Parietal Alpha Oscillatory Peak Frequency Mediates the Effect of Practice on Visuospatial Working Memory Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
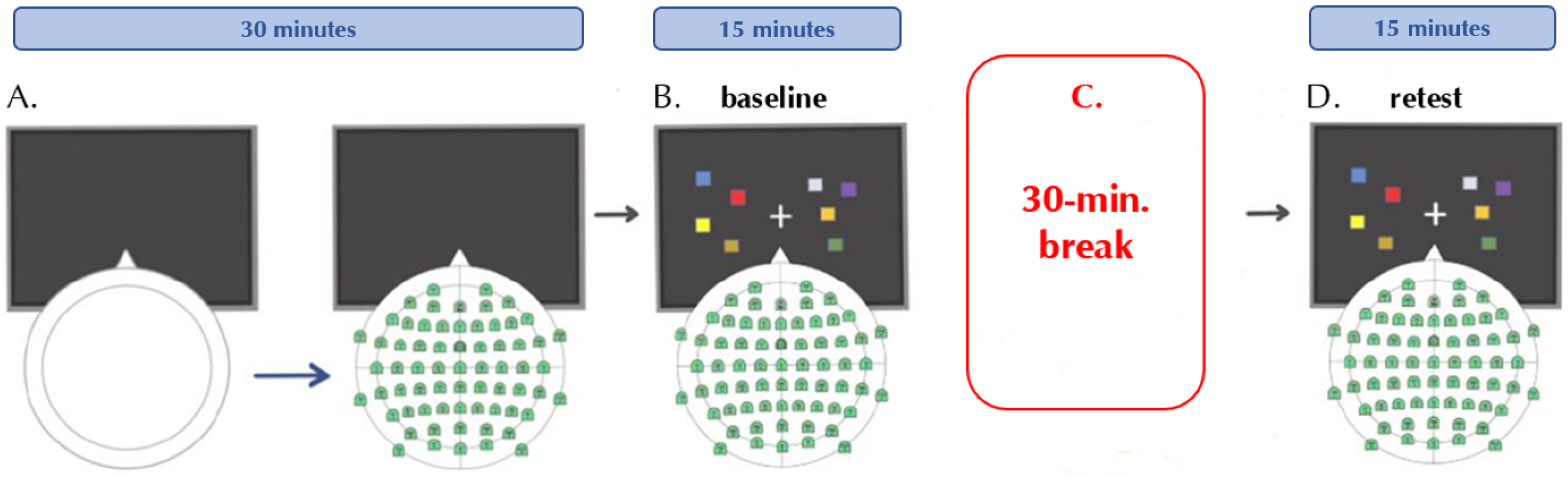
2.2. Procedure
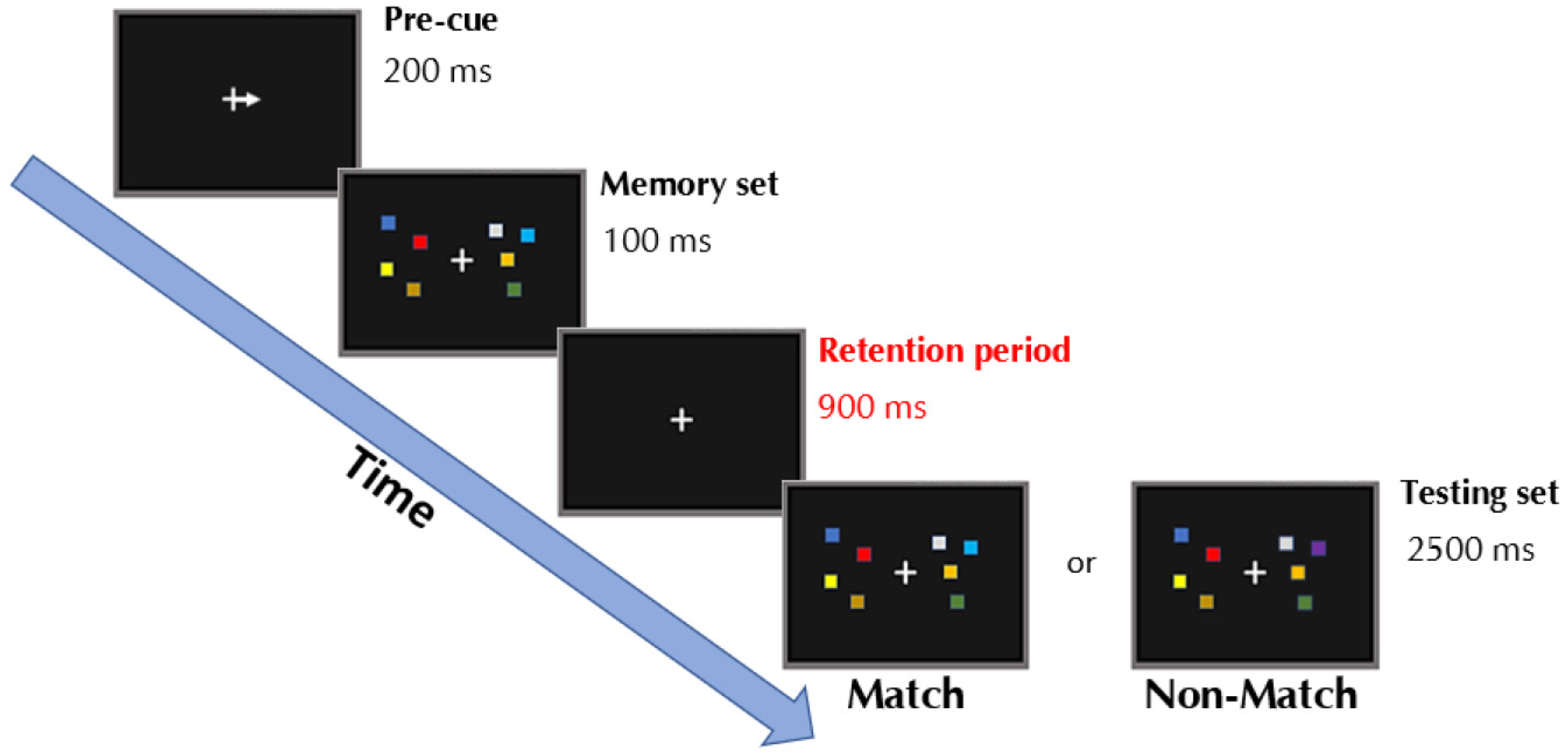
2.3. Change-Detection Task
2.4. EEG Recordings
2.5. Data Analyses
2.5.1. Behavioral Data
2.5.2. EEG Data: Preprocessing
2.5.3. EEG Data: Peak Frequency Analyses
2.6. Statistical Analyses
3. Results
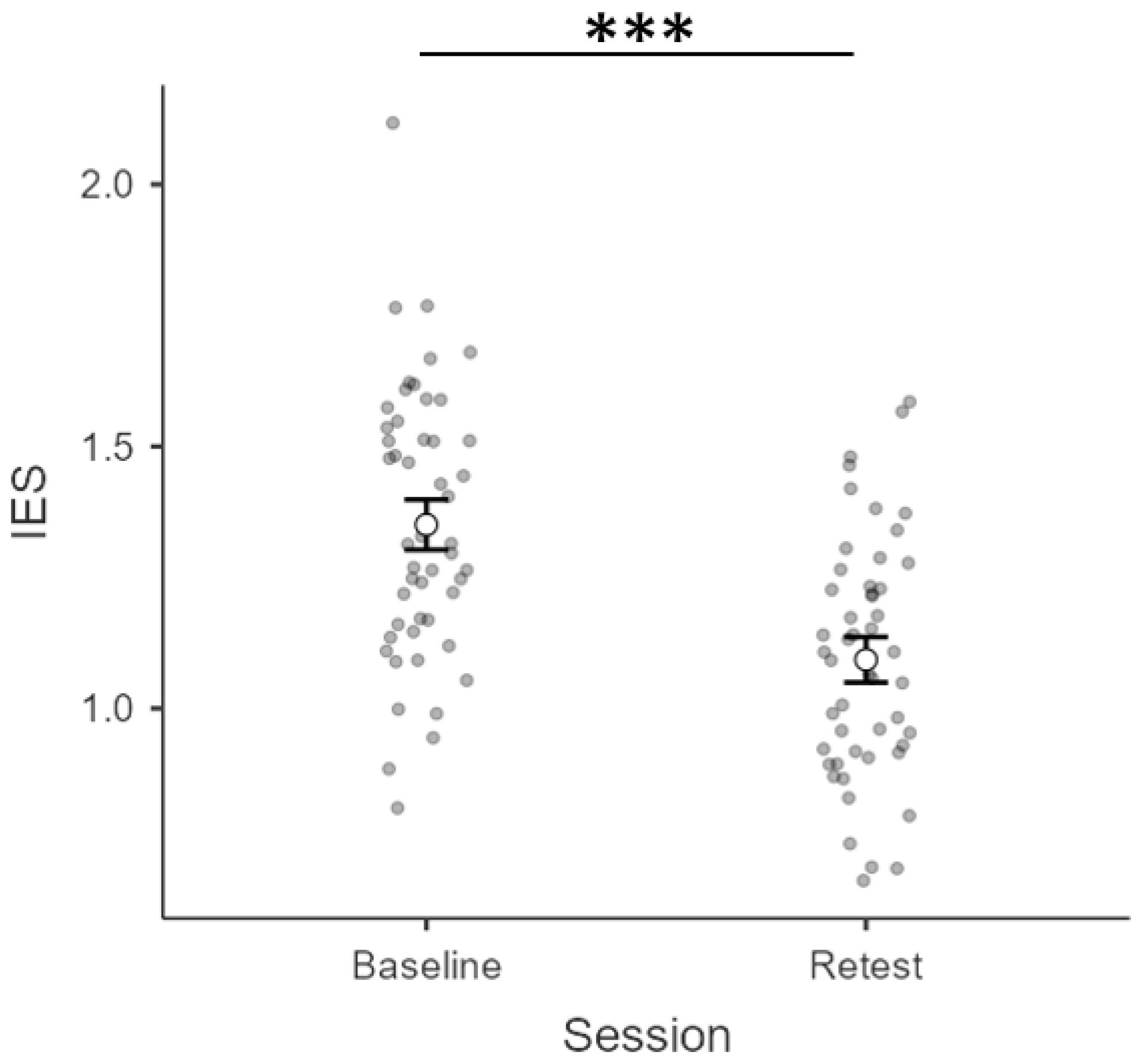
3.1. Inverse Efficiency Scores (IES)
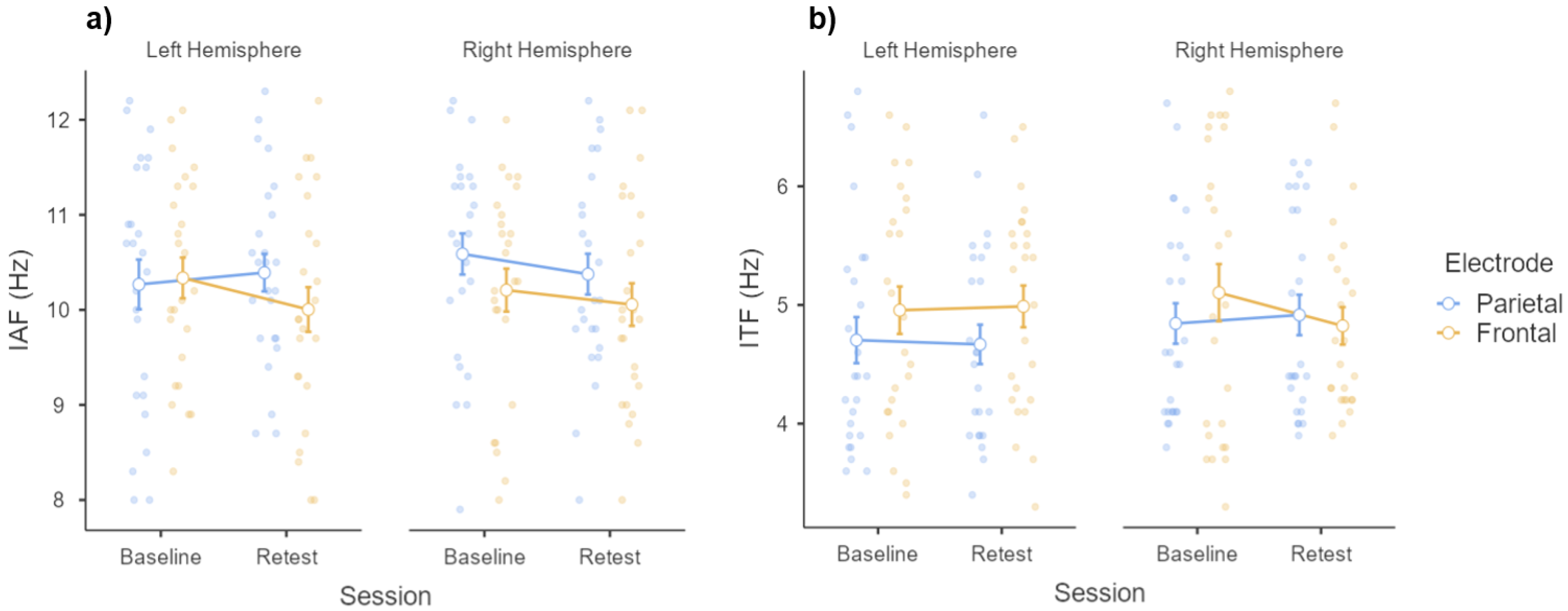
3.2. Individual Frequency Peaks
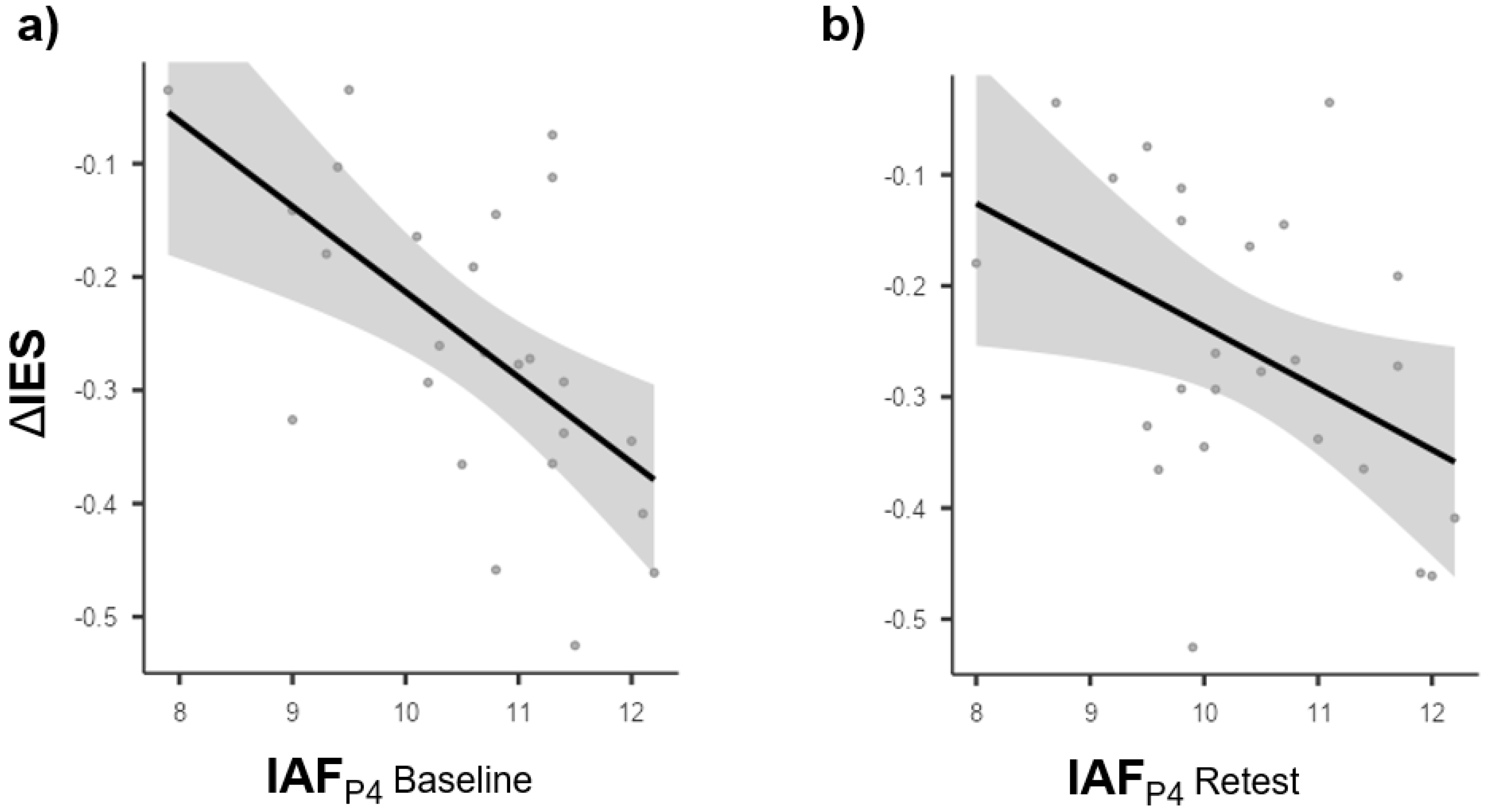
3.3. Brain Behavior Relationships
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddeley, A.; Hitch, G. Working Memory. In The Psychology of Learning and Motivation: Advances in Research and Theory; Academic: New York, NY, USA, 1974; pp. 47–88. [Google Scholar]
- Baddeley, A. Working Memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory: Theories, Models, and Controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef]
- De Renzi, E.; Nichelli, P. Verbal and Non-Verbal Short-Term Memory Impairment Following Hemispheric Damage. Cortex 1975, 11, 341–354. [Google Scholar] [CrossRef]
- Basso, A.; Spinnler, H.; Vallar, G.; Zanobio, M.E. Left Hemisphere Damage and Selective Impairment of Auditory Verbal Short-Term Memory. A Case Study. Neuropsychol. 1982, 20, 263–274. [Google Scholar] [CrossRef]
- Shallice, T.; Warrington, E.K. Independent Functioning of Verbal Memory Stores: A Neuropsychological Study. Q. J. Exp. Psychol. 1970, 22, 261–273. [Google Scholar] [CrossRef]
- Baddeley, A.; Hitch, G.; Allen, R. A Multicomponent Model of Working Memory. In Working Memory: State of the Science; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Logie, R. The Functional Organization and Capacity Limits of Working Memory. Curr. Dir. Psychol. Sci. 2011, 20, 240–245. [Google Scholar] [CrossRef]
- Jonides, J.; Smith, E.E.; Koeppe, R.A.; Awh, E.; Minoshima, S.; Mintun, M.A. Spatial Working Memory in Humans as Revealed by PET. Nature 1993, 363, 623–625. [Google Scholar] [CrossRef]
- Curtis, C.E. Prefrontal and Parietal Contributions to Spatial Working Memory. Neuroscience 2006, 139, 173–180. [Google Scholar] [CrossRef]
- Postle, B.R.; Stern, C.E.; Rosen, B.R.; Corkin, S. An FMRI Investigation of Cortical Contributions to Spatial and Nonspatial Visual Working Memory. NeuroImage 2000, 11, 409–423. [Google Scholar] [CrossRef]
- Constantinidis, C.; Wang, X.J. A Neural Circuit Basis for Spatial Working Memory. Neuroscientist 2004, 10, 553–565. [Google Scholar] [CrossRef]
- van Asselen, M.; Kessels, R.P.C.; Neggers, S.F.W.; Kappelle, L.J.; Frijns, C.J.M.; Postma, A. Brain Areas Involved in Spatial Working Memory. Neuropsychologia 2006, 44, 1185–1194. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Carpenter, P.A.; Just, M.A. Collaborative Activity between Parietal and Dorso-Lateral Prefrontal Cortex in Dynamic Spatial Working Memory Revealed by FMRI. NeuroImage 2000, 12, 85–99. [Google Scholar] [CrossRef]
- Awh, E.; Jonides, J. Overlapping Mechanisms of Attention and Spatial Working Memory. Trends Cogn. Sci. 2001, 5, 119–126. [Google Scholar] [CrossRef]
- Linden, D.E.J.; Bittner, R.A.; Muckli, L.; Waltz, J.A.; Kriegeskorte, N.; Goebel, R.; Singer, W.; Munk, M.H.J. Cortical Capacity Constraints for Visual Working Memory: Dissociation of FMRI Load Effects in a Fronto-Parietal Network. NeuroImage 2003, 20, 1518–1530. [Google Scholar] [CrossRef]
- Wallis, G.; Stokes, M.; Cousijn, H.; Woolrich, M.; Nobre, A.C. Frontoparietal and Cingulo-Opercular Networks Play Dissociable Roles in Control of Working Memory George. J. Cogn. Neurosci. 2015, 27, 2019–2034. [Google Scholar] [CrossRef]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling Neural Correlates of Working Memory: A Coordinate-Based Meta-Analysis. NeuroImage 2012, 60, 830–846. [Google Scholar] [CrossRef]
- Coull, J.T.; Frith, C.D.; Frackowiak, R.S.J.; Grasby, P.M. A Fronto-Parietal Network for Rapid Visual Information Processing: A PET Study of Sustained Attention and Working Memory. Neuropsychologia 1996, 34, 1085–1095. [Google Scholar] [CrossRef]
- Sarnthein, J.; Petsche, H.; Rappelsberger, P.; Shaw, G.L.; Von Stein, A. Synchronization between Prefrontal and Posterior Association Cortex during Human Working Memory. Proc. Natl. Acad. Sci. USA 1998, 95, 7092–7096. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, N.; Stokes, M.; Nobre, A.C.; Rushworth, M.F.S. Frontal and Parietal Cortical Interactions with Distributed Visual Representations during Selective Attention and Action Selection. J. Neurosci. 2013, 33, 16443–16458. [Google Scholar] [CrossRef]
- Todd, J.J.; Marois, R. Posterior Parietal Cortex Activity Predicts Individual Differences in Visual Short-Term Memory Capacity. Cogn. Affect. Behav. Neurosci. 2005, 5, 144–155. [Google Scholar] [CrossRef]
- Silver, M.A.; Ress, D.; Heeger, D.J. Topographic Maps of Visual Spatial Attention in Human Parietal Cortex. J. Neurophysiol. 2005, 94, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Dewar, C.D.; Solbakk, A.K.; Endestad, T.; Meling, T.R.; Knight, R.T. Bidirectional Frontoparietal Oscillatory Systems Support Working Memory. Curr. Biol. 2017, 27, 1829–1835.e4. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Barbey, A.K.; Postle, B.R.; Grafman, J. Superior Parietal Cortex Is Critical for the Manipulation of Information in Working Memory. J. Neurosci. 2009, 29, 14980–14986. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, M.E.; Olson, I.R. Is the Posterior Parietal Lobe Involved in Working Memory Retrieval? Evidence from Patients with Bilateral Parietal Lobe Damage. Neuropsychologia 2008, 46, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Pisella, L.; Berberovic, N.; Mattingley, J.B. Impaired Working Memory for Location but Not for Colour or Shape in Visual Neglect: A Comparison of Parietal and Non-Parietal Lesions. Cortex 2004, 40, 379–390. [Google Scholar] [CrossRef]
- De Renzi, E. Disorders of Space Exploration and Cognition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1982. [Google Scholar]
- Vallar, G.; Perani, D. The Anatomy of Unilateral Neglect after Right-Hemisphere Stroke Lesions. A Clinical/CT-Scan Correlation Study in Man. Neuropsychologia 1986, 24, 609–622. [Google Scholar] [CrossRef]
- Mesulam, M. Spatial Attention and Neglect: Parietal, Frontal and Cingulate Contributions to the Mental Representation and Attentional Targeting of Salient Extrapersonal Events. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 2083. [Google Scholar] [CrossRef][Green Version]
- Babiloni, C.; Babiloni, F.; Carducci, F.; Cappa, S.F.; Cincotti, F.; Del Percio, C.; Miniussi, C.; Moretti, D.V.; Rossi, S.; Sosta, K.; et al. Human Cortical Rhythms during Visual Delayed Choice Reaction Time Tasks: A High-Resolution EEG Study on Normal Aging. Behav. Brain Res. 2004, 153, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Freunberger, R.; Werkle-Bergner, M.; Griesmayr, B.; Lindenberger, U.; Klimesch, W. Brain Oscillatory Correlates of Working Memory Constraints. Brain Res. 2011, 1375, 93–102. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Heise, K.F.; Gruber, W.R.; Holz, E.; Karim, A.A.; Glennon, M.; Gerloff, C.; Birbaumer, N.; Hummel, F.C. Brain Oscillatory Substrates of Visual Short-Term Memory Capacity. Curr. Biol. 2009, 19, 1846–1852. [Google Scholar] [CrossRef]
- Hsieh, L.T.; Ekstrom, A.D.; Ranganath, C. Neural Oscillations Associated with Item and Temporal Order Maintenance in Working Memory. J. Neurosci. 2011, 31, 10803–10810. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Hsieh, L.T.; Ranganath, C. Oscillatory Activity during Maintenance of Spatial and Temporal Information in Working Memory. Neuropsychologia 2013, 51, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Schack, B.; Klimesch, W.; Sauseng, P. Phase Synchronization between Theta and Upper Alpha Oscillations in a Working Memory Task. Int. J. Psychophysiol. 2005, 57, 105–114. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Gruber, W.; Doppelmayr, M.; Stadler, W.; Schabus, M. The Interplay between Theta and Alpha Oscillations in the Human Electroencephalogram Reflects the Transfer of Information between Memory Systems. Neurosci. Lett. 2002, 324, 121–124. [Google Scholar] [CrossRef]
- Roux, F.; Uhlhaas, P.J. Working Memory and Neural Oscillations: Alpha-Gamma versus Theta-Gamma Codes for Distinct WM Information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liesefeld, H.R.; Liesefeld, A.M.; Sauseng, P.; Jacob, S.N.; Müller, H.J. How Visual Working Memory Handles Distraction: Cognitive Mechanisms and Electrophysiological Correlates. Vis. Cogn. 2020, 28, 372–387. [Google Scholar] [CrossRef]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control Mechanisms in Working Memory: A Possible Function of EEG Theta Oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef]
- Sauseng, P.; Liesefeld, H.R. Cognitive Control: Brain Oscillations Coordinate Human Working Memory. Curr. Biol. 2020, 30, R405–R407. [Google Scholar] [CrossRef]
- Bonnefond, M.; Jensen, O. Alpha Oscillations Serve to Protect Working Memory Maintenance against Anticipated Distracters. Curr. Biol. 2012, 22, 1969–1974. [Google Scholar] [CrossRef]
- de Vries, I.E.J.; van Driel, J.; Olivers, C.N.L. Posterior α EEG Dynamics Dissociate Current from Future Goals in Working Memory-Guided Visual Search. J. Neurosci. 2017, 37, 1591–1603. [Google Scholar] [CrossRef]
- D’Esposito, M.; Postle, B.R. The Cognitive Neuroscience of Working Memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Freunberger, R.; Fellinger, R.; Sauseng, P.; Gruber, W.; Klimesch, W. Dissociation between Phase-Locked and Nonphase-Locked Alpha Oscillations in a Working Memory Task. Hum. Brain Mapp. 2009, 30, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Göddertz, A.; Haase, H.; Hickey, C.; Wascher, E. Hemispheric Asymmetries in EEG Alpha Oscillations Indicate Active Inhibition during Attentional Orienting within Working Memory. Behav. Brain Res. 2019, 359, 38–46. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Riddle, J.; Scimeca, J.M.; Cellier, D.; Dhanani, S.; D’Esposito, M. Causal Evidence for a Role of Theta and Alpha Oscillations in the Control of Working Memory. Curr. Biol. 2020, 30, 1748–1754.e4. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, N.; Cooper, N.R.; Sauseng, P.; Romei, V. The Speed of Parietal Theta Frequency Drives Visuospatial Working Memory Capacity. PLoS Biol. 2018, 16, e2005348. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Korman, C.; Batsikadze, G.; Paulus, W. The Importance of Timing in Segregated Theta Phase-Coupling for Cognitive Performance. Curr. Biol. 2012, 22, 1314–1318. [Google Scholar] [CrossRef]
- Jaušovec, N.; Jaušovec, K. Increasing Working Memory Capacity with Theta Transcranial Alternating Current Stimulation (TACS). Biol. Psychol. 2014, 96, 42–47. [Google Scholar] [CrossRef]
- Bender, M.; Romei, V.; Sauseng, P. Slow Theta TACS of the Right Parietal Cortex Enhances Contralateral Visual Working Memory Capacity. Brain Topogr. 2019, 32, 477–481. [Google Scholar] [CrossRef]
- Hamidi, M.; Tononi, G.; Postle, B.R. Evaluating Frontal and Parietal Contributions to Spatial Working Memory with Repetitive Transcranial Magnetic Stimulation. Brain Res. 2008, 1230, 202–210. [Google Scholar] [CrossRef]
- Lisman, J.; Idiart, M. Storage of 7 ± 2 Short-Term Memories in Oscillatory Subcycles. Science 1995, 267, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Gerloff, C. Enhancing Cognitive Performance with Repetitive Transcranial Magnetic Stimulation at Human Individual Alpha Frequency. Eur. J. Neurosci. 2003, 17, 1129–1133. [Google Scholar] [CrossRef]
- Cecere, R.; Rees, G.; Romei, V. Individual Differences in Alpha Frequency Drive Crossmodal Illusory Perception. Curr. Biol. 2015, 25, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Venskus, A.; Ferri, F.; Migliorati, D.; Spadone, S.; Costantini, M.; Hughes, G. Temporal Binding Window and Sense of Agency Are Related Processes Modifiable via Occipital TACS. PLoS ONE 2021, 16, e0256987. [Google Scholar] [CrossRef] [PubMed]
- Venskus, A.; Hughes, G. Individual Differences in Alpha Frequency Are Associated with the Time Window of Multisensory Integration, but Not Time Perception. Neuropsychologia 2021, 159, 107919. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Poch, C.; Gillmeister, H.; Costantini, M.; Romei, V. Oscillatory Properties of Functional Connections between Sensory Areas Mediate Cross-Modal Illusory Perception. J. Neurosci. 2019, 39, 5711–5718. [Google Scholar] [CrossRef]
- Samaha, J.; Postle, B.R. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr. Biol. 2015, 25, 2985–2990. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Trajkovic, J.; Roperti, C.; Marcantoni, E.; Di Luzio, P.; Avenanti, A.; Thut, G.; Romei, V. Tuning Alpha Rhythms to Shape Conscious Visual Perception. Curr. Biol. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Schmiedek, F.; Lövdén, M.; Lindenberger, U. Peak Individual Alpha Frequency Qualifies as a Stable Neurophysiological Trait Marker in Healthy Younger and Older Adults. Psychophysiology 2013, 50, 570–582. [Google Scholar] [CrossRef]
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Moran, R.J.; Campo, P.; Maestu, F.; Reilly, R.B.; Dolan, R.J.; Strange, B.A. Peak Frequency in the Theta and Alpha Bands Correlates with Human Working Memory Capacity. Front. Hum. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Jolles, D.D.; Grol, M.J.; Van Buchem, M.A.; Rombouts, S.A.R.B.; Crone, E.A. NeuroImage Practice Effects in the Brain: Changes in Cerebral Activation after Working Memory Practice Depend on Task Demands. NeuroImage 2010, 52, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Schmiedek, F.; Huxhold, O.; Röcke, C.; Smith, J.; Lindenberger, U. Working Memory Plasticity in Old Age: Practice Gain, Transfer, and Maintenance. Psychol. Aging 2008, 23, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Melby-Lervåg, M.; Redick, T.S.; Hulme, C. Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of “Far Transfer”: Evidence From a Meta-Analytic Review. Perspect. Psychol. Sci. 2016, 11, 512–534. [Google Scholar] [CrossRef] [PubMed]
- Olesen, P.J.; Westerberg, H.; Klingberg, T. Increased Prefrontal and Parietal Activity after Training of Working Memory. Nat. Neurosci. 2004, 7, 75–79. [Google Scholar] [CrossRef]
- Xu, Z.; Adam, K.C.S.; Fang, X.; Vogel, E.K. The Reliability and Stability of Visual Working Memory Capacity. Behav. Res. Methods 2018, 50, 576–588. [Google Scholar] [CrossRef]
- Adam, K.C.S.; Vogel, E.K. Improvements to Visual Working Memory Performance with Practice and Feedback. PLoS ONE 2018, 13, e0203279. [Google Scholar] [CrossRef]
- Garavan, H.; Kelley, D.A.N.; Rosen, A.; Rao, S.M.; Words, K.E.Y. Practice-Related Functional Activation Changes in a Working Memory Task. Microsc. Res. Tech. 2000, 63, 54–63. [Google Scholar] [CrossRef]
- Landau, S.M.; Schumacher, E.H.; Garavan, H.; Druzgal, T.J.; D’Esposito, M. A Functional MRI Study of the Influence of Practice on Component Processes of Working Memory. NeuroImage 2004, 22, 211–221. [Google Scholar] [CrossRef]
- Sayala, S.; Sala, J.B.; Courtney, S.M. Increased Neural Efficiency with Repeated Performance of a Working Memory Task Is Information-Type Dependent. Cereb. Cortex 2006, 16, 609–617. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-Resolution EEG Mapping of Cortical Activation Related to Working Memory: Effects of Task Difficulty, Type of Processing, and Practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, L.K.; Smith, M.E.; Gevins, A. Dynamic Cortical Networks of Verbal and Spatial Working Memory: Effects of Memory Load and Task Practice. Cereb. Cortex 1998, 8, 563–574. [Google Scholar] [CrossRef]
- Bruyer, R.; Brysbaert, M. Combining Speed and Accuracy in Cognitive Psychology: Is the Inverse Efficiency Score (IES) a Better Dependent Variable than the Mean Reaction Time (RT) and the Percentage of Errors (PE)? Psychol. Belg. 2011, 51, 5–13. [Google Scholar] [CrossRef]
- Townsend, J.; Ashby, G. Methods of Modeling Capacity in Simple Processing Systems. In Cognitive Theory; Castellan, J., Restle, F., Eds.; Lawrence ErlbaumAssociates Ltd.: Hillsdale, NJ, USA, 1978; pp. 200–239. [Google Scholar]
- Vogel, E.K.; Machizawa, M.G. Neural Activity Predicts Individual Differences in Visual Working Memory Capacity. Nature 2004, 428, 748–751. [Google Scholar] [CrossRef] [PubMed]
- De Renzi, E.; Faglioni, P.; Previdi, P. Spatial Memory and Hemispheric Locus of Lesion. Cortex 1977, 13, 424–433. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; Jaap Kappelle, L.; De Haan, E.H.F.; Postma, A. Lateralization of Spatial-Memory Processes: Evidence on Spatial Span, Maze Learning, and Memory for Object Locations. Neuropsychologia 2002, 40, 1465–1473. [Google Scholar] [CrossRef]
- Heilman, K.M.; Abell, T. Van Den Right Hemisphere Dominance for Attention: The Mechanism Underlying Hemispheric Asymmetries of Inattention (Neglect). Neurology 1980, 327–330. [Google Scholar] [CrossRef]
- Mesulam, M. A Cortical Network for Directed Attention and Unilateral Neglect. Ann. Neurol. 1981, 10, 309–325. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J.; Koeppe, R.A. Dissociating Verbal and Spatial Working Memory Using PET. Cereb. Cortex 1996, 6, 11–20. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Thut, G.; Mok, R.M.; Schyns, P.G.; Driver, J. Causal Implication by Rhythmic Transcranial Magnetic Stimulation of Alpha Frequency in Feature-Based Local vs. Global Attention. Eur. J. Neurosci. 2012, 35, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Driver, J.; Schyns, P.G.; Thut, G. Rhythmic TMS over Parietal Cortex Links Distinct Brain Frequencies to Global versus Local Visual Processing. Curr. Biol. 2011, 21, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Coles, M.G.H.; Donchin, E. A New Method for Off-Line Removal of Ocular Artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Pritchard, W.S. The Brain in Fractal Time: 1/f-like Power Spectrum Scaling of the Human Electroencephalogram. Int. J. Neurosci. 1992, 66, 119–129. [Google Scholar] [CrossRef]
- Klimesch, W.; Schimke, H.; Pfurtscheller, G. Alpha Frequency, Cognitive Load and Memory Performance. Brain Topogr. 1993, 5, 241–251. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Hanslmayr, S. Upper Alpha ERD and Absolute Power: Their Meaning for Memory Performance. Prog. Brain Res. 2006, 159, 151–165. [Google Scholar] [CrossRef]
- Rihs, T.A.; Michel, C.M.; Thut, G. Mechanisms of Selective Inhibition in Visual Spatial Attention Are Indexed by α-Band EEG Synchronization. Eur. J. Neurosci. 2007, 25, 603–610. [Google Scholar] [CrossRef]
- Wianda, E.; Ross, B. The Roles of Alpha Oscillation in Working Memory Retention. Brain Behav. 2019, 9, e01263. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Doppelmayr, M.; Pecherstorfer, T.; Freunberger, R.; Hanslmayr, S. EEG Alpha Synchronization and Functional Coupling during Top-down Processing in a Working Memory Task. Hum. Brain Mapp. 2005, 26, 148–155. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated Cortical Projection of EEG Sensors: Anatomical Correlation via the International 10-10 System. NeuroImage 2009, 46, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Rubens, M.T.; Thangavel, A.; Gazzaley, A. Causal Role of the Prefrontal Cortex in Top-down Modulation of Visual Processing and Working Memory. Nat. Neurosci. 2011, 14, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Violante, I.R.; Li, L.M.; Carmichael, D.W.; Lorenz, R.; Leech, R.; Hampshire, A.; Rothwell, J.C.; Sharp, D.J. Externally Induced Frontoparietal Synchronization Modulates Network Dynamics and Enhances Working Memory Performance. eLife 2017, 6, e22001. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, M.R.; Zanto, T.P.; Gazzaley, A. Spatial Attention and the Effects of Frontoparietal Alpha Band Stimulation. Front. Hum. Neurosci. 2017, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Mengotti, P.; Käsbauer, A.S.; Fink, G.R.; Vossel, S. Lateralization, Functional Specialization, and Dysfunction of Attentional Networks. Cortex 2020, 132, 206–222. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Seidel Malkinson, T. Hemispheric Lateralization of Attention Processes in the Human Brain. Curr. Opin. Psychol. 2019, 29, 90–96. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. New Vistas for α-Frequency Band Oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- VanRullen, R.; Reddy, L.; Koch, C. Attention-Driven Discrete Sampling of Motion Perception. Proc. Natl. Acad. Sci. USA 2005, 102, 5291–5296. [Google Scholar] [CrossRef]
- VanRullen, R.; Koch, C. Is Perception Discrete or Continuous? Trends Cogn. Sci. 2003, 7, 207–213. [Google Scholar] [CrossRef]
- Varela, F.J.; Toro, A.; John, E.R.; Schwartz, E.L. Perceptual Framing and Cortical Alpha Rhythm. Neuropsychologia 1981, 19, 675–686. [Google Scholar]
- Borghini, G.; Candini, M.; Filannino, C.; Hussain, M.; Walsh, V.; Romei, V.; Zokaei, N.; Cappelletti, M. Alpha Oscillations Are Causally Linked to Inhibitory Abilities in Ageing. J. Neurosci. 2018, 38, 4418–4429. [Google Scholar] [CrossRef] [PubMed]
- VanRullen, R. Perceptual Cycles. Trends Cogn. Sci. 2016, 20, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Dustman, R.E.; Beck, E.C. Phase of Alpha Brain Waves, Reaction Time and Visually Evoked Potentials. Electroencephalogr. Clin. Neurophysiol. 1965, 18, 433–440. [Google Scholar] [CrossRef]
- Jin, Y.; O’Halloran, J.; Plon, L.; Sandman, C.; Potkin, S. Alpha EEG Predicts Visual Reaction Time. Int. J. Neurosci. 2006, 116, 1035–1044. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Schimke, H.; Packinger, T. Alpha Frequency Reaction Time and the Speed of Processing Information. J. Clin. Neurophysiol. 1996, 13, 511–518. [Google Scholar] [CrossRef]
- Linkenkaer-Hansen, K.; Nikulin, V.V.; Palva, S.; Ilmoniemi, R.J.; Palva, J.M. Prestimulus Oscillations Enhance Psychophysical Performance in Humans. J. Neurosci. 2004, 24, 10186–10190. [Google Scholar] [CrossRef]
- Jensen, O.; Bonnefond, M.; VanRullen, R. An Oscillatory Mechanism for Prioritizing Salient Unattended Stimuli. Trends Cogn. Sci. 2012, 16, 200–206. [Google Scholar] [CrossRef]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-Frequency Coupling Supports Multi-Item Working Memory in the Human Hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and Functions of Theta Rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef]
- Hsieh, L.T.; Ranganath, C. Frontal Midline Theta Oscillations during Working Memory Maintenance and Episodic Encoding and Retrieval. NeuroImage 2013, 85, 721–729. [Google Scholar] [CrossRef]
- Lisman, J.; Jensen, O. The Theta-Gamma Neural Code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G. A Neural Coding Scheme Formed by the Combined Function of Gamma and Theta Oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. The Magical Mystery Four: How Is Working Memory Capacity Limited, and Why? Curr. Dir. Psychol. Sci. 2010, 19, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Tesche, C.D. Frontal Theta Activity in Humans Increases with Memory Load in a Working Memory Task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-Parietal EEG Coherence in Theta and Upper Alpha Reflect Central Executive Functions of Working Memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Zambrano-Vazquez, L.; Allen, J.J.B. Theta Lingua Franca: A Common Mid-Frontal Substrate for Action Monitoring Processes. Psychophysiology 2012, 49, 220–238. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal Theta as a Mechanism for Cognitive Control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertaccini, R.; Ellena, G.; Macedo-Pascual, J.; Carusi, F.; Trajkovic, J.; Poch, C.; Romei, V. Parietal Alpha Oscillatory Peak Frequency Mediates the Effect of Practice on Visuospatial Working Memory Performance. Vision 2022, 6, 30. https://doi.org/10.3390/vision6020030
Bertaccini R, Ellena G, Macedo-Pascual J, Carusi F, Trajkovic J, Poch C, Romei V. Parietal Alpha Oscillatory Peak Frequency Mediates the Effect of Practice on Visuospatial Working Memory Performance. Vision. 2022; 6(2):30. https://doi.org/10.3390/vision6020030
Chicago/Turabian StyleBertaccini, Riccardo, Giulia Ellena, Joaquin Macedo-Pascual, Fabrizio Carusi, Jelena Trajkovic, Claudia Poch, and Vincenzo Romei. 2022. "Parietal Alpha Oscillatory Peak Frequency Mediates the Effect of Practice on Visuospatial Working Memory Performance" Vision 6, no. 2: 30. https://doi.org/10.3390/vision6020030
APA StyleBertaccini, R., Ellena, G., Macedo-Pascual, J., Carusi, F., Trajkovic, J., Poch, C., & Romei, V. (2022). Parietal Alpha Oscillatory Peak Frequency Mediates the Effect of Practice on Visuospatial Working Memory Performance. Vision, 6(2), 30. https://doi.org/10.3390/vision6020030





