Minimally Invasive Glaucoma Surgery: A Review of the Literature
Abstract
:1. Introduction
1.1. Terminology
1.2. Advantages and Limitations
2. Literature Search Details
Indications and Contraindications
3. Devices, Procedures, and Surgical Techniques
3.1. Trabectome and Kahook Dual Blade
3.2. iStent and iStent Inject
3.3. High-Frequency Deep Sclerotomy
3.4. TRAB360, OMNI, Streamline, and ABiC
3.5. Hydrus Microstent
3.6. Gonioscopy-Assisted Transluminal Trabeculotomy (GATT)
3.7. iStent Supra and CyPass
3.8. EX-PRESS Glaucoma Filtration Device, Xen Gel Stent, and PreserFlo MicroShunt
4. Postoperative Course and Outcomes
5. Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavia, C.; Dallorto, L.; Maule, M.; Ceccarelli, M.; Fea, A.M. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0183142. [Google Scholar] [CrossRef] [PubMed]
- SooHoo, J.R.; Seibold, L.K.; Radcliffe, N.M.; Kahook, M.Y. Minimally invasive glaucoma surgery: Current implants and future innovations. Can. J. Ophthalmol. 2014, 49, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.J.; Buys, Y.M. Minimally invasive glaucoma surgery: A critical appraisal of the literature. Annu. Rev. Vis. Sci. 2020, 6, 47–89. [Google Scholar] [CrossRef] [PubMed]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2017, 52, 114–124. [Google Scholar] [CrossRef]
- Ahmed, I.I.K. MIGS and the FDA: What’s in a Name? Ophthalmology 2015, 122, 1737–1739. [Google Scholar] [CrossRef]
- Bloom, P.; Au, L. “Minimally Invasive Glaucoma Surgery (MIGS) Is a Poor Substitute for Trabeculectomy”—The Great Debate. Ophthalmol. Ther. 2018, 7, 203–210. [Google Scholar] [CrossRef]
- Vijaya, L.; Manish, P.; Ronnie, G.; Shantha, B. Management of complications in glaucoma surgery. Indian J. Ophthalmol. 2011, 59, S131–S140. [Google Scholar] [CrossRef]
- Gedde, S.J.; Chen, P.P.; Heuer, D.K.; Singh, K.; Wright, M.M.; Feuer, W.J.; Schiffman, J.C.; Shi, W.; Group, P.T.V.T.S. The primary tube versus trabeculectomy study: Methodology of a multicenter randomized clinical trial comparing tube shunt surgery and trabeculectomy with mitomycin C. Ophthalmology 2018, 125, 774–781. [Google Scholar] [CrossRef]
- Yook, E.; Vinod, K.; Panarelli, J.F. Complications of micro-invasive glaucoma surgery. Curr. Opin. Ophthalmol. 2018, 29, 147–154. [Google Scholar] [CrossRef]
- Bicket, A.K.; Le, J.T.; Azuara-Blanco, A.; Gazzard, G.; Wormald, R.; Bunce, C.; Hu, K.; Jayaram, H.; King, A.; Otárola, F. Minimally invasive glaucoma surgical techniques for open-angle glaucoma: An overview of cochrane systematic reviews and network meta-analysis. JAMA Ophthalmol. 2021, 139, 983–989. [Google Scholar] [CrossRef]
- Agrawal, P.; Bradshaw, S.E. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol. Ther. 2018, 7, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Porter, A.J.; Vincent, R.A.; Makk, J.; Vincent, S.J. Combined phacoemulsification and microinvasive glaucoma surgery in comparison to phacoemulsification alone for open angle glaucoma. Eye 2020, 34, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.K.; Gupta, S.; Chachanidze, M.; Hall, N.; Chang, T.C.; Valle, S.-D. Safety and efficacy of microinvasive glaucoma surgery with cataract extraction in patients with normal-tension glaucoma. Sci. Rep. 2021, 11, 8910. [Google Scholar] [CrossRef] [PubMed]
- Young, C.E.C.; Seibold, L.K.; SooHoo, J.R.; Kahook, M.Y. Microinvasive Glaucoma Surgeries and When to Use Them. Adv. Ophthalmol. Optom. 2019, 4, 223–243. [Google Scholar] [CrossRef]
- Minckler, D.S.; Baerveldt, G.; Alfaro, M.R.; Francis, B.A. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology 2005, 112, 962–967. [Google Scholar] [CrossRef]
- Dorairaj, S.K.; Seibold, L.K.; Radcliffe, N.M.; Aref, A.A.; Jimenez-Román, J.; Lazcano-Gomez, G.S.; Darlington, J.K.; Mansouri, K.; Berdahl, J.P. 12-month outcomes of goniotomy performed using the kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv. Ther. 2018, 35, 1460–1469. [Google Scholar] [CrossRef]
- Francis, B.A.; See, R.F.; Rao, N.A.; Minckler, D.S.; Baerveldt, G. Ab interno trabeculectomy: Development of a novel device (Trabectome™) and surgery for open-angle glaucoma. J. Glaucoma 2006, 15, 68–73. [Google Scholar] [CrossRef]
- Jordan, J.F.; Wecker, T.; van Oterendorp, C.; Anton, A.; Reinhard, T.; Boehringer, D.; Neuburger, M. Trabectome surgery for primary and secondary open angle glaucomas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2753–2760. [Google Scholar] [CrossRef]
- Maeda, M.; Watanabe, M.; Ichikawa, K. Evaluation of trabectome in open-angle glaucoma. J. Glaucoma 2013, 22, 205–208. [Google Scholar] [CrossRef]
- Seibold, L.K.; SooHoo, J.R.; Ammar, D.A.; Kahook, M.Y. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am. J. Ophthalmol. 2013, 155, 524–529.e2. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Gallardo, M.J.; ElMallah, M.K.; Williamson, B.K.; Kahook, M.Y.; Mahootchi, A.; Rappaport, L.A.; Lazcano-Gomez, G.S.; Díaz-Robles, D.; Dorairaj, S.K. Six-month outcomes of goniotomy performed with the Kahook dual blade as a stand-alone glaucoma procedure. Adv. Ther. 2018, 35, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Alahmadi, M.W.; Alahmadi, M.; AlMuzaini, A.; AlMohammadi, M. The safety of the Kahook dual blade in the surgical treatment of glaucoma. Cureus 2020, 12, e6682. [Google Scholar] [CrossRef] [PubMed]
- Fliney, G.D.; Kim, E.; Sarwana, M.; Wong, S.; Tai, T.Y.T.; Liu, J.; Sarrafpour, S.; Chadha, N.; Teng, C.C. Kahook Dual Blade versus Trabectome (KVT): Comparing Outcomes in Combination with Cataract Surgery. Clin. Ophthalmol. 2023, 17, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Okeke, C.O.; Miller-Ellis, E.; Rojas, M. Trabectome success factors. Medicine 2017, 96, e7061. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Kakimoto, H.; Orii, Y.; Arimura, S.; Takamura, Y.; Inatani, M. Long-term outcomes of a Kahook Dual Blade procedure combined with phacoemulsification in Japanese patients with open-angle glaucoma. J. Clin. Med. 2022, 11, 1354. [Google Scholar] [CrossRef]
- Nichamin, L.D. Glaukos iStent® trabecular micro-bypass. Middle East Afr. J. Ophthalmol. 2009, 16, 138. [Google Scholar] [CrossRef]
- Shalaby, W.S.; Jia, J.; Katz, L.J.; Lee, D. iStent inject: Comprehensive review. J. Cataract Refract. Surg. 2021, 47, 385–399. [Google Scholar] [CrossRef]
- Malvankar-Mehta, M.S.; Chen, Y.N.; Iordanous, Y.; Wang, W.W.; Costella, J.; Hutnik, C.M. iStent as a solo procedure for glaucoma patients: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0128146. [Google Scholar] [CrossRef]
- Arriola-Villalobos, P.; Martínez-de-la-Casa, J.M.; Díaz-Valle, D.; Fernández-Pérez, C.; García-Sánchez, J.; García-Feijoó, J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br. J. Ophthalmol. 2012, 96, 645–649. [Google Scholar] [CrossRef]
- Hooshmand, J.; Rothschild, P.; Allen, P.; Kerr, N.M.; Vote, B.J.; Toh, T.Y. Minimally invasive glaucoma surgery: Comparison of iStent with iStent inject in primary open angle glaucoma. Clin. Exp. Ophthalmol. 2019, 47, 898–903. [Google Scholar] [CrossRef]
- Shalaby, W.S.; Lam, S.S.; Arbabi, A.; Myers, J.S.; Moster, M.R.; Kolomeyer, N.N.; Razeghinejad, R.; Shukla, A.G.; Hussein, T.R.; Eid, T.M. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J. Ophthalmol. 2021, 69, 2488. [Google Scholar] [CrossRef] [PubMed]
- Abushanab, M.M.I.; El-Shiaty, A.; El-Beltagi, T.; Hassan Salah, S. The efficacy and safety of high-frequency deep sclerotomy in treatment of chronic open-angle glaucoma patients. BioMed Res. Int. 2019, 2019, 1850141. [Google Scholar] [CrossRef] [PubMed]
- Pajic, B.; Cvejic, Z.; Mansouri, K.; Resan, M.; Allemann, R. High-frequency deep sclerotomy, a minimal invasive Ab interno glaucoma procedure combined with cataract surgery: Physical properties and clinical outcome. Appl. Sci. 2019, 10, 218. [Google Scholar] [CrossRef]
- Pajic, B.; Pallas, G.; Heinrich, G.; Böhnke, M. A novel technique of ab interno glaucoma surgery: Follow-up results after 24 months. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 22–27. [Google Scholar] [CrossRef]
- Kontic, M.; Todorovic, D.; Zecevic, R.; Vulovic, T.S. High-Frequency Deep Sclerotomy as Adjunctive Therapy in Open-Angle Glaucoma Patients. Ophthalmic Res. 2023, 66, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, S.R.; Mathews, B.; Ding, K.; Patel, A.; Nicek, Z. 360 ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin. Ophthalmol. 2019, 13, 161. [Google Scholar] [CrossRef]
- Areaux, R.G., Jr.; Grajewski, A.L.; Balasubramaniam, S.; Brandt, J.D.; Jun, A.; Edmunds, B.; Shyne, M.T.; Bitrian, E. Trabeculotomy ab interno with the Trab360 device for childhood glaucomas. Am. J. Ophthalmol. 2020, 209, 178–186. [Google Scholar] [CrossRef]
- Vold, S.D.; Williamson, B.K.; Hirsch, L.; Aminlari, A.E.; Cho, A.S.; Nelson, C.; Dickerson, J.E., Jr. Canaloplasty and trabeculotomy with the OMNI system in pseudophakic patients with open-angle glaucoma: The ROMEO study. Ophthalmol. Glaucoma 2021, 4, 173–181. [Google Scholar] [CrossRef]
- Klabe, K.; Kaymak, H. Standalone trabeculotomy and viscodilation of Schlemm’s canal and collector channels in open-angle glaucoma using the OMNI surgical system: 24-month outcomes. Clin. Ophthalmol. 2021, 15, 3121–3129. [Google Scholar] [CrossRef]
- Lazcano-Gomez, G.; Garg, S.J.; Yeu, E.; Kahook, M.Y. Interim analysis of STREAMLINE® surgical system clinical outcomes in eyes with glaucoma. Clin. Ophthalmol. 2022, 16, 1313–1320. [Google Scholar] [CrossRef]
- Körber, N. Ab interno canaloplasty for the treatment of glaucoma: A case series study. Spektrum Der Augenheilkd. 2018, 32, 223. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.J.; Supnet, R.A.; Ahmed, I.I.K. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin. Ophthalmol. 2018, 12, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Davids, A.-M.; Pahlitzsch, M.; Boeker, A.; Winterhalter, S.; Maier-Wenzel, A.-K.; Klamann, M. Ab interno canaloplasty (ABiC)—12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1947–1953. [Google Scholar] [CrossRef]
- Laspas, P.; Pfeiffer, N. Hydrus Microstent. In Minimally Invasive Glaucoma Surgery; Springer: Singapore, 2021; pp. 59–71. [Google Scholar]
- Samet, S.; Ong, J.A.; Ahmed, I.I.K. Hydrus microstent implantation for surgical management of glaucoma: A review of design, efficacy and safety. Eye Vis. 2019, 6, 32. [Google Scholar] [CrossRef]
- Ahmed, I.I.K.; De Francesco, T.; Rhee, D.; McCabe, C.; Flowers, B.; Gazzard, G.; Samuelson, T.W.; Singh, K.; HORIZON Investigators. Long-term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology 2022, 129, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.I.K.; Rhee, D.J.; Jones, J.; Singh, I.P.; Radcliffe, N.; Gazzard, G.; Samuelson, T.W.; Ong, J.; Singh, K.; HORIZON Investigators. Three-year findings of the HORIZON trial: A Schlemm canal microstent for pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology 2021, 128, 857–865. [Google Scholar] [CrossRef]
- Salimi, A.; Kassem, R.; Santhakumaran, S.; Harasymowycz, P. Three-Year Outcomes of a Schlemm Canal Microstent (Hydrus Microstent) with Concomitant Phacoemulsification in Open-Angle Glaucoma. Ophthalmol. Glaucoma 2023, 6, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.P.; Clement, C.I.; Nguyen, V.; Healey, P.R.; Lim, R.; White, A.; Yuen, J.; Lawlor, M. Comparative study of 2-year outcomes for Hydrus or iStent inject microinvasive glaucoma surgery implants with cataract surgery. Clin. Exp. Ophthalmol. 2022, 50, 303–311. [Google Scholar] [CrossRef]
- Coventon, J.; Cronin, B. The Hydrus Microstent in Pseudophakic Patients With Medically Refractory Open-angle Glaucoma. J. Glaucoma 2021, 30, 192–194. [Google Scholar] [CrossRef]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; de Oca, I.M.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [CrossRef]
- Aboalazayem, F.; Elhusseiny, A.M.; El Sayed, Y.M. Gonioscopy-Assisted Transluminal Trabeculotomy: A Review. Curr. Eye Res. 2023, 48, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Y.; Qi, X.-H.; Qi, J.-M. Systematic review and meta-analysis of treating open angle glaucoma with gonioscopy-assisted transluminal trabeculotomy. Int. J. Ophthalmol. 2020, 13, 317. [Google Scholar] [CrossRef]
- Sharkawi, E.; Lindegger, D.J.; Artes, P.H.; Lehmann-Clarke, L.; El Wardani, M.; Misteli, M.; Pasquier, J.; Guarnieri, A. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br. J. Ophthalmol. 2021, 105, 977–982. [Google Scholar] [CrossRef]
- Ćwiklińska-Haszcz, A.; Żarnowski, T.; Wróbel-Dudzińska, D.; Kosior-Jarecka, E. The Efficacy and Safety of the GATT Procedure in Open-Angle Glaucoma—6-Month Results. Int. J. Environ. Res. Public Health 2023, 20, 2759. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy (GATT): Thermal suture modification with a dye-stained rounded tip. J. Glaucoma 2016, 25, 501–504. [Google Scholar] [CrossRef]
- Hill, R.A.; Haffner, D.; Voskanyan, L. The iStent® MIGS Family: iStent®, iStent Inject®, and iStent Supra®. In Surgical Innovations in Glaucoma; Springer: New York, NY, USA, 2014; pp. 147–156. [Google Scholar]
- Junemann, A. Twelve-month outcomes following ab interno implantation of suprachoroidal stent and postoperative administration of travoprost to treat open angle glaucoma. In Proceedings of the 31st Congress of the European Society of Cataract and Refractive Surgeons, Amsterdam, The Netherlands, 5 October 2013. [Google Scholar]
- Meyers, J.; Katz, L. Open angle glaucoma treated with a suprachoroidal stent and topical travoprost. In Proceedings of the 23rd Annual American Glaucoma Society Meeting, San Francisco, CA, USA, 28 February–3 March 2013. [Google Scholar]
- Saheb, H.; Ahmed, I.I.K. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hoeh, H.; Vold, S.D.; Ahmed, I.K.; Anton, A.; Rau, M.; Singh, K.; Chang, D.F.; Shingleton, B.J.; Ianchulev, T. Initial clinical experience with the CyPass micro-stent: Safety and surgical outcomes of a novel supraciliary microstent. J. Glaucoma 2016, 25, 106–112. [Google Scholar] [CrossRef]
- Reiss, G.; Clifford, B.; Vold, S.; He, J.; Hamilton, C.; Dickerson, J.; Lane, S. Safety and effectiveness of CyPass supraciliary micro-stent in primary open-angle glaucoma: 5-year results from the COMPASS XT study. Am. J. Ophthalmol. 2019, 208, 219–225. [Google Scholar] [CrossRef]
- Lass, J.H.; Benetz, B.A.; He, J.; Hamilton, C.; Von Tress, M.; Dickerson, J.; Lane, S. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass Micro-Stent. Am. J. Ophthalmol. 2019, 208, 211–218. [Google Scholar] [CrossRef]
- Green, W.; Lind, J.T.; Sheybani, A. Review of the Xen gel stent and InnFocus MicroShunt. Curr. Opin. Ophthalmol. 2018, 29, 162–170. [Google Scholar] [CrossRef]
- Salim, S. Ex-PRESS glaucoma filtration device—Surgical technique and outcomes. Int. Ophthalmol. Clin. 2011, 51, 83–94. [Google Scholar] [CrossRef]
- Salim, S. The role of the Ex-PRESS glaucoma filtration device in glaucoma surgery. Semin. Ophthalmol. 2013, 28, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.E.; Netland, P.A. EX-PRESS Glaucoma Filtration Device: Efficacy, safety, and predictability. Med. Devices Evid. Res. 2015, 8, 381–388. [Google Scholar]
- Netland, P.A.; Sarkisian, S.R., Jr.; Moster, M.R.; Ahmed, I.I.K.; Condon, G.; Salim, S.; Sherwood, M.B.; Siegfried, C.J. Randomized, prospective, comparative trial of EX-PRESS glaucoma filtration device versus trabeculectomy (XVT study). Am. J. Ophthalmol. 2014, 157, 433–440.e3. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.; Lafuma, A.; Aguadé, A.-S.; Berdeaux, G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin. Ophthalmol. 2011, 5, 527–533. [Google Scholar]
- Dahan, E.; Ben Simon, G.; Lafuma, A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: A prospective, randomised study. Eye 2012, 26, 703–710. [Google Scholar] [CrossRef]
- Khouri, A.S.; Khan, M.N.; Fechtner, R.D.; Vold, S.D. Technique for removal of malpositioned Ex-PRESS glaucoma device. J. Glaucoma 2014, 23, 435–436. [Google Scholar] [CrossRef]
- Tavolato, M.; Babighian, S.; Galan, A. Spontaneous Extrusion of a Stainless Steel Glaucoma Drainage Implant (Ex-PRESS); SAGE Publications Sage UK: London, UK, 2006. [Google Scholar]
- Song, Y.J.; Kim, S.; Yoon, G.J. Impending extrusion of Ex-PRESS shunt treated by shunt-position adjustment: A case report. BMC Ophthalmol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Garg, S.J.; Kanitkar, K.; Weichel, E.; Fischer, D. Trauma-induced extrusion of an Ex-PRESS glaucoma shunt presenting as an intraocular foreign body. Arch. Ophthalmol. 2005, 123, 1270–1272. [Google Scholar] [CrossRef]
- Nicolai, M.; Franceschi, A.; Pelliccioni, P.; Pirani, V.; Mariotti, C. EX-PRESS glaucoma filtration device: Management of complications. Vision 2020, 4, 39. [Google Scholar] [CrossRef]
- Fea, A.M.; Durr, G.M.; Marolo, P.; Malinverni, L.; Economou, M.A.; Ahmed, I. XEN® gel stent: A comprehensive review on its use as a treatment option for refractory glaucoma. Clin. Ophthalmol. 2020, 14, 1805–1832. [Google Scholar] [CrossRef] [PubMed]
- Vera, V.I.; Horvath, C. XEN gel stent: The solution designed by AqueSys®. In Surgical Innovations in Glaucoma; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–198. [Google Scholar] [CrossRef]
- Tan, S.; Walkden, A.; Au, L. One-year result of XEN45 implant for glaucoma: Efficacy, safety, and postoperative management. Eye 2018, 32, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Flynn, W.J.; Bashford, K.P.; Lewis, R.A.; Duh, Y.-J.; Nangia, R.S.; Niksch, B. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am. J. Ophthalmol. 2017, 183, 25–36. [Google Scholar] [CrossRef]
- De Gregorio, A.; Pedrotti, E.; Russo, L.; Morselli, S. Minimally invasive combined glaucoma and cataract surgery: Clinical results of the smallest ab interno gel stent. Int. Ophthalmol. 2018, 38, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hengerer, F.H.; Kohnen, T.; Mueller, M.; Conrad-Hengerer, I. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J. Glaucoma 2017, 26, 1130–1136. [Google Scholar] [CrossRef]
- Widder, R.A.; Dietlein, T.S.; Dinslage, S.; Kühnrich, P.; Rennings, C.; Rössler, G. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: Success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 765–771. [Google Scholar] [CrossRef]
- Sng, C.C.; Wang, J.; Hau, S.; Htoon, H.M.; Barton, K. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin. Exp. Ophthalmol. 2018, 46, 339–345. [Google Scholar] [CrossRef]
- Olgun, A.; Imamoglu, S.; Karapapak, M.; Düzgün, E.; Kaçar, H. Endophthalmitis after XEN gel stent implantation: 2 cases. J. Glaucoma 2018, 27, e191–e194. [Google Scholar] [CrossRef]
- Dervenis, N.; Mikropoulou, A.M.; Dervenis, P.; Lewis, A. Dislocation of a previously successful XEN glaucoma implant into the anterior chamber: A case report. BMC Ophthalmol. 2017, 17, 148. [Google Scholar] [CrossRef]
- Fea, A.M.; Spinetta, R.; Cannizzo, P.M.L.; Consolandi, G.; Lavia, C.; Aragno, V.; Germinetti, F.; Rolle, T. Evaluation of bleb morphology and reduction in IOP and glaucoma medication following implantation of a novel gel stent. J. Ophthalmol. 2017, 2017, 9364910. [Google Scholar] [CrossRef]
- Karimi, A.; Lindfield, D.; Turnbull, A.; Dimitriou, C.; Bhatia, B.; Radwan, M.; Gouws, P.; Hanifudin, A.; Amerasinghe, N.; Jacob, A. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye 2019, 33, 469–477. [Google Scholar] [CrossRef]
- Midha, N.; Rao, H.L.; Mermoud, A.; Mansouri, K. Identifying the predictors of needling after XEN gel implant. Eye 2019, 33, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Midha, N.; Gillmann, K.; Chaudhary, A.; Mermoud, A.; Mansouri, K. Efficacy of needling revision after XEN gel stent implantation: A prospective study. J. Glaucoma 2020, 29, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gambini, G.; Carlà, M.M.; Giannuzzi, F.; Caporossi, T.; De Vico, U.; Savastano, A.; Baldascino, A.; Rizzo, C.; Kilian, R.; Caporossi, A. PreserFlo® MicroShunt: An overview of this minimally invasive device for open-angle glaucoma. Vision 2022, 6, 12. [Google Scholar] [CrossRef]
- Saeed, E.; Gołaszewska, K.; Dmuchowska, D.A.; Zalewska, R.; Konopińska, J. The PreserFlo MicroShunt in the Context of Minimally Invasive Glaucoma Surgery: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 2904. [Google Scholar] [CrossRef]
- Barberá, M.I.; Martínez-Galdón, F.; Caballero-Magro, E.; Rodríguez-Piñero, M.; Tañá-Rivero, P. Efficacy and Safety of the Preserflo Microshunt With Mitomycin C for the Treatment of Open Angle Glaucoma. J. Glaucoma 2022, 31, 557. [Google Scholar] [CrossRef]
- Batlle, J.F.; Fantes, F.; Riss, I.; Pinchuk, L.; Alburquerque, R.; Kato, Y.P.; Arrieta, E.; Peralta, A.C.; Palmberg, P.; Parrish, R.K. Three-year follow-up of a novel aqueous humor microshunt. J. Glaucoma 2016, 25, e58–e65. [Google Scholar] [CrossRef]
- Schlenker, M.B.; Durr, G.M.; Michaelov, E.; Ahmed, I.I.K. Intermediate outcomes of a novel standalone ab externo SIBS microshunt with mitomycin C. Am. J. Ophthalmol. 2020, 215, 141–153. [Google Scholar] [CrossRef]
- Beckers, H.J.; Aptel, F.; Webers, C.A.; Bluwol, E.; Martínez-de-la-Casa, J.M.; García-Feijoó, J.; Lachkar, Y.; Méndez-Hernández, C.D.; Riss, I.; Shao, H. Safety and effectiveness of the PRESERFLO® MicroShunt in primary open-angle glaucoma: Results from a 2-year multicenter study. Ophthalmol. Glaucoma 2022, 5, 195–209. [Google Scholar] [CrossRef]
- Scheres, L.M.; Kujovic-Aleksov, S.; Ramdas, W.D.; de Crom, R.M.; Roelofs, L.C.; Berendschot, T.T.; Webers, C.A.; Beckers, H.J. XEN® Gel Stent compared to PRESERFLO™ MicroShunt implantation for primary open-angle glaucoma: Two-year results. Acta Ophthalmol. 2021, 99, e433–e440. [Google Scholar] [CrossRef] [PubMed]
- Gambini, G.; Carlà, M.M.; Giannuzzi, F.; Boselli, F.; Grieco, G.; Caporossi, T.; De Vico, U.; Savastano, A.; Baldascino, A.; Rizzo, C. Anterior Segment-Optical Coherence Tomography Bleb Morphology Comparison in Minimally Invasive Glaucoma Surgery: XEN Gel Stent vs. PreserFlo MicroShunt. Diagnostics 2022, 12, 1250. [Google Scholar] [CrossRef] [PubMed]
- Kyari, F.; Abdull, M.M. The basics of good postoperative care after glaucoma surgery. Community Eye Health 2016, 29, 29. [Google Scholar] [PubMed]
- Radcliffe, N. The case for standalone micro-invasive glaucoma surgery: Rethinking the role of surgery in the glaucoma treatment paradigm. Curr. Opin. Ophthalmol. 2022, 34, 138–145. [Google Scholar] [CrossRef]
- Al Habash, A.; Nagshbandi, A.A. Quality of life after combined cataract and minimally invasive glaucoma surgery in glaucoma patients. Clin. Ophthalmol. 2020, 14, 3049–3056. [Google Scholar] [CrossRef]
- Samuelson, T.W.; Singh, I.P.; Williamson, B.K.; Falvey, H.; Lee, W.C.; Odom, D.; McSorley, D.; Katz, L.J. Quality of life in primary open-angle glaucoma and cataract: An analysis of VFQ-25 and OSDI from the iStent inject® pivotal trial. Am. J. Ophthalmol. 2021, 229, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Durr, G.M.; Samet, S.; Marolo, P.; Ahmed, I.I.K. Minimally Invasive Glaucoma Surgery (MIGS). In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–67. [Google Scholar] [CrossRef]
- Nichani, P.; Popovic, M.M.; Schlenker, M.B.; Park, J.; Ahmed, I.I.K. Microinvasive glaucoma surgery: A review of 3476 eyes. Surv. Ophthalmol. 2021, 66, 714–742. [Google Scholar] [CrossRef]
- Chen, D.Z.; Sng, C.C. Safety and efficacy of microinvasive glaucoma surgery. J. Ophthalmol. 2017, 2017, 3182935. [Google Scholar] [CrossRef]
- de Las Matas, R.B.-S.; Such-Irusta, L.; Alfonso-Muñoz, E.A.; Mascarós-Mena, H.; Lanzagorta-Aresti, A.; Mataix-Boronat, J.; Font-Julià, C. Late-onset Endophthalmitis after XEN45® Implantation: A retrospective case series and literature review. J. Curr. Glaucoma Pract. 2021, 15, 153. [Google Scholar] [CrossRef]
- Prokosch-Willing, V.; Vossmerbaeumer, U.; Hoffmann, E.; Pfeiffer, N. Suprachoroidal bleeding after XEN gel implantation. J. Glaucoma 2017, 26, e261–e263. [Google Scholar] [CrossRef]
- Vinod, K.; Gedde, S.J. Safety profile of minimally invasive glaucoma surgery. Curr. Opin. Ophthalmol. 2021, 32, 160–168. [Google Scholar] [CrossRef]
- Dhingra, D.; Bhartiya, S. Evaluating glaucoma surgeries in the MIGS context. Rom. J. Ophthalmol. 2020, 64, 85. [Google Scholar] [CrossRef]
- Carter, L.; Herndon, L.W. Minimally Invasive Glaucoma Surgery: Past, Present, and Future. Adv. Ophthalmol. Optom. 2023, 8, 239–248. [Google Scholar] [CrossRef]
- Pereira, I.C.; van de Wijdeven, R.; Wyss, H.M.; Beckers, H.J.; den Toonder, J.M. Conventional glaucoma implants and the new MIGS devices: A comprehensive review of current options and future directions. Eye 2021, 35, 3202–3221. [Google Scholar] [CrossRef]
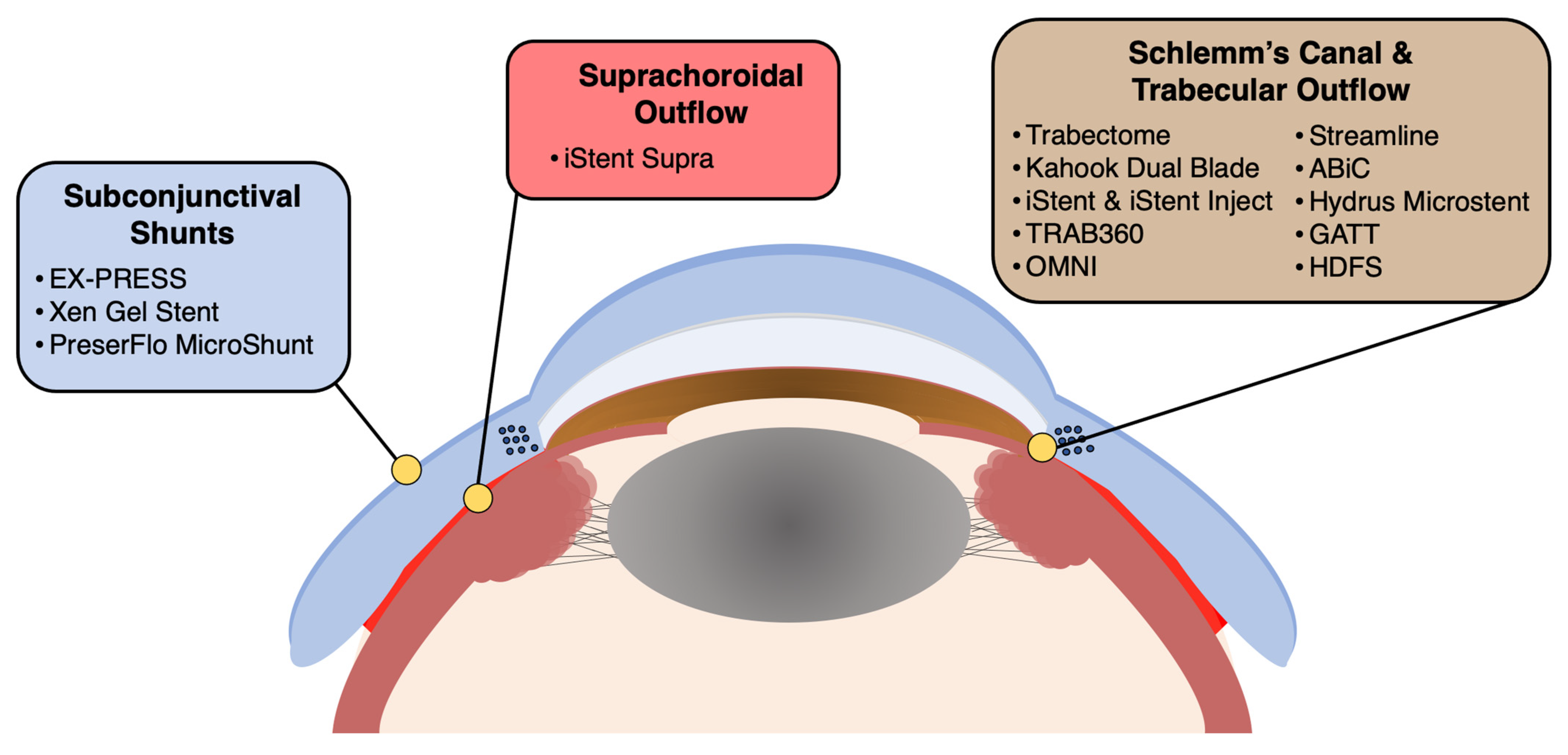
| Device Category | Device(s) (Manufacturer(s)) | FDA Approval Year | Brief Description | Mechanism of Action | Indication |
|---|---|---|---|---|---|
| Trabecular Outflow | Trabectome (NeoMedix Corporation) | 2004 | A microelectrocautery device that ablates and removes a portion of the trabecular meshwork and the inner wall of Schlemm’s canal. | Trabecular Meshwork Removal | Moderate-to-severe glaucoma |
| Kahook Dual Blade (New World Medical) | 2015 | An instrument with a dual-sided tip for precise incisions to create a trabecular meshwork window. | Trabecular Meshwork Removal | Moderate-to-severe glaucoma | |
| iStent (Glaukos Corporation) | 2012 | The first trabecular micro-bypass stent implanted to enhance aqueous humor outflow. | Trabecular Bypass | Mild-to-moderate OAG | |
| iStent Inject (Glaukos Corporation) | 2018 | A preloaded injector system that implants two stents to enhance IOP reduction. | Trabecular Bypass | Mild-to-moderate OAG | |
| HFDS (Oertli Instrumente) | NA | Creates six pockets penetrating 1 mm deep into the trabecular meshwork and Schlemm’s canal. | Deep Sclerotomy | Moderate-to-severe OAG | |
| Schlemm’s Canal Targeted | TRAB360 (Sight Sciences) | 2013 | A device utilizing a flexible microcatheter to cannulate and viscodilate Schlemm’s canal. | Trabecular Bypass | Mild-to-moderate OAG |
| OMNI Surgical System (Sight Sciences) | 2017 | A two-step device that combines viscodilation of Schlemm’s canal and trabeculotomy to enhance aqueous humor outflow. | Viscodilation and Trabeculotomy | Mild-to-moderate OAG | |
| Streamline (New World Medical) | 2021 | A single-use device that creates a goniotomy and delivers viscoelastic material to dilate Schlemm’s canal. | Goniotomy and Canal Dilation | Mild-to-moderate OAG | |
| ABiC (Ellex iScience) | NA | Uses a microcatheter system to cannulate and dilate Schlemm’s canal and distal outflow channels with viscoelastic material. | Canal Dilation | Mild-to-moderate primary OAG | |
| Schlemm’s Canal and Trabecular Outflow | Hydrus Microstent (Ivantis Inc.,) | 2018 | A flexible, biocompatible nitinol stent implanted via a clear corneal incision. | Canal Dilation and Trabecular Bypass | Mild-to-moderate OAG |
| Gonioscopy-Assisted Procedures | GATT | Not Device-Dependent | A procedure that utilizes a microcatheter and flexible suture for a 360-degree trabeculotomy. | 360-degree Trabeculotomy | Moderate-to-severe glaucoma |
| Suprachoroidal Outflow | iStent Supra (Glaukos Corporation) | NA | A PES device with a porous titanium coating implanted to facilitate aqueous humor drainage into the suprachoroidal space. | Suprachoroidal Bypass | Moderate-to-severe glaucoma |
| Subconjunctival Shunts | EX-PRESS Glaucoma Filtration Device (Alcon Laboratories) | 2002 | Small, stainless-steel shunt that is available in two sizes: P-50 (50 microns) and P-200 (200 microns). | Bypasses the Trabecular Meshwork | Moderate-to-severe glaucoma |
| Xen Gel Stent (Allergan) | 2016 | Hydrophilic, porcine-derived gelatin material, implanted to create a new drainage pathway that bypasses the trabecular meshwork. | New Drainage Pathway | Moderate-to-severe glaucoma | |
| PreserFlo MicroShunt (Santen) | 2021 | A small, flexible SIBS tube diverting aqueous humor from the anterior chamber to the subconjunctival space. | Diverts Aqueous Humor | Moderate-to-severe glaucoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balas, M.; Mathew, D.J. Minimally Invasive Glaucoma Surgery: A Review of the Literature. Vision 2023, 7, 54. https://doi.org/10.3390/vision7030054
Balas M, Mathew DJ. Minimally Invasive Glaucoma Surgery: A Review of the Literature. Vision. 2023; 7(3):54. https://doi.org/10.3390/vision7030054
Chicago/Turabian StyleBalas, Michael, and David J. Mathew. 2023. "Minimally Invasive Glaucoma Surgery: A Review of the Literature" Vision 7, no. 3: 54. https://doi.org/10.3390/vision7030054
APA StyleBalas, M., & Mathew, D. J. (2023). Minimally Invasive Glaucoma Surgery: A Review of the Literature. Vision, 7(3), 54. https://doi.org/10.3390/vision7030054






