Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells
Abstract
1. Introduction
2. Materials and Methods
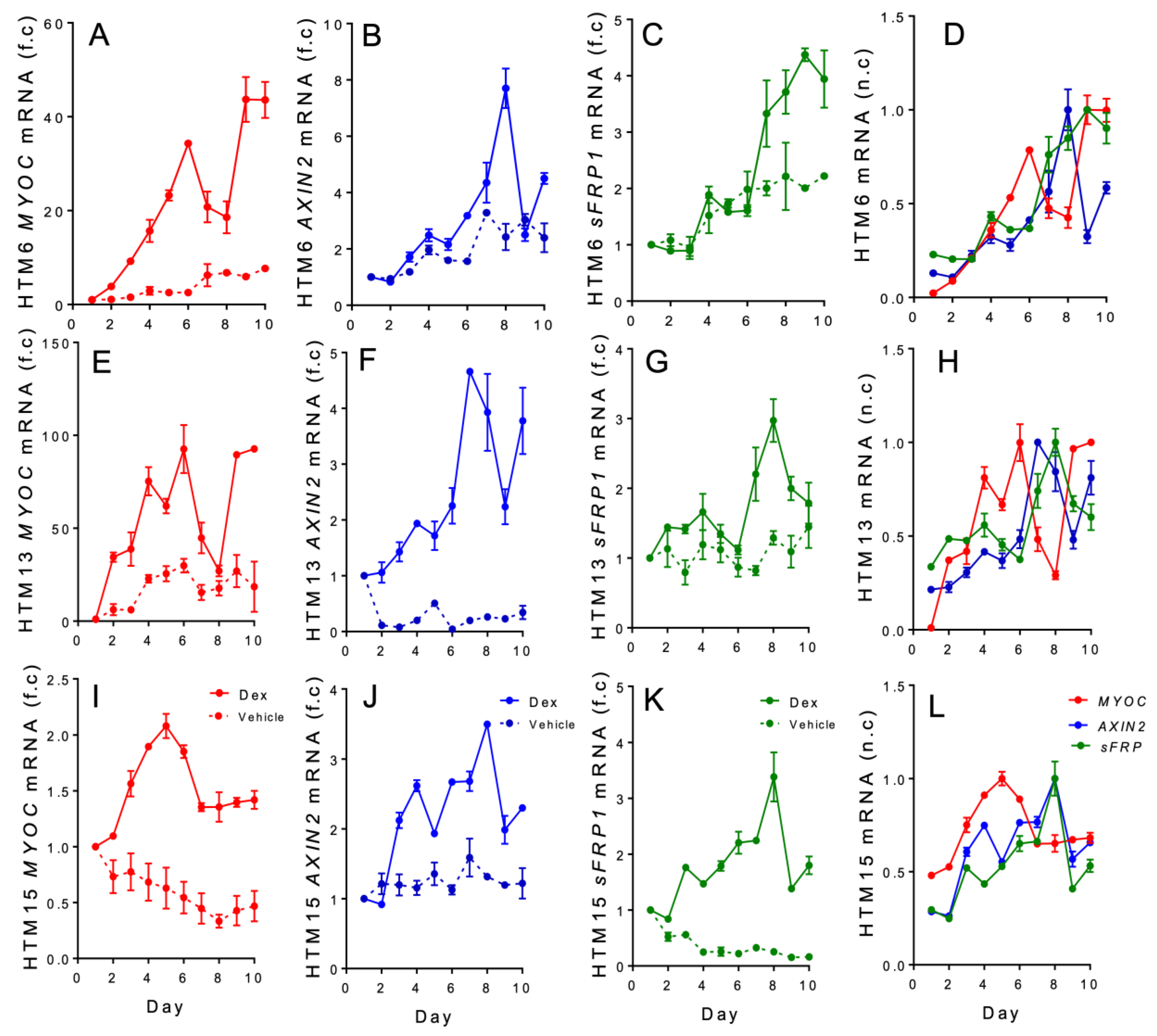
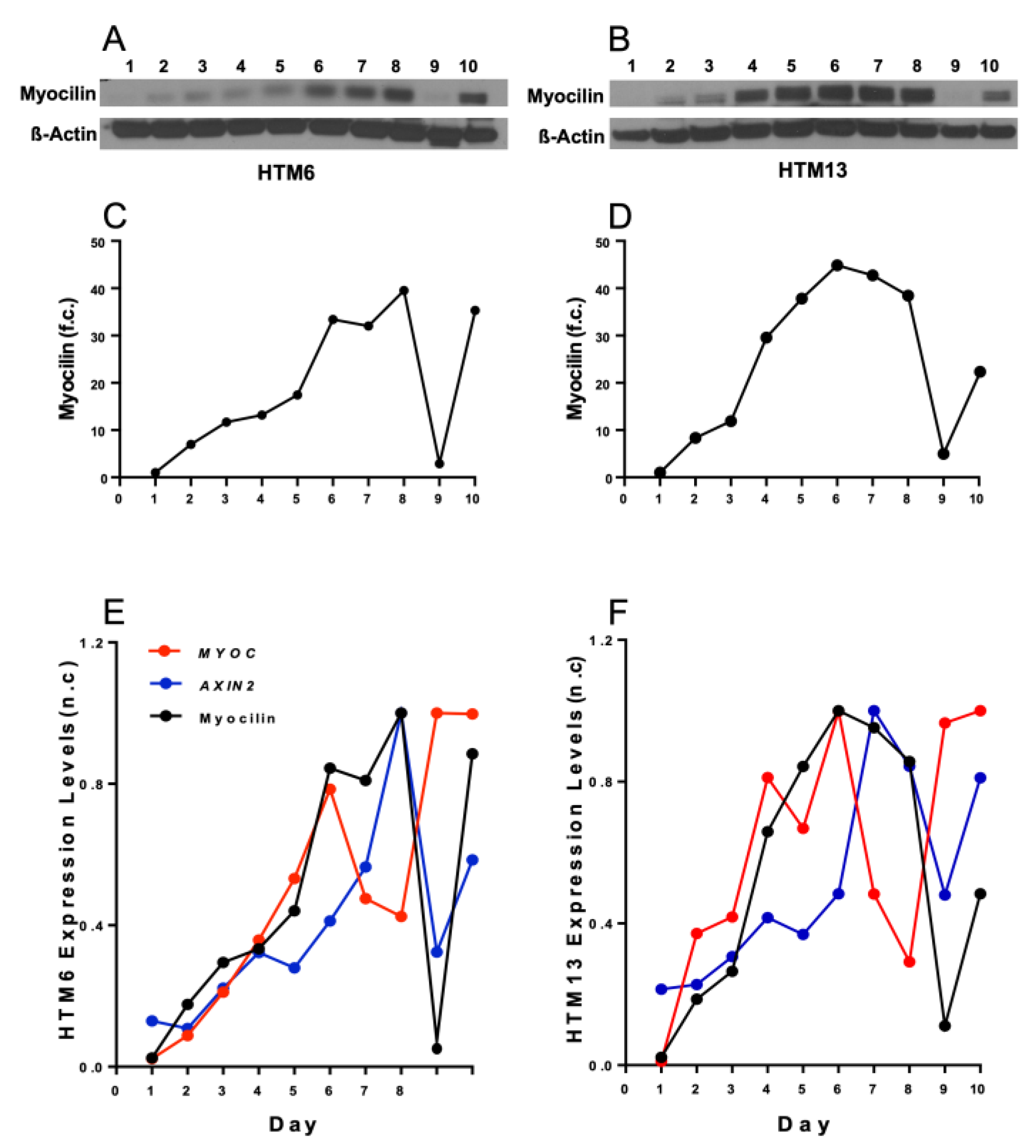
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Smith, R.A. Overview of age-related ocular conditions. Am. J. Manag. Care 2013, 19, S67–S75. [Google Scholar] [PubMed]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, U.; Ursell, P.G.; Rama, P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: Are they all the same? Ophthalmol. Ther. 2013, 2, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Call, M.K.; Yuan, Y.; Zhang, Y.; Fischesser, K.; Liu, C.Y.; Kao, W.W. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6502–6509. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cui, G.; Dismuke, W.M.; Navarro, I.; Perkumas, K.; Woodward, D.F.; Stamer, W.D. Differential response and withdrawal profile of glucocorticoid-treated human trabecular meshwork cells. Exp. Eye Res. 2017, 155, 38–46. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Z.; Li, M.; Chen, W.; Zhang, H.; Wang, J. Effect of topical application of adrenaline on schlemm canal, trabecular meshwork and intraocular pressure. Medicine 2019, 98, e15558. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Li, M.; Chen, W.; Liu, S.; Cai, Z.; Chen, L.; Xiang, Y.; Zhang, H.; Wang, J. Schlemm’s canal and trabecular meshwork morphology in high myopia. Ophthalmic Physiol. Opt. 2018, 38, 266–272. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of k-115 (ripasudil), a novel rock inhibitor, on trabecular meshwork and schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef]
- Gordon, D.M. Prednisone and prednisolone in ocular disease. Am. J. Ophthalmol. 1956, 41, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Armaly, M.F. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch. Ophthalmol. 1963, 70, 482–491. [Google Scholar] [CrossRef]
- Armaly, M.F. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch. Ophthalmol. 1963, 70, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Wordinger, R.J.; Clark, A.F. Effects of glucocorticoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog. Retin. Eye Res. 1999, 18, 629–667. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F.; Wordinger, R.J. The role of steroids in outflow resistance. Exp. Eye Res. 2009, 88, 752–759. [Google Scholar] [CrossRef]
- Fini, M.E.; Schwartz, S.G.; Gao, X.; Jeong, S.; Patel, N.; Itakura, T.; Price, M.O.; Price, F.W., Jr.; Varma, R.; Stamer, W.D. Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine. Prog. Retin. Eye Res. 2017, 56, 58–83. [Google Scholar] [CrossRef]
- Tektas, O.Y.; Lutjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Katz, L.J. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012, 47, 66–80. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Gasiorowski, J.Z.; Russell, P. Biological properties of trabecular meshwork cells. Exp. Eye Res. 2009, 88, 671–675. [Google Scholar] [CrossRef]
- Stamer, W.D.; Seftor, R.E.; Williams, S.K.; Samaha, H.A.; Snyder, R.W. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr. Eye Res. 1995, 14, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Carreon, T.; van der Merwe, E.; Fellman, R.L.; Johnstone, M.; Bhattacharya, S.K. Aqueous outflow—A continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res. 2017, 57, 108–133. [Google Scholar] [CrossRef]
- Keller, K.E.; Acott, T.S. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J. Ocul. Biol. 2013, 1, 3. [Google Scholar]
- Roy Chowdhury, U.; Hann, C.R.; Stamer, W.D.; Fautsch, M.P. Aqueous humor outflow: Dynamics and disease. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hassan, D.W.; Acott, T.S.; Kelley, M.J. The trabecular meshwork: A basic review of form and function. J. Ocul. Biol. 2014, 2, 9. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Phuc Le, P.; Friedman, J.R.; Schug, J.; Brestelli, J.E.; Parker, J.B.; Bochkis, I.M.; Kaestner, K.H. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet. 2005, 1, e16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, J.; Li, M.; Liu, S.; Chen, L.; Jing, S.; Cai, Z.; Xiang, Y.; Song, Y.; Zhang, H.; et al. Effect of age on the morphologies of the human schlemm’s canal and trabecular meshwork measured with swept-source optical coherence tomography. Eye 2018, 32, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Gottanka, J.; Flugel, C.; Hoffmann, F.; Futa, R.; Lutjen-Drecoll, E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch. Ophthalmol. 1997, 115, 375–383. [Google Scholar] [CrossRef]
- Bollinger, K.E.; Crabb, J.S.; Yuan, X.; Putliwala, T.; Clark, A.F.; Crabb, J.W. Proteomic similarities in steroid responsiveness in normal and glaucomatous trabecular meshwork cells. Mol. Vis. 2012, 18, 2001–2011. [Google Scholar]
- Liesenborghs, I.; Eijssen, L.M.T.; Kutmon, M.; Gorgels, T.; Evelo, C.T.; Beckers, H.J.M.; Webers, C.A.B.; Schouten, J. The molecular processes in the trabecular meshwork after exposure to corticosteroids and in corticosteroid-induced ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef]
- Chu, E.R.; Gonzalez, J.M., Jr.; Tan, J.C. Tissue-based imaging model of human trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014, 30, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.F.; Wilson, K.; McCartney, M.D.; Miggans, S.T.; Kunkle, M.; Howe, W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 281–294. [Google Scholar]
- Mao, W.; Rubin, J.S.; Anoruo, N.; Wordinger, R.J.; Clark, A.F. Sfrp1 promoter methylation and expression in human trabecular meshwork cells. Exp. Eye Res. 2012, 97, 130–136. [Google Scholar] [CrossRef]
- Stamer, D.W.; Roberts, B.C.; Epstein, D.L.; Allingham, R.R. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr. Eye Res. 2000, 20, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Ahadome, S.D.; Zhang, C.; Tannous, E.; Shen, J.; Zheng, J.J. Small-molecule inhibition of wnt signaling abrogates dexamethasone-induced phenotype of primary human trabecular meshwork cells. Exp. Cell Res. 2017, 357, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G., Jr.; Chatterjee, A.; Oh, S.S.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7433–7440. [Google Scholar] [CrossRef]
- Bermudez, J.Y.; Webber, H.C.; Brown, B.; Braun, T.A.; Clark, A.F.; Mao, W. A comparison of gene expression profiles between glucocorticoid responder and non-responder bovine trabecular meshwork cells using rna sequencing. PLoS ONE 2017, 12, e0169671. [Google Scholar] [CrossRef]
- Grigoryan, T.; Wend, P.; Klaus, A.; Birchmeier, W. Deciphering the function of canonical wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008, 22, 2308–2341. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nusse, R. Towards an integrated view of wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Nusse, R. Wnt signaling: A common theme in animal development. Genes Dev. 1997, 11, 3286–3305. [Google Scholar] [CrossRef] [PubMed]
- Tran, F.H.; Zheng, J.J. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017, 26, 650–661. [Google Scholar] [CrossRef]
- Wong, H.C.; Bourdelas, A.; Krauss, A.; Lee, H.J.; Shao, Y.M.; Wu, D.; Mlodzik, M.; Shi, D.L.; Zheng, J. Direct binding of the pdz domain of dishevelled to a conserved internal sequence in the c-terminal region of frizzled. Mol. Cell 2003, 12, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, Y.G. Dishevelled: The hub of wnt signaling. Cell. Signal. 2009, 22, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, S.; Li, L. New insights into the regulation of axin function in canonical wnt signaling pathway. Protein Cell 2014, 5, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Clements, W.K.; Kimelman, D.; Xu, W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 2003, 17, 2753–2764. [Google Scholar] [CrossRef]
- Law, S.M.; Zheng, J.J. Premise and peril of wnt signaling activation through gsk-3beta inhibition. iScience 2022, 25, 104159. [Google Scholar] [CrossRef]
- Wu, D.; Pan, W. Gsk3: A multifaceted kinase in wnt signaling. Trends Biochem. Sci. 2009, 35, 161–168. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kypta, R. Secreted antagonists of the wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; McNatt, L.G.; Pang, I.H.; Millar, J.C.; Hellberg, P.E.; Hellberg, M.H.; Steely, H.T.; Rubin, J.S.; Fingert, J.H.; Sheffield, V.C.; et al. Increased expression of the wnt antagonist sfrp-1 in glaucoma elevates intraocular pressure. J. Clin. Investig. 2008, 118, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Park, B.C.; Yue, B.Y.; Tomarev, S.; Surguchov, A. Interaction of myocilin with gamma-synuclein affects its secretion and aggregation. Cell. Mol. Neurobiol. 2005, 25, 1009–1033. [Google Scholar] [CrossRef] [PubMed]
- Polansky, J.R.; Wood, I.S.; Maglio, M.T.; Alvarado, J.A. Trabecular meshwork cell culture in glaucoma research: Evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology 1984, 91, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2016, 158, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Bhattacharya, S.K.; Borras, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Shepard, A.R.; Jacobson, N.; Fingert, J.H.; Stone, E.M.; Sheffield, V.C.; Clark, A.F. Delayed secondary glucocorticoid responsiveness of myoc in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3173–3181. [Google Scholar]
- Faralli, J.A.; Clark, R.W.; Filla, M.S.; Peters, D.M. Nfatc1 activity regulates the expression of myocilin induced by dexamethasone. Exp. Eye Res. 2015, 130, 9–16. [Google Scholar] [CrossRef]
- Polansky, J.R.; Weinreb, R.N.; Baxter, J.D.; Alvarado, J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Investig. Ophthalmol. Vis. Sci. 1979, 18, 1043–1049. [Google Scholar]
- Polansky, J.R.; Fauss, D.J.; Zimmerman, C.C. Regulation of tigr/myoc gene expression in human trabecular meshwork cells. Eye 2000, 14, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Lee, H.S.; Ji, Y.; Rubin, J.S.; Tomarev, S.I. Myocilin is a modulator of wnt signaling. Mol. Cell. Biol. 2009, 29, 2139–2154. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ying, H.; Yue, B.Y. Wnt activation by wild type and mutant myocilin in cultured human trabecular meshwork cells. PLoS ONE 2012, 7, e44902. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone stiffens trabecular meshwork, trabecular meshwork cells, and matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef]
- Mao, W.; Millar, J.C.; Wang, W.H.; Silverman, S.M.; Liu, Y.; Wordinger, R.J.; Rubin, J.S.; Pang, I.H.; Clark, A.F. Existence of the canonical wnt signaling pathway in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7043–7051. [Google Scholar] [CrossRef]
- Gibb, N.; Lavery, D.L.; Hoppler, S. Sfrp1 promotes cardiomyocyte differentiation in xenopus via negative-feedback regulation of wnt signalling. Development 2013, 140, 1537–1549. [Google Scholar] [CrossRef]
- Jain, A.; Wordinger, R.J.; Yorio, T.; Clark, A.F. Role of the alternatively spliced glucocorticoid receptor isoform grbeta in steroid responsiveness and glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 121–127. [Google Scholar] [CrossRef]
- Zhang, X.; Clark, A.F.; Yorio, T. Interactions of endothelin-1 with dexamethasone in primary cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5301–5308. [Google Scholar] [CrossRef]
- Dhamodaran, K.; Baidouri, H.; Sandoval, L.; Raghunathan, V. Wnt activation after inhibition restores trabecular meshwork cells toward a normal phenotype. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef]
- Morgan, J.T.; Raghunathan, V.K.; Chang, Y.R.; Murphy, C.J.; Russell, P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 2015, 6, 15362–15374. [Google Scholar] [CrossRef] [PubMed]
- Dibas, A.; Yorio, T. Glucocorticoid therapy and ocular hypertension. Eur. J. Pharmacol. 2016, 787, 57–71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Tannous, E.; Thomas, A.; Jung, N.; Ma, E.; Zheng, J.J. Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells. Vision 2023, 7, 43. https://doi.org/10.3390/vision7020043
Zhang C, Tannous E, Thomas A, Jung N, Ma E, Zheng JJ. Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells. Vision. 2023; 7(2):43. https://doi.org/10.3390/vision7020043
Chicago/Turabian StyleZhang, Chi, Elizabeth Tannous, Alseena Thomas, Natalia Jung, Edmond Ma, and Jie J. Zheng. 2023. "Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells" Vision 7, no. 2: 43. https://doi.org/10.3390/vision7020043
APA StyleZhang, C., Tannous, E., Thomas, A., Jung, N., Ma, E., & Zheng, J. J. (2023). Dexamethasone Modulates the Dynamics of Wnt Signaling in Human Trabecular Meshwork Cells. Vision, 7(2), 43. https://doi.org/10.3390/vision7020043




