with type 2 membranoproliferative glomerulonephritis. BMJ Case Rep. 2021, 14, e241666. [Google Scholar] [CrossRef] [PubMed]
- Ulbig, M.R.; Riordan-Eva, P.; Holz, F.G.; Rees, H.C.; Hamilton, P.A. Membranoproliferative glomerulonephritis type II associated with central serous retinopathy. Am. J. Ophthalmol. 1993, 116, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Mishra, K.; Nguyen, H.V.; Miller, P.P.; Ghoraba, H.; Karaca, I.; Matsumiya, W.; Nguyen, Q.D.; Leung, L.S. C3 glomerulopathy associated with both hypertensive retinopathy and purtscher-like retinopathy. Am. J. Ophthalmol. Case Rep. 2022, 27, 101683. [Google Scholar] [CrossRef]
- NHS. Eye Health 2025. Available online: https://www.england.nhs.uk/primary-care/eye-health/ (accessed on 23 February 2025).
- Ervin, A.M.; Solomon, S.D.; Shoge, R.Y. Access to Eye Care in the United States: Evidence-Informed Decision-Making Is Key to Improving Access for Underserved Populations. Ophthalmology 2022, 129, 1079–1080. [Google Scholar] [CrossRef]
- Fung, A.T.; Yang, Y.; Kam, A.W. Central serous chorioretinopathy: A review. Clin. Exp. Ophthalmol. 2023, 51, 243–270. [Google Scholar] [CrossRef]
- Gass, J.D.M.; Slamovits, T.L.; Fuller, D.G.; Gieser, R.G. Posterior Chorioretinopathy and Retinal-Detachment after Organ-Transplantation. Arch. Ophthalmol. 1992, 110, 1717–1722. [Google Scholar] [CrossRef]
- Bomback, A.S.; Smith, R.J.; Barile, G.R.; Zhang, Y.; Heher, E.C.; Herlitz, L.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D.; Canetta, P.A.; et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012, 7, 748–756. [Google Scholar] [CrossRef]
- Khandhadia, S.; Hakobyan, S.; Heng, L.Z.; Gibson, J.; Adams, D.H.; Alexander, G.J.; Gibson, J.M.; Martin, K.R.; Menon, G.; Nash, K.; et al. Age-related macular degeneration and modification of systemic complement factor H production through liver transplantation. Ophthalmology 2013, 120, 1612–1618. [Google Scholar] [CrossRef]
- D’Souza, Y.; Short, C.D.; McLeod, D.; Bonshek, R.E. Long-term follow-up of drusen-like lesions in patients with type II mesangiocapillary glomerulonephritis. Br. J. Ophthalmol. 2008, 92, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Gruppo, R.A.; Rother, R.P. Eculizumab for congenital atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 360, 544–546. [Google Scholar] [CrossRef]
- Alexander, J.J.; Quigg, R.J. The simple design of complement factor H: Looks can be deceiving. Mol. Immunol. 2007, 44, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.C.; Cook, H.T. Translational mini-review series on complement factor H: Renal diseases associated with complement factor H: Novel insights from humans and animals. Clin. Exp. Immunol. 2008, 151, 210–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundh von Leithner, P.; Kam, J.H.; Bainbridge, J.; Catchpole, I.; Gough, G.; Coffey, P.; Jeffery, G. Complement factor h is critical in the maintenance of retinal perfusion. Am. J. Pathol. 2009, 175, 412–421, Erratum in Am. J. Pathol. 2010, 176, 2581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Wang, F.; Rendahl, K.G.; Manning, W.C.; Quiroz, D.; Coyne, M.; Miller, S.S. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Investig. Ophthalmol. Vis. Sci. 2003, 44, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.; Hu, Z.; Tezel, T.H.; Sohn, J.H.; Kang, S.G.; Cruz, J.M.; Bora, N.S.; Garen, A.; Kaplan, H.J. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Sepp, T.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Moore, A.T.; Bird, A.C.; Yates, J.R. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Investig. Ophthalmol. Vis. Sci. 2006, 47, 536–540. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Ebrahem, Q.; Renganathan, K.; Sears, J.; Vasanji, A.; Gu, X.; Lu, L.; Salomon, R.G.; Crabb, J.W.; Anand-Apte, B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 13480–13484. [Google Scholar] [CrossRef]
- Moore, D.J.; Hussain, A.A.; Marshall, J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1290–1297. [Google Scholar]
- Spraul, C.W.; Lang, G.E.; Grossniklaus, H.E. Morphometric analysis of the choroid. Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1996, 37, 2724–2735. [Google Scholar] [PubMed]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
| Country of Study | n (%) |
|---|---|
| United States | 21 (55%) |
| Europe | 17 (45%) |
| Geographic Breakdown of European Studies | |
| England | 9 (24%) |
| Germany | 3 (8%) |
| Netherlands | 2 (5%) |
| Spain | 1 (3%) |
| Switzerland | 1 (3%) |
| Turkey | 1 (3%) |
| LogMAR | Total | % |
|---|---|---|
| 0.0 | 43 | 40.19 |
| 0.1 | 7 | 6.54 |
| 0.2 | 15 | 14.02 |
| 0.3 | 19 | 17.76 |
| 0.4 | 2 | 1.87 |
| 0.5 | 2 | 1.87 |
| 0.6 | 1 | 0.93 |
| 0.7 | 1 | 0.93 |
| 0.8 | 1 | 0.93 |
| 0.9 | 1 | 0.93 |
| 1.0 | 4 | 3.74 |
| 1.2 | 1 | 0.93 |
| 1.5 | 1 | 0.93 |
| Counting Fingers (CF) | 3 | 2.80 |
| Hand movements (HM) | 1 | 0.93 |
| Eye Disorders | n (%) |
|---|---|
| Hypertensive Retinopathy | 7 (7%) |
| Central Serous Chorioretinopathy (CSCR) | 6 (4.7%) |
| Retinoschisis | 2 (1%) |
| Glaucoma | 1 (0.7%) |
| Amblyopia | 2 (1%) |
| Macular Degeneration (undefined) | 2 (1%) |
| Purtscher-like retinopathy | 1 (0.7%) |
| Clinical Features | n (%) |
|---|---|
| Drusen-like Deposits | 103 (75%) |
| RPE Detachment | 24 (18%) |
| Choroidal Neovascularisation | 22 (16%) |
| Macular Atrophy | 17 (12%) |
| Retinal Haemorrhage | 15 (11%) |
| Bruch’s Membrane Irregularities | 15 (11%) |
| RPE Elevations | 13 (9%) |
| Subretinal Fluid | 10 (7%) |
| Intraretinal Fluid | 2 (1%) |
| Retinal Atrophy | 7 (5%) |
| RPE Mottling | 5 (4%) |
| Retinal Pigment Migration | 4 (3%) |
| Retinal Vascular Changes | 3 (2%) |
| Maculopathy | 2 (1%) |
| Cotton Wool Spots | 2 (1%) |
| Macular Oedema | 1 (0.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarney, J.; Curran, K.; Peto, T.; Silvestri, G.; Cushley, L.N. The Retinal Complications of C3 Dense Deposit Disease: A Scoping Review. Vision 2025, 9, 64. https://doi.org/10.3390/vision9030064
McCarney J, Curran K, Peto T, Silvestri G, Cushley LN. The Retinal Complications of C3 Dense Deposit Disease: A Scoping Review. Vision. 2025; 9(3):64. https://doi.org/10.3390/vision9030064
Chicago/Turabian StyleMcCarney, Jolene, Katie Curran, Tunde Peto, Giuliana Silvestri, and Laura N. Cushley. 2025. "The Retinal Complications of C3 Dense Deposit Disease: A Scoping Review" Vision 9, no. 3: 64. https://doi.org/10.3390/vision9030064
APA StyleMcCarney, J., Curran, K., Peto, T., Silvestri, G., & Cushley, L. N. (2025). The Retinal Complications of C3 Dense Deposit Disease: A Scoping Review. Vision, 9(3), 64. https://doi.org/10.3390/vision9030064






