Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration
Abstract
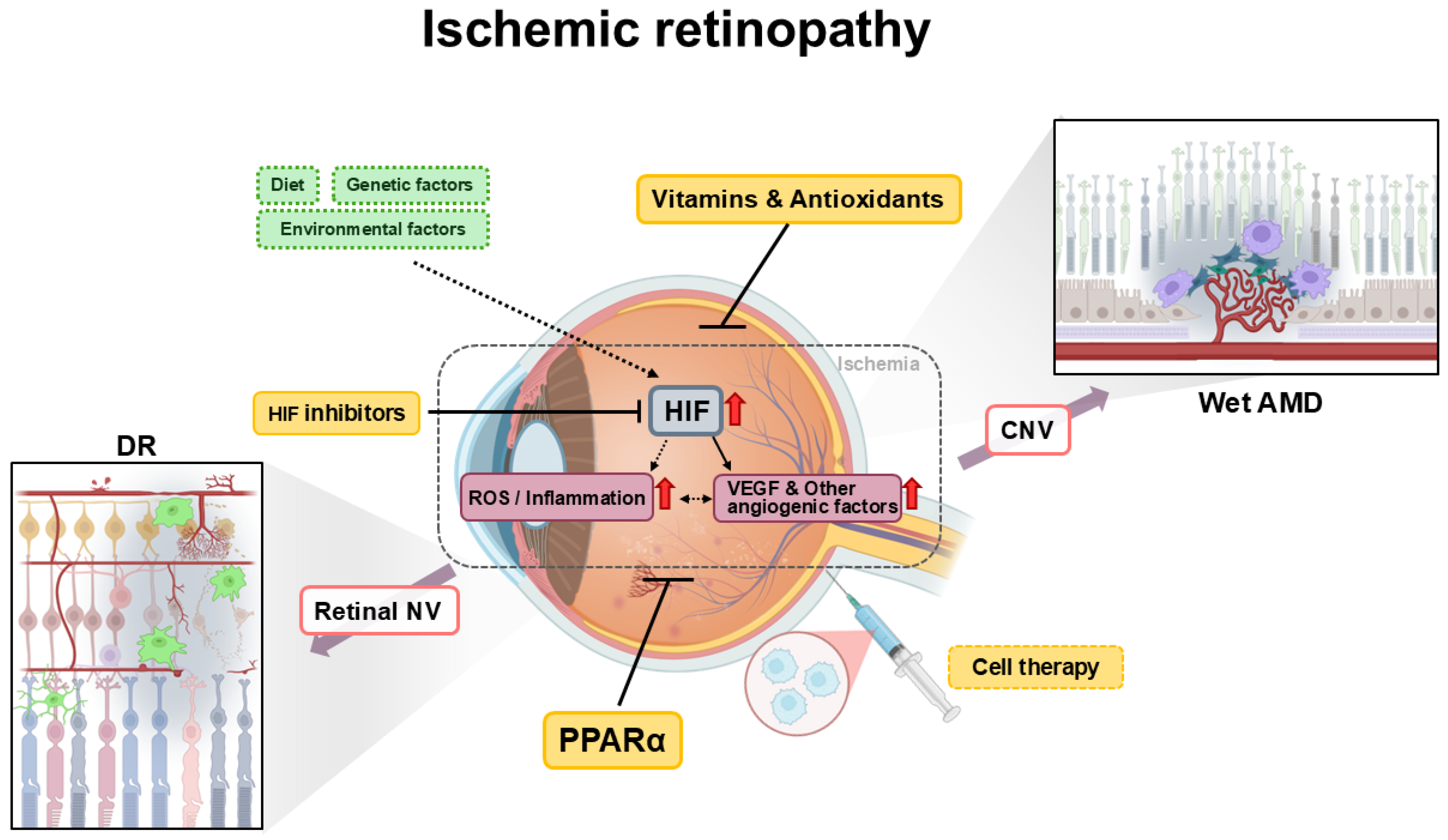
1. Introduction
2. Diabetic Retinopathy
2.1. Pathophysiology
2.2. Therapeutic Approaches
2.2.1. Peroxisome Proliferator-Activator Receptor Alpha
2.2.2. Vitamins
2.2.3. Cell Therapy
2.2.4. Hypoxia-Inducible Factors
3. Age-Related Macular Degeneration
3.1. Pathophysiology
3.2. Therapeutic Approaches
3.2.1. Antioxidants
3.2.2. Peroxisome Proliferator-Activator Receptor Alpha
3.2.3. Cell Therapy
3.2.4. Hypoxia-Inducible Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| PDGFR | Platelet-derived growth factor receptor |
| GA | Geographic atrophy |
| MNV | Macular neovascularization |
| AREDS | Age-Related Eye Disease Study |
| DR | Diabetic retinopathy |
| VEGF | Vascular endothelial growth factor |
| HIF | Hypoxia-inducible factor |
| PPAR | Peroxisome proliferator-activator receptor |
| FGF21 | Fibroblast growth factor 21 |
| OIR | Oxygen-induced retinopathy |
| SPPARMα | Selective peroxisome proliferator-activated receptor alpha modulator |
| SGLT2 | Sodium-glucose cotransporter 2 |
| TGF | Transforming growth factor |
| AGE | Advanced glycation end product |
| PKC | Protein kinase C |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 |
| TNF | Tumor necrosis factor |
| ANG | Angiopoietin |
| STZ | Streptozotocin |
| IL | Interleukin |
| CNV | Choroidal neovascularization |
| ROS | Reactive oxygen species |
References
- Lee, D.; Tomita, Y.; Miwa, Y.; Kunimi, H.; Nakai, A.; Shoda, C.; Negishi, K.; Kurihara, T. Recent Insights into Roles of Hypoxia-Inducible Factors in Retinal Diseases. Int. J. Mol. Sci. 2024, 25, 10140. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.41–45.47.20. [Google Scholar] [CrossRef]
- Lelyte, I.; Ahmed, Z.; Kaja, S.; Kalesnykas, G. Structure-Function Relationships in the Rodent Streptozotocin-Induced Model for Diabetic Retinopathy: A Systematic Review. J. Ocul. Pharmacol. Ther. 2022, 38, 271–286. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef]
- Lam, C.H.; Zou, B.; Chan, H.H.; Tse, D.Y. Functional and structural changes in the neuroretina are accompanied by mitochondrial dysfunction in a type 2 diabetic mouse model. Eye Vis. 2023, 10, 37. [Google Scholar] [CrossRef]
- Han, Z.; Guo, J.; Conley, S.M.; Naash, M.I. Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 574–584. [Google Scholar] [CrossRef]
- Wisniewska-Kruk, J.; Klaassen, I.; Vogels, I.M.; Magno, A.L.; Lai, C.M.; Van Noorden, C.J.; Schlingemann, R.O.; Rakoczy, E.P. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp. Eye Res. 2014, 122, 123–131. [Google Scholar] [CrossRef]
- Lai, A.K.; Lo, A.C. Animal models of diabetic retinopathy: Summary and comparison. J. Diabetes Res. 2013, 2013, 106594. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, A.; Liang, J.; Meng, H.; Chang, B.; Gao, H.; Qiao, X. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s retinal angiomatous proliferation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hellinen, L.; Hagström, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 2019, 9, 13761. [Google Scholar] [CrossRef]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef]
- Souto, E.B.; Campos, J.R.; Da Ana, R.; Martins-Gomes, C.; Silva, A.M.; Souto, S.B.; Lucarini, M.; Durazzo, A.; Santini, A. Ocular Cell Lines and Genotoxicity Assessment. Int. J. Environ. Res. Public Health 2020, 17, 2046. [Google Scholar] [CrossRef]
- Watanabe, S.; Morisaki, N.; Tezuka, M.; Fukuda, K.; Ueda, S.; Koyama, N.; Yokote, K.; Kanzaki, T.; Yoshida, S.; Saito, Y. Cultured retinal pericytes stimulate in vitro angiogenesis of endothelial cells through secretion of a fibroblast growth factor-like molecule. Atherosclerosis 1997, 130, 101–107. [Google Scholar] [CrossRef]
- Lan, X.; Jiang, H.; Wang, Q.; Shiqi, Q.; Xiong, Y. The application of retinal organoids in ophthalmic regenerative medicine: A mini-review. Regen. Ther. 2024, 26, 382–386. [Google Scholar] [CrossRef]
- Scholler, J.; Groux, K.; Goureau, O.; Sahel, J.A.; Fink, M.; Reichman, S.; Boccara, C.; Grieve, K. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci. Appl. 2020, 9, 140. [Google Scholar] [CrossRef]
- Campa, C. New Anti-VEGF Drugs in Ophthalmology. Curr. Drug Targets 2020, 21, 1194–1200. [Google Scholar] [CrossRef]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Kwong, T.Q.; Mohamed, M. Anti-vascular endothelial growth factor therapies in ophthalmology: Current use, controversies and the future. Br. J. Clin. Pharmacol. 2014, 78, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Oishi, A.; Hata, M.; Takahashi, A.; Tsujikawa, A. Visual acuity outcomes of anti-VEGF treatment for neovascular age-related macular degeneration in clinical trials. Jpn. J. Ophthalmol. 2021, 65, 741–760. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Armendariz, B.G.; Fauser, S. 15 years of anti-VEGF treatment for nAMD: Success or failure or something in between? Eye 2022, 36, 2232–2233. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Hwang, J.C.; Del Priore, L.V.; Freund, K.B.; Chang, S.; Iranmanesh, R. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging 2011, 42, 6–11. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J. Neovascular Remodeling and Subretinal Fibrosis as Biomarkers for Predicting Incomplete Response to Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration. Front. Biosci. 2022, 27, 135. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Deschler, E.K.; Sun, J.K.; Silva, P.S. Side-effects and complications of laser treatment in diabetic retinal disease. Semin. Ophthalmol. 2014, 29, 290–300. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Musa, M.; Gagliano, C.; Zeppieri, M. The Treatment of Diabetic Retinal Edema with Intravitreal Steroids: How and When. J. Clin. Med. 2024, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Chen, S.J.; Wu, T.T.; Wu, W.C.; Yang, C.H.; Yang, C.M. Refining vitrectomy for proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3659–3670. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H. Why do patients still require surgery for the late complications of Proliferative Diabetic Retinopathy? Eye 2010, 24, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018, 42, 364–376. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Li, G.; Gao, J.; Ding, P.; Gao, Y. The role of endothelial cell-pericyte interactions in vascularization and diseases. J. Adv. Res. 2025, 67, 269–288. [Google Scholar] [CrossRef]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. 2014, 163, 296–306. [Google Scholar] [CrossRef]
- von Tell, D.; Armulik, A.; Betsholtz, C. Pericytes and vascular stability. Exp. Cell Res. 2006, 312, 623–629. [Google Scholar] [CrossRef]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Kunimi, H.; Ibuki, M.; Shoda, C.; Nakai, A.; Kurihara, T. HIF Inhibition Therapy in Ocular Diseases. Keio J. Med. 2022, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, Y.; Hassanshahi, G.; Kounis, N.G.; Koniari, I.; Khorramdelazad, H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: Latest evidence and clinical considerations. J. Cell Commun. Signal. 2019, 13, 451–462. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Du, J.; Li, X.; Zhao, L.; Long, L.; Li, D.; Lu, S. Tumor necrosis factor-α and diabetic retinopathy: Review and meta-analysis. Clin. Chim. Acta 2018, 485, 210–217. [Google Scholar] [CrossRef]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Shah, J.; Tan, B.; Wong, D.; Abdul Gani, N.F.B.; Hu, Q.; Liu, X.; Chua, J. Evaluation of thickness of individual macular retinal layers in diabetic eyes from optical coherence tomography. Sci. Rep. 2024, 14, 17909. [Google Scholar] [CrossRef]
- Frizziero, L.; Parrozzani, R.; Londei, D.; Pilotto, E.; Midena, E. Quantification of vascular and neuronal changes in the peripapillary retinal area secondary to diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 1577–1583. [Google Scholar] [CrossRef]
- Scott, R.; Best, J.; Forder, P.; Taskinen, M.R.; Simes, J.; Barter, P.; Keech, A. Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: Baseline characteristics and short-term effects of fenofibrate [ISRCTN64783481]. Cardiovasc. Diabetol. 2005, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Dodson, P.M. Medical management of diabetic retinopathy: Fenofibrate and ACCORD Eye studies. Eye 2011, 25, 843–849. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, M.; Ren, J.; Qiu, Y.; Li, S.; Cao, W. Association Between Increased Lipid Profiles and Risk of Diabetic Retinopathy in a Population-Based Case-Control Study. J. Inflamm. Res. 2022, 15, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Nie, Y.; Gong, Z.; Sivaprasad, S.; Fung, A.T.; Wang, Q.; Qiu, B.; Xie, R.; Wang, Y. Circulating level of homocysteine contributes to diabetic retinopathy associated with dysregulated lipid profile and impaired kidney function in patients with type 2 diabetes mellitus. Eye 2023, 37, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V. Lipid metabolism dysregulation in diabetic retinopathy. J. Lipid Res. 2021, 62, 100017. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Allen, W.; Tsubota, K.; Negishi, K.; Kurihara, T. PPARα Modulation-Based Therapy in Central Nervous System Diseases. Life 2021, 11, 1168. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X.; et al. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, R.; Hu, Y.; Takahashi, Y.; Jenkins, A.J.; Keech, A.C.; Humphries, K.M.; Gu, X.; Elliott, M.H.; Xia, X.; et al. Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina. Am. J. Pathol. 2014, 184, 2709–2720. [Google Scholar] [CrossRef]
- Yuan, T.; Dong, L.; Pearsall, E.A.; Zhou, K.; Cheng, R.; Ma, J.X. The Protective Role of Microglial PPARα in Diabetic Retinal Neurodegeneration and Neurovascular Dysfunction. Cells 2022, 11, 3869. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, R.; Ma, X.; Liang, W.; Hong, Y.; Li, H.; Zhou, K.; Du, Y.; Takahashi, Y.; Zhang, X.; et al. Regulation of Monocyte Activation by PPARα Through Interaction With the cGAS-STING Pathway. Diabetes 2023, 72, 958–972. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Negishi, K.; Kurihara, T. Therapeutic roles of PPARα activation in ocular ischemic diseases. Histol. Histopathol. 2023, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liang, W.; Zhou, K.; Wassel, R.A.; Ridge, Z.D.; Ma, J.X.; Wang, B. Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization. Biology 2021, 10, 1328. [Google Scholar] [CrossRef]
- Hanaguri, J.; Nagai, N.; Yokota, H.; Kushiyama, A.; Watanabe, M.; Yamagami, S.; Nagaoka, T. Fenofibrate Nano-Eyedrops Ameliorate Retinal Blood Flow Dysregulation and Neurovascular Coupling in Type 2 Diabetic Mice. Pharmaceutics 2022, 14, 384. [Google Scholar] [CrossRef]
- Preiss, D.; Logue, J.; Sammons, E.; Zayed, M.; Emberson, J.; Wade, R.; Wallendszus, K.; Stevens, W.; Cretney, R.; Harding, S.; et al. Effect of Fenofibrate on Progression of Diabetic Retinopathy. NEJM Evid. 2024, 3, EVIDoa2400179. [Google Scholar] [CrossRef]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef]
- Khan, M.S.; Ghumman, G.M.; Baqi, A.; Shah, J.; Aziz, M.; Mir, T.; Tahir, A.; Katragadda, S.; Singh, H.; Taleb, M.; et al. Efficacy of Pemafibrate Versus Fenofibrate Administration on Serum Lipid Levels in Patients with Dyslipidemia: Network Meta-Analysis and Systematic Review. Am. J. Cardiovasc. Drugs 2023, 23, 547–558. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhou, Y.; Liu, J.; Wang, F.; Zhao, Q. Pemafibrate Tends to have Better Efficacy in Treating Dyslipidemia than Fenofibrate. Curr. Pharm. Des. 2019, 25, 4725–4734. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Negishi, K.; Kurihara, T. Pemafibrate, a potent selective peroxisome proliferator-activated receptor α modulator, a promising novel treatment for ischemic retinopathy? Neural Regen. Res. 2023, 18, 1495–1496. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, Y.; Asahiyama, M.; Yano, W.; Takizawa, T.; Kamiya, W.; Matsumura, Y.; Anai, M.; Osawa, T.; Fruchart, J.C.; et al. Selective PPARα Modulator Pemafibrate and Sodium-Glucose Cotransporter 2 Inhibitor Tofogliflozin Combination Treatment Improved Histopathology in Experimental Mice Model of Non-Alcoholic Steatohepatitis. Cells 2022, 11, 720. [Google Scholar] [CrossRef]
- Kimura, A.; Kamimura, K.; Ohkoshi-Yamada, M.; Shinagawa-Kobayashi, Y.; Goto, R.; Owaki, T.; Oda, C.; Shibata, O.; Morita, S.; Sakai, N.; et al. Effects of a novel selective PPARα modulator, statin, sodium-glucose cotransporter 2 inhibitor, and combinatorial therapy on the liver and vasculature of medaka nonalcoholic steatohepatitis model. Biochem. Biophys Res. Commun. 2022, 596, 76–82. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 inhibitors beyond glycaemic control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Marilly, E.; Cottin, J.; Cabrera, N.; Cornu, C.; Boussageon, R.; Moulin, P.; Lega, J.C.; Gueyffier, F.; Cucherat, M.; Grenet, G. SGLT2 inhibitors in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials balancing their risks and benefits. Diabetologia 2022, 65, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Tielsch, J.M.; Wang, J.J.; Wong, T.Y.; Mitchell, P.; Tano, Y.; Tominaga, M.; Oizumi, T.; Daimon, M.; Kato, T.; et al. The metabolic syndrome and retinal microvascular signs in a Japanese population: The Funagata study. Br. J. Ophthalmol. 2008, 92, 161–166. [Google Scholar] [CrossRef]
- Wong, T.Y.; Duncan, B.B.; Golden, S.H.; Klein, R.; Couper, D.J.; Klein, B.E.; Hubbard, L.D.; Sharrett, A.R.; Schmidt, M.I. Associations between the metabolic syndrome and retinal microvascular signs: The Atherosclerosis Risk In Communities study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2949–2954. [Google Scholar] [CrossRef]
- Gutfreund, S.; Izkhakov, E.; Pokroy, R.; Yaron, M.; Yeshua, H.; Burgansky-Eliash, Z.; Barak, A.; Rubinstein, A. Retinal blood flow velocity in metabolic syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1507–1513. [Google Scholar] [CrossRef]
- Ryu, T.; Chae, S.Y.; Lee, J.; Han, J.W.; Yang, H.; Chung, B.S.; Yang, K. Multivitamin supplementation and its impact in metabolic dysfunction-associated steatotic liver disease. Sci. Rep. 2025, 15, 8675. [Google Scholar] [CrossRef]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kwon, J.W.; Jee, D. The relationship between blood vitamin A levels and diabetic retinopathy: A population-based study. Sci. Rep. 2024, 14, 491. [Google Scholar] [CrossRef]
- Rostamkhani, H.; Mellati, A.A.; Tabaei, B.S.; Alavi, M.; Mousavi, S.N. Association of Serum Zinc and Vitamin A Levels with Severity of Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Study. Biol. Trace Elem. Res. 2019, 192, 123–128. [Google Scholar] [CrossRef]
- Cinici, E.; Dilekmen, N.; Senol, O.; Arpalı, E.; Cinici, O.; Tanas, S. Blood thiamine pyrophosphate concentration and its correlation with the stage of diabetic retinopathy. Int. Ophthalmol. 2020, 40, 3279–3284. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Aida, R.; Kamada, C.; Fujihara, K.; Tanaka, S.; Tanaka, S.; Araki, A.; Yoshimura, Y.; Moriya, T.; Akanuma, Y.; et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: Analysis of data from the Japan Diabetes Complications Study (JDCS). Eur. J. Nutr. 2020, 59, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.; Chen, Y. Mediation of endothelial activation and stress index in the association between vitamin B6 turnover rate and diabetic retinopathy: An analysis of the National Health and Nutrition Examination Survey. Front. Nutr. 2024, 11, 1490340. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Zhang, P.; Jia, X.; Hua, S.; Yao, D. Association of vitamin B6 intake with the risk and prognosis of diabetic retinopathy: A NHANES-based study. Clin. Exp. Optom. 2024, 107, 847–856. [Google Scholar] [CrossRef]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef]
- May, J.M. Ascorbic acid repletion: A possible therapy for diabetic macular edema? Free Radic. Biol. Med. 2016, 94, 47–54. [Google Scholar] [CrossRef]
- Tecilazich, F.; Formenti, A.M.; Giustina, A. Role of vitamin D in diabetic retinopathy: Pathophysiological and clinical aspects. Rev. Endocr. Metab. Disord. 2021, 22, 715–727. [Google Scholar] [CrossRef]
- Ho, J.I.; Ng, E.Y.; Chiew, Y.; Koay, Y.Y.; Chuar, P.F.; Phang, S.C.W.; Ahmad, B.; Kadir, K.A. The effects of vitamin E on non-proliferative diabetic retinopathy in type 2 diabetes mellitus: Are they sustainable with 12 months of therapy. SAGE Open Med. 2022, 10, 20503121221095324. [Google Scholar] [CrossRef]
- Reddy, S.S.; Prabhakar, Y.K.; Kumar, C.U.; Reddy, P.Y.; Reddy, G.B. Effect of vitamin B12 supplementation on retinal lesions in diabetic rats. Mol. Vis. 2020, 26, 311–325. [Google Scholar]
- Wang, X.D.; Kashii, S.; Zhao, L.; Tonchev, A.B.; Katsuki, H.; Akaike, A.; Honda, Y.; Yamashita, J.; Yamashima, T. Vitamin B6 protects primate retinal neurons from ischemic injury. Brain Res. 2002, 940, 36–43. [Google Scholar] [CrossRef]
- Mehta, R.; Dedina, L.; O’Brien, P.J. Rescuing hepatocytes from iron-catalyzed oxidative stress using vitamins B1 and B6. Toxicol. Vitr. 2011, 25, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; Kolaja, K.; Petersen, T.; Weber, K.; McVean, M.; Funk, K.A. Stem Cell Research. Int. J. Toxicol. 2015, 34, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Dezawa, M. A new age of regenerative medicine: Fusion of tissue engineering and stem cell research. Anat. Rec. 2014, 297, 4–5. [Google Scholar] [CrossRef]
- Rong, L.; Wei, W.; Fang, Y.; Liu, Y.; Gao, T.; Wang, L.; Hao, J.; Gu, X.; Wu, J.; Wu, W. Clinical-grade human embryonic stem cell-derived mesenchymal stromal cells ameliorate diabetic retinopathy in db/db mice. Cytotherapy 2024, 26, 606–615. [Google Scholar] [CrossRef]
- Cheung, K.W.; Yazdanyar, A.; Dolf, C.; Cary, W.; Marsh-Armstrong, N.; Nolta, J.A.; Park, S.S. Analysis of the retinal capillary plexus layers in a murine model with diabetic retinopathy: Effect of intravitreal injection of human CD34+ bone marrow stem cells. Ann. Transl. Med. 2021, 9, 1273. [Google Scholar] [CrossRef]
- Scalinci, S.Z.; Scorolli, L.; Corradetti, G.; Domanico, D.; Vingolo, E.M.; Meduri, A.; Bifani, M.; Siravo, D. Potential role of intravitreal human placental stem cell implants in inhibiting progression of diabetic retinopathy in type 2 diabetes: Neuroprotective growth factors in the vitreous. Clin. Ophthalmol. 2011, 5, 691–696. [Google Scholar] [CrossRef]
- Harding, J.; Roberts, R.M.; Mirochnitchenko, O. Large animal models for stem cell therapy. Stem Cell Res. Ther. 2013, 4, 23. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen homeostasis. Wiley Interdiscip Rev. Syst. Biol. Med. 2010, 2, 336–361. [Google Scholar] [CrossRef]
- Wert, K.J.; Mahajan, V.B.; Zhang, L.; Yan, Y.; Li, Y.; Tosi, J.; Hsu, C.W.; Nagasaki, T.; Janisch, K.M.; Grant, M.B.; et al. Neuroretinal hypoxic signaling in a new preclinical murine model for proliferative diabetic retinopathy. Signal Transduct. Target. Ther. 2016, 1, 16005. [Google Scholar] [CrossRef]
- Lim, J.I.; Spee, C.; Hinton, D.R. A comparison of hypoxia-inducible factor-α in surgically excised neovascular membranes of patients with diabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina 2010, 30, 1472–1478. [Google Scholar] [CrossRef]
- Mazzeo, A.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M.; Beltramo, E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp. Eye Res. 2019, 184, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, J.; Ayuso, E.; Navarro, M.; Carretero, A.; Nacher, V.; Haurigot, V.; George, M.; Llombart, C.; Casellas, A.; Costa, C.; et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J. Clin. Investig. 2004, 113, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Tobe, T.; Hackett, S.F.; Ozaki, H.; Vinores, M.A.; LaRochelle, W.; Zack, D.J.; Campochiaro, P.A. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: A new model of intraretinal and subretinal neovascularization. Am. J. Pathol. 1997, 151, 281–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis. Model. Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef]
- Modrzejewska, M.; Zdanowska, O.; Połubiński, P. The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature. J. Clin. Med. 2024, 13, 4034. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Aguilar, E.; Murinello, S.; Prins, M.; Gantner, M.L.; Wright, P.E.; Berlow, R.B.; Friedlander, M. An allosteric peptide inhibitor of HIF-1α regulates hypoxia-induced retinal neovascularization. Proc. Natl. Acad. Sci. USA 2020, 117, 28297–28306. [Google Scholar] [CrossRef]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef]
- Yoshida, T.; Zhang, H.; Iwase, T.; Shen, J.; Semenza, G.L.; Campochiaro, P.A. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010, 24, 1759–1767. [Google Scholar] [CrossRef]
- Zeng, M.; Shen, J.; Liu, Y.; Lu, L.Y.; Ding, K.; Fortmann, S.D.; Khan, M.; Wang, J.; Hackett, S.F.; Semenza, G.L.; et al. The HIF-1 antagonist acriflavine: Visualization in retina and suppression of ocular neovascularization. J. Mol. Med. 2017, 95, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sharma, D.; Dinabandhu, A.; Sanchez, J.; Applewhite, B.; Jee, K.; Deshpande, M.; Flores-Bellver, M.; Hu, M.W.; Guo, C.; et al. Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, A.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Retinal Diseases Regulated by Hypoxia-Basic and Clinical Perspectives: A Comprehensive Review. J. Clin. Med. 2021, 10, 5496. [Google Scholar] [CrossRef]
- Mares, J.A.; Voland, R.P.; Sondel, S.A.; Millen, A.E.; Larowe, T.; Moeller, S.M.; Klein, M.L.; Blodi, B.A.; Chappell, R.J.; Tinker, L.; et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 470–480. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Chen, M.; Cao, Y.; Lu, W.; Li, X. The retinal pigment epithelium: Functions and roles in ocular diseases. Fundam. Res. 2024, 4, 1710–1718. [Google Scholar] [CrossRef]
- Lee, K.S.; Lin, S.; Copland, D.A.; Dick, A.D.; Liu, J. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J. Neuroinflammation 2021, 18, 32. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Agrón, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow Geographic Atrophy Progression to the Fovea in Age-Related Macular Degeneration. Ophthalmology 2025, 132, 14–29. [Google Scholar] [CrossRef]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Tudor, C.; Pintea, A. A Brief Overview of Dietary Zeaxanthin Occurrence and Bioaccessibility. Molecules 2020, 25, 4067. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A. Zeaxanthin: Review of Toxicological Data and Acceptable Daily Intake. J. Ophthalmol. 2016, 2016, 3690140. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Li, B.; Blount, J.D.; Nwagbo, U.; Kim, H.J.; Sparrow, J.R.; Bernstein, P.S. Lutein and zeaxanthin reduce A2E and iso-A2E levels and improve visual performance in Abca4−/−/Bco2−/− double knockout mice. Exp. Eye Res. 2021, 209, 108680. [Google Scholar] [CrossRef]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef]
- Parmar, U.P.S.; Surico, P.L.; Mori, T.; Singh, R.B.; Cutrupi, F.; Premkishore, P.; Gallo Afflitto, G.; Di Zazzo, A.; Coassin, M.; Romano, F. Antioxidants in Age-Related Macular Degeneration: Lights and Shadows. Antioxidants 2025, 14, 152. [Google Scholar] [CrossRef]
- Gong, Y.; Shao, Z.; Fu, Z.; Edin, M.L.; Sun, Y.; Liegl, R.G.; Wang, Z.; Liu, C.H.; Burnim, S.B.; Meng, S.S.; et al. Fenofibrate Inhibits Cytochrome P450 Epoxygenase 2C Activity to Suppress Pathological Ocular Angiogenesis. eBioMedicine 2016, 13, 201–211. [Google Scholar] [CrossRef]
- Zhao, J.F.; Hua, H.R.; Chen, Q.B.; Guan, M.; Yang, J.H.; Xi, X.T.; Li, Y.; Geng, Y. Impact of fenofibrate on choroidal neovascularization formation and VEGF-C plus VEGFR-3 in Brown Norway rats. Exp. Eye Res. 2018, 174, 152–160. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, N.; Zhang, Y.; Ye, S.; Liang, X.; Wang, X.; Lin, X.; Zong, R.; Chen, H.; Liu, Z. Fenofibrate Inhibits Subretinal Fibrosis Through Suppressing TGF-β-Smad2/3 signaling and Wnt signaling in Neovascular Age-Related Macular Degeneration. Front. Pharmacol. 2020, 11, 580884. [Google Scholar] [CrossRef]
- Tenbrock, L.; Wolf, J.; Boneva, S.; Schlecht, A.; Agostini, H.; Wieghofer, P.; Schlunck, G.; Lange, C. Subretinal fibrosis in neovascular age-related macular degeneration: Current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022, 387, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Nakai, A.; Miwa, Y.; Negishi, K.; Tomita, Y.; Kurihara, T. Pemafibrate prevents choroidal neovascularization in a mouse model of neovascular age-related macular degeneration. PeerJ 2023, 11, e14611. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gong, Y.; Liegl, R.; Wang, Z.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Saba, N.J.; Fredrick, T.W.; Morss, P.C.; et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Rep. 2017, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Schneeberger, M.; Fan, W.; Bugde, A.; Gautron, L.; Vale, K.; Hammer, R.E.; Zhang, Y.; Friedman, J.M.; Mangelsdorf, D.J.; et al. FGF21 counteracts alcohol intoxication by activating the noradrenergic nervous system. Cell Metab. 2023, 35, 429–437.e425. [Google Scholar] [CrossRef]
- Kang, K.; Xu, P.; Wang, M.; Chunyu, J.; Sun, X.; Ren, G.; Xiao, W.; Li, D. FGF21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress. Biomed. Pharmacother. 2020, 129, 110439. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, H.; Ni, Z.; Shen, Y.; Wang, D.; Li, W.; Zhao, L.; Li, C.; Gao, H. Fibroblast growth factor 21 alleviates diabetes-induced cognitive decline. Cereb. Cortex. 2024, 34. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Deng, L.; Du, J.; Fan, Z.; Ma, T.; Xiong, J.; Xiuyun, X.; Gu, N.; Di, Z.; et al. FGF21 attenuates neuroinflammation following subarachnoid hemorrhage through promoting mitophagy and inhibiting the cGAS-STING pathway. J. Transl. Med. 2024, 22, 436. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflammation 2020, 17, 257. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Zhang, Z.; Ma, B.; Sun, S.; Gao, L.; Gao, G. FGF21 alleviates endothelial mitochondrial damage and prevents BBB from disruption after intracranial hemorrhage through a mechanism involving SIRT6. Mol. Med. 2023, 29, 165. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Lee, D.; Miwa, Y.; Yamagami, S.; Nakashizuka, H.; Nimura, K.; Okamoto, K.; Kawagishi, H.; Negishi, K.; Kurihara, T. Inhibition of hypoxia-inducible factors suppresses subretinal fibrosis. FASEB J. 2024, 38, e23792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Lau, E.; Qin, Y.; Jee, K.; Rodrigues, M.; Guo, C.; Dinabandhu, A.; McIntyre, E.; Salman, S.; Hwang, Y.; et al. VEGF inhibition increases expression of HIF-regulated angiogenic genes by the RPE limiting the response of wet AMD eyes to aflibercept. Proc. Natl. Acad. Sci. USA 2024, 121, e2322759121. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Qin, Y.; Flores-Bellver, M.; Niu, Y.; Bhutto, I.A.; Aparicio-Domingo, S.; Guo, C.; Rodrigues, M.; Domashevich, T.; Deshpande, M.; et al. Pathologic vs. protective roles of hypoxia-inducible factor 1 in RPE and photoreceptors in wet vs. dry age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2302845120. [Google Scholar] [CrossRef]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Gawryluk, A.; Cybulska-Klosowicz, A.; Charzynska, A.; Zakrzewska, R.; Sobolewska, A.; Kossut, M.; Liguz-Lecznar, M. Mitigation of aging-related plasticity decline through taurine supplementation and environmental enrichment. Sci. Rep. 2024, 14, 19546. [Google Scholar] [CrossRef]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. [Google Scholar] [CrossRef]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Lactoferrin Has a Therapeutic Effect via HIF Inhibition in a Murine Model of Choroidal Neovascularization. Front. Pharmacol. 2020, 11, 174. [Google Scholar] [CrossRef]
- Iwase, T.; Fu, J.; Yoshida, T.; Muramatsu, D.; Miki, A.; Hashida, N.; Lu, L.; Oveson, B.; Lima e Silva, R.; Seidel, C.; et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J. Control. Release 2013, 172, 625–633. [Google Scholar] [CrossRef]
- Hackett, S.F.; Fu, J.; Kim, Y.C.; Tsujinaka, H.; Shen, J.; Lima, E.S.R.; Khan, M.; Hafiz, Z.; Wang, T.; Shin, M.; et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials 2020, 243, 119935. [Google Scholar] [CrossRef] [PubMed]
- Barben, M.; Ail, D.; Storti, F.; Klee, K.; Schori, C.; Samardzija, M.; Michalakis, S.; Biel, M.; Meneau, I.; Blaser, F.; et al. Hif1a inactivation rescues photoreceptor degeneration induced by a chronic hypoxia-like stress. Cell Death Differ. 2018, 25, 2071–2085. [Google Scholar] [CrossRef] [PubMed]
- Kast, B.; Schori, C.; Grimm, C. Hypoxic preconditioning protects photoreceptors against light damage independently of hypoxia inducible transcription factors in rods. Exp. Eye Res. 2016, 146, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Thiersch, M.; Lange, C.; Joly, S.; Heynen, S.; Le, Y.Z.; Samardzija, M.; Grimm, C. Retinal neuroprotection by hypoxic preconditioning is independent of hypoxia-inducible factor-1 alpha expression in photoreceptors. Eur. J. Neurosci. 2009, 29, 2291–2302. [Google Scholar] [CrossRef]
- Thiersch, M.; Raffelsberger, W.; Frigg, R.; Samardzija, M.; Wenzel, A.; Poch, O.; Grimm, C. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genom. 2008, 9, 73. [Google Scholar] [CrossRef]
- Chandra, S.; Tan, E.Y.; Empeslidis, T.; Sivaprasad, S. Tyrosine Kinase Inhibitors and their role in treating neovascular age-related macular degeneration and diabetic macular oedema. Eye 2023, 37, 3725–3733. [Google Scholar] [CrossRef]
| Treatment | Advantages | Disadvantages; Limitations |
|---|---|---|
| Hypoxia-inducible factor (HIF) inhibition | Strong efficacy in inhibiting diverse inflammatory cytokines and angiogenic factors including VEGF; modulation of HIF-mediated apoptosis; selective targeting of pathologic HIF expression under hypoxic conditions | Side effects for systemic HIF inhibition; a lack of capability of prolonged drug release for the long-term effects |
| Peroxisome proliferator-activator receptor alpha (PPARα) activation | Improvements of lipid metabolism under systemic metabolic dysregulation; anti-inflammation and anti-vascular damage; potent neuroprotective effects via the FGF21/PPARα pathway | Uncertainty about the direct or indirect therapeutic effects; a lack of identification of the target cell type in the eye under the disease condition |
| Cell therapy | Ocular protection as well as regeneration/replacement; the potential for personalized medicine | limited availability and accessibility; unexpected complications; a lack of experimental evidence; ethical issue; high cost |
| Antioxidants (lutein, zeaxanthin, and vitamins) | Strong efficacy to enzymatically scavenge ROS; direct reduction of oxidative stress-mediated retinal cell death or dysfunction; anti-inflammation under oxidative stress conditions | Potential interference with important physiologic functions in cells; the suitable antioxidants that remain unknown, depending on the disease states |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, S.J.; Lee, J. Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration. Vision 2025, 9, 35. https://doi.org/10.3390/vision9020035
Lee D, Kim SJ, Lee J. Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration. Vision. 2025; 9(2):35. https://doi.org/10.3390/vision9020035
Chicago/Turabian StyleLee, Deokho, Soo Jin Kim, and Junyeop Lee. 2025. "Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration" Vision 9, no. 2: 35. https://doi.org/10.3390/vision9020035
APA StyleLee, D., Kim, S. J., & Lee, J. (2025). Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration. Vision, 9(2), 35. https://doi.org/10.3390/vision9020035







