Behavioral Interference by Emotional Stimuli: Sequential Modulation by Perceptual Conditions but Not by Emotional Primes
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. Stimuli
2.3. Procedure
2.4. Analysis
3. Results
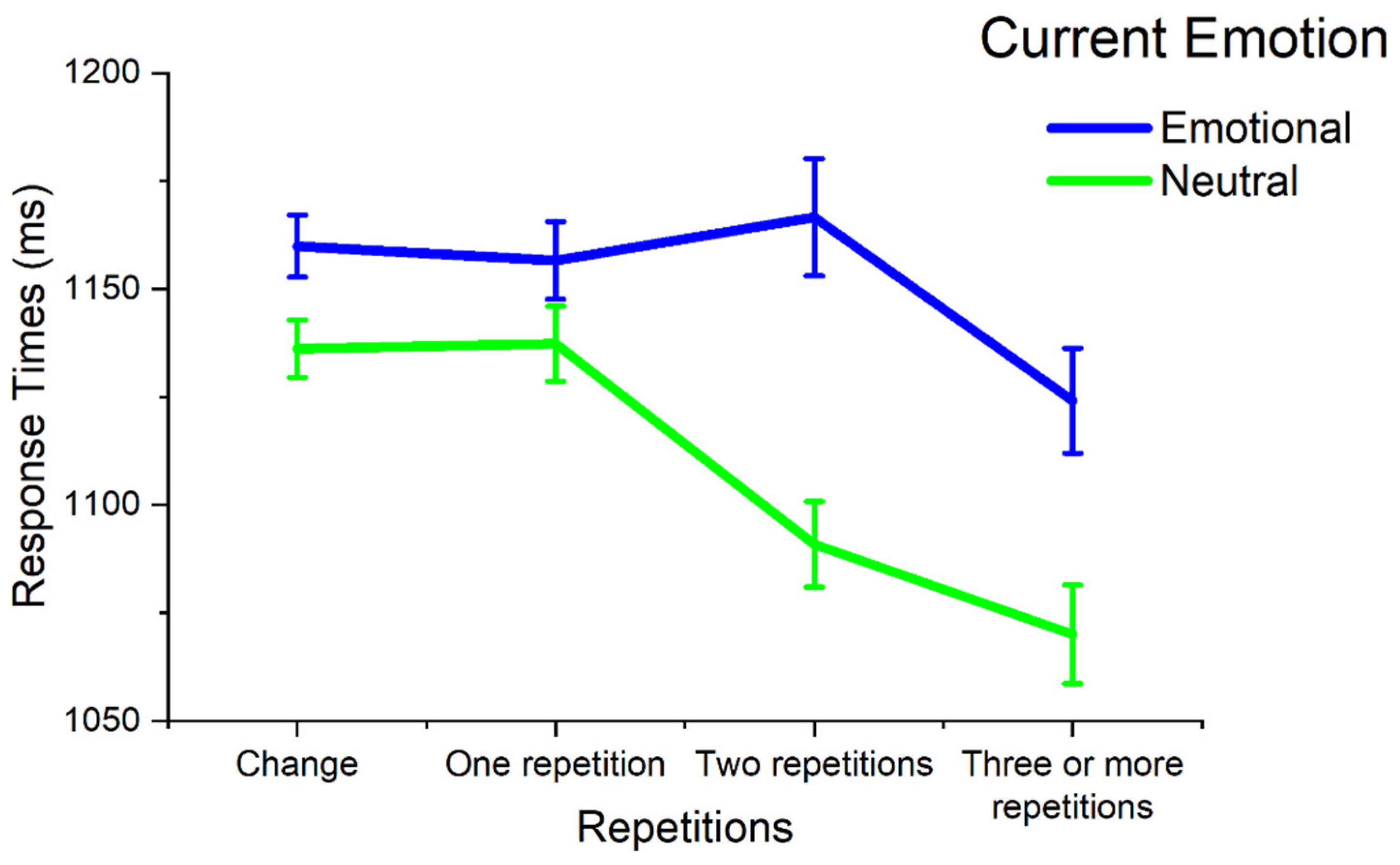
3.1. Effects of Current Emotion
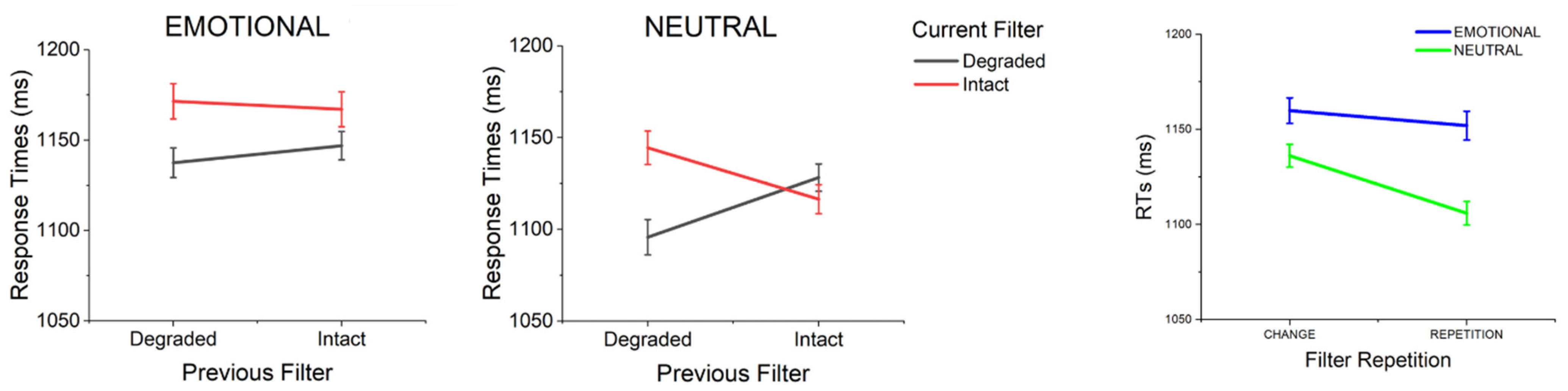
3.2. Effects of Previous Emotion and Filter
3.3. Further Analysis: Filter Repetition/Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, M.M. Natural Selective Attention: Orienting and Emotion. Psychophysiology 2009, 46, 1–11. [Google Scholar] [CrossRef]
- Codispoti, M.; Ferrari, V.; De Cesarei, A.; Cardinale, R. Implicit and Explicit Categorization of Natural Scenes. Prog. Brain Res. 2006, 156, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Flaisch, T.; Stockburger, J.; Junghöfer, M. Emotion and Attention: Event-Related Brain Potential Studies. Prog. Brain Res. 2006, 156, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Drobes, D.; Lang, P.J. A Probe for All Reasons: Reflex and RT Measures in Perception. Psychophysiology 1996, 33, S25. [Google Scholar] [CrossRef]
- Bradley, M.M.; Cuthbert, B.N.; Lang, P.J. Affect and the Startle Reflex. In Startle Modification: Implications for Neuroscience, Cognitive Science, and Clinical Science; Dawson, M.E., Schell, A.M., Böhmelt, A.H., Eds.; Cambridge University Press: New York, NY, USA, 1999; pp. 157–184. [Google Scholar] [CrossRef]
- De Cesarei, A.; Codispoti, M. Fuzzy Picture Processing: Effects of Size Reduction and Blurring on Emotional Processing. Emotion 2008, 8, 352–363. [Google Scholar] [CrossRef]
- Ferrari, V.; Mastria, S.; Bruno, N. Crossmodal Interactions during Affective Picture Processing. PLoS ONE 2014, 9, e89858. [Google Scholar] [CrossRef][Green Version]
- Micucci, A.; Ferrari, V.; De Cesarei, A.; Codispoti, M. Contextual Modulation of Emotional Distraction: Attentional Capture and Motivational Significance. J. Cogn. Neurosci. 2020, 32, 621–633. [Google Scholar] [CrossRef]
- Okon-Singer, H.; Tzelgov, J.; Henik, A. Distinguishing between Automaticity and Attention in the Processing of Emotionally Significant Stimuli. Emotion 2007, 7, 147–157. [Google Scholar] [CrossRef]
- Pereira, M.G.; Volchan, E.; de Souza, G.G.L.; Oliveira, L.; Campagnoli, R.R.; Pinheiro, W.M.; Pessoa, L. Sustained and Transient Modulation of Performance Induced by Emotional Picture Viewing. Emotion 2006, 6, 622–634. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hyönä, J.; Calvo, M.G. Eye Movement Assessment of Selective Attentional Capture by Emotional Pictures. Emotion 2006, 6, 257–268. [Google Scholar] [CrossRef]
- Schimmack, U. Response Latencies of Pleasure and Displeasure Ratings: Further Evidence for Mixed Feelings. Cogn. Emot. 2005, 19, 671–691. [Google Scholar] [CrossRef]
- Moors, A.; De Houwer, J. Automaticity: A Theoretical and Conceptual Analysis. Psychol. Bull. 2006, 132, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Kastner, S.; Ungerleider, L.G. Attentional Control of the Processing of Neutral and Emotional Stimuli. Cogn. Brain Res. 2002, 15, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, M.; De Cesarei, A.; Biondi, S.; Ferrari, V. The Fate of Unattended Stimuli and Emotional Habituation: Behavioral Interference and Cortical Changes. Cogn. Affect. Behav. Neurosci. 2016, 16, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, M.; Micucci, A.; De Cesarei, A. Time Will Tell: Object Categorization and Emotional Engagement during Processing of Degraded Natural Scenes. Psychophysiology 2021, 58, e13704. [Google Scholar] [CrossRef] [PubMed]
- De Cesarei, A.; Mastria, S.; Codispoti, M. Early Spatial Frequency Processing of Natural Images: An ERP Study. PLoS ONE 2013, 8, e65103. [Google Scholar] [CrossRef]
- De Cesarei, A.; Loftus, G.R.; Mastria, S.; Codispoti, M. Understanding Natural Scenes: Contributions of Image Statistics. Neurosci. Biobehav. Rev. 2017, 74, 44–57. [Google Scholar] [CrossRef]
- Reisenzein, R.; Franikowski, P. On the Latency of Object Recognition and Affect: Evidence from Temporal Order and Simultaneity Judgments. J. Exp. Psychol. Gen. 2022, 151, 3060–3078. [Google Scholar] [CrossRef]
- Storbeck, J.; Robinson, M.D.; McCourt, M.E. Semantic Processing Precedes Affect Retrieval: The Neurological Case for Cognitive Primacy in Visual Processing. Rev. Gen. Psychol. 2006, 10, 41–55. [Google Scholar] [CrossRef]
- De Cesarei, A.; Loftus, G.R. Global and Local Vision in Natural Scene Identification. Psychon. Bull. Rev. 2011, 18, 840–847. [Google Scholar] [CrossRef]
- Charbonneau, I.; Duncan, J.; Blais, C.; Guérette, J.; Plouffe-Demers, M.P.; Smith, F.; Fiset, D. Facial Expression Categorization Predominantly Relies on Mid-Spatial Frequencies. Vis. Res. 2025, 231, 108611. [Google Scholar] [CrossRef]
- Mastria, S.; Codispoti, M.; Tronelli, V.; De Cesarei, A. Subjective Affective Responses to Natural Scenes Require Understanding, Not Spatial Frequency Bands. Vision 2024, 8, 36. [Google Scholar] [CrossRef]
- Fiorentini, A.; Berardi, N. Perceptual Learning Specific for Orientation and Spatial Frequency. Nature 1980, 287, 43–44. [Google Scholar] [CrossRef]
- Sowden, P.T.; Schyns, P.G. Channel Surfing in the Visual Brain. Trends Cogn. Sci. 2006, 10, 538–545. [Google Scholar] [CrossRef]
- Kamitani, Y.; Tong, F. Decoding the Visual and Subjective Contents of the Human Brain. Nat. Neurosci. 2005, 8, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Özgen, E.; Payne, H.E.; Sowden, P.T.; Schyns, P.G. Retinotopic Sensitisation to Spatial Scale: Evidence for Flexible Spatial Frequency Processing in Scene Perception. Vis. Res. 2006, 46, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Schyns, P.G.; Oliva, A. Dr. Angry and Mr. Smile: When Categorization Flexibly Modifies the Perception of Faces in Rapid Visual Presentations. Cognition 1999, 69, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Sowden, P.T.; Özgen, E.; Schyns, P.G.; Daoutis, C. Expectancy Effects on Spatial Frequency Processing. Vis. Res. 2003, 43, 2759–2772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manassi, M.; Murai, Y.; Whitney, D. Serial Dependence in Visual Perception: A Meta-Analysis and Review. J. Vis. 2023, 23, 18. [Google Scholar] [CrossRef]
- De Cesarei, A.; Codispoti, M. Scene Identification and Emotional Response: Which Spatial Frequencies Are Critical? J. Neurosci. 2011, 31, 17052–17057. [Google Scholar] [CrossRef][Green Version]
- De Cesarei, A.; Codispoti, M. Spatial Frequencies and Emotional Perception. Rev. Neurosci. 2013, 24, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Coles, M.G.; Donchin, E. Optimizing the Use of Information: Strategic Control of Activation of Responses. J. Exp. Psychol. Gen. 1992, 121, 480. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B.; Proctor, R.W.; Vu, K.P.L. A Feature-Integration Account of Sequential Effects in the Simon Task. Psychol. Res. 2004, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, E.; Braem, S.; Notebaert, W.; Verguts, T. Grounding Cognitive Control in Associative Learning. Psychol. Bull. 2016, 142, 693. [Google Scholar] [CrossRef]
- Dignath, D.; Johannsen, L.; Hommel, B.; Kiesel, A. Reconciling Cognitive-Control and Episodic-Retrieval Accounts of Sequential Conflict Modulation: Binding of Control-States into Event-Files. J. Exp. Psychol. Hum. Percept. Perform. 2019, 45, 1265. [Google Scholar] [CrossRef]
- Egner, T. Creatures of Habit (and Control): A Multi-Level Learning Perspective on the Modulation of Congruency Effects. Front. Psychol. 2014, 5, 1247. [Google Scholar] [CrossRef]
- Frings, C.; Hommel, B.; Koch, I.; Rothermund, K.; Dignath, D.; Giesen, C.; Philipp, A. Binding and Retrieval in Action Control (BRAC). Trends Cogn. Sci. 2020, 24, 375–387. [Google Scholar] [CrossRef]
- Theeuwes, J. Cross-Dimensional Perceptual Selectivity. Percept. Psychophys. 1991, 50, 184–193. [Google Scholar] [CrossRef]
- Hickey, C.; Chelazzi, L.; Theeuwes, J. Reward Guides Vision When It’s Your Thing: Trait Reward-Seeking in Reward-Mediated Visual Priming. PLoS ONE 2010, 5, e14087. [Google Scholar] [CrossRef]
- Hickey, C.; Chelazzi, L.; Theeuwes, J. Reward-Priming of Location in Visual Search. PLoS ONE 2014, 9, e103372. [Google Scholar] [CrossRef]
- Brown, S.B.; Van Steenbergen, H.; Kedar, T.; Nieuwenhuis, S. Effects of Arousal on Cognitive Control: Empirical Tests of the Conflict-Modulated Hebbian-Learning Hypothesis. Front. Hum. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- De Cesarei, A.; D’Ascenzo, S.; Nicoletti, R.; Codispoti, M. The Role of Novelty and Probability in Cognitive Control: Evidence from the Simon Task. Psychol. Res. 2023, 87, 2390–2406. [Google Scholar] [CrossRef] [PubMed]
- Dignath, D.; Janczyk, M.; Eder, A.B. Phasic Valence and Arousal Do Not Influence Post-Conflict Adjustments in the Simon Task. Acta Psychol. 2017, 174, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, Technical Report A-7; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An Open-Source, Graphical Experiment Builder for the Social Sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Kühn, S.; Filevich, E. “Just Another Tool for Online Studies” (JATOS): An Easy Solution for Setup and Management of Web Servers Supporting Online Studies. PLoS ONE 2015, 10, e0130834. [Google Scholar] [CrossRef]
- Rabbitt, P.M. Errors and Error Correction in Choice-Response Tasks. J. Exp. Psychol. 1966, 71, 264–272. [Google Scholar] [CrossRef]
- King, J.A.; Korb, F.M.; Von Cramon, D.Y.; Ullsperger, M. Post-Error Behavioral Adjustments Are Facilitated by Activation and Suppression of Task-Relevant and Task-Irrelevant Information Processing. J. Neurosci. 2010, 30, 12759–12769. [Google Scholar] [CrossRef]
- Cousineau, D. Confidence Intervals in Within-Subject Designs: A Simpler Solution to Loftus and Masson’s Method. Tutor. Quant. Methods Psychol. 2005, 1, 42–45. [Google Scholar] [CrossRef]
- Flaisch, T.; Junghöfer, M.; Bradley, M.M.; Schupp, H.T.; Lang, P.J. Rapid Picture Processing: Affective Primes and Targets. Psychophysiology 2008, 45, 1–10. [Google Scholar] [CrossRef]
- Flaisch, T.; Stockburger, J.; Schupp, H.T. Affective Prime and Target Picture Processing: An ERP Analysis of Early and Late Interference Effects. Brain Topogr. 2008, 20, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, S. Learned Reward Association Improves Visual Working Memory. J. Exp. Psychol. Hum. Percept. Perform. 2014, 40, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Infanti, E.; Hickey, C.; Menghi, N.; Turatto, M. Reward-Priming Impacts Visual Working Memory Maintenance: Evidence from Human Electrophysiology. Vis. Cogn. 2017, 25, 956–971. [Google Scholar] [CrossRef]
- Infanti, E.; Hickey, C.; Turatto, M. Reward Associations Impact Both Iconic and Visual Working Memory. Vis. Res. 2015, 107, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wallis, G.; Stokes, M.G.; Arnold, C.; Nobre, A.C. Reward Boosts Working Memory Encoding over a Brief Temporal Window. Vis. Cogn. 2015, 23, 291–312. [Google Scholar] [CrossRef]
- De Cesarei, A.; Mastria, S.; Codispoti, M. Distraction Driven by Reward History: Attentional Capture and Sequential Effects. Atten. Percept. Psychophys. 2025. submitted. [Google Scholar]
- Mastria, S.; Codispoti, M.; De Cesarei, A. We Care a Lot! Individual Attitudes and Value-Based Attentional Capture. In Proceedings of the ESCOP 2023—23rd Conference of the European Society for Cognitive Psychology, Porto, Portugal, 6–9 September 2023. [Google Scholar]
- Simon, J.R.; Rudell, A.P. Auditory S-R Compatibility: The Effect of an Irrelevant Cue on Information Processing. J. Appl. Psychol. 1967, 51, 300–304. [Google Scholar] [CrossRef]
- Harris, C.R.; Pashler, H. Attention and the Processing of Emotional Words and Names: Not So Special After All. Psychol. Sci. 2004, 15, 171–178. [Google Scholar] [CrossRef]
- Braver, T.S. The Variable Nature of Cognitive Control: A Dual Mechanisms Framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Ásgeirsson, Á.G.; Kristjánsson, Á. Random Reward Priming Is Task-Contingent: The Robustness of the 1-Trial Reward Priming Effect. Front. Psychol. 2014, 5, 309. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cesarei, A.; Tronelli, V.; Mastria, S.; Ferrari, V.; Codispoti, M. Behavioral Interference by Emotional Stimuli: Sequential Modulation by Perceptual Conditions but Not by Emotional Primes. Vision 2025, 9, 66. https://doi.org/10.3390/vision9030066
De Cesarei A, Tronelli V, Mastria S, Ferrari V, Codispoti M. Behavioral Interference by Emotional Stimuli: Sequential Modulation by Perceptual Conditions but Not by Emotional Primes. Vision. 2025; 9(3):66. https://doi.org/10.3390/vision9030066
Chicago/Turabian StyleDe Cesarei, Andrea, Virginia Tronelli, Serena Mastria, Vera Ferrari, and Maurizio Codispoti. 2025. "Behavioral Interference by Emotional Stimuli: Sequential Modulation by Perceptual Conditions but Not by Emotional Primes" Vision 9, no. 3: 66. https://doi.org/10.3390/vision9030066
APA StyleDe Cesarei, A., Tronelli, V., Mastria, S., Ferrari, V., & Codispoti, M. (2025). Behavioral Interference by Emotional Stimuli: Sequential Modulation by Perceptual Conditions but Not by Emotional Primes. Vision, 9(3), 66. https://doi.org/10.3390/vision9030066




