Abstract
A growing body of literature offers exciting perspectives on the use of brain stimulation to boost training-related perceptual improvements in humans. Recent studies suggest that combining visual perceptual learning (VPL) training with concomitant transcranial electric stimulation (tES) leads to learning rate and generalization effects larger than each technique used individually. Both VPL and tES have been used to induce neural plasticity in brain regions involved in visual perception, leading to long-lasting visual function improvements. Despite being more than a century old, only recently have these techniques been combined in the same paradigm to further improve visual performance in humans. Nonetheless, promising evidence in healthy participants and in clinical population suggests that the best could still be yet to come for the combined use of VPL and tES. In the first part of this perspective piece, we briefly discuss the history, the characteristics, the results and the possible mechanisms behind each technique and their combined effect. In the second part, we discuss relevant aspects concerning the use of these techniques and propose a perspective concerning the combined use of electric brain stimulation and perceptual learning in the visual system, closing with some open questions on the topic.
1. Introduction
The idea of improving one’s eyesight, hearing or tactile perception has fascinated humans for centuries. In this perspective paper, we discuss the combined use of two techniques, perceptual learning and brain stimulation, whose individual use dates back more than 100 years ago. Perceptual improvements through repeated practice was first reported in scientific papers in the second half of the XIX century [1], while the use of devices to deliver electric stimulation to the brain as a means to improve perceptual functions has been reported as early as the XVIII century [2]. Both these techniques went on to greatly contribute to our understanding of the mechanisms through which our brain responds, learns, and adapts to sensory stimulation.
1.1. Improvements through Practice
Behavioral studies investigating practice-induced sensory improvements commonly fall under the name of perceptual learning [3]. Over the last decades, perceptual learning has been applied to every sensory domain, building a rich literature that includes numerous models [4,5,6], proposed mechanisms [7,8,9,10], and sophisticated paradigms [11,12,13]. In the visual domain, visual perceptual learning (VPL) effects have been observed for a large variety of tasks, such as contrast detection [14,15,16], motion perception [17,18,19], visual search [20,21,22], texture discrimination [23,24], and more. Foundational VPL studies suggested that the observed improvements following extensive practice were due to neural plasticity changes at the earlier stages of sensory processing [23,25] that could in turn lead to improvements at later stages of perception, relying on input signals originating from these early areas [26]. In this context, VPL-mediated improvements were understood to be limited by the properties of neurons at the early stages of visual perception, thus leading to learning gains specific for stimulus features such as orientation, spatial frequency, retinal location, and the eye [23,24,27,28]. However, more recent studies and models depict a rather complex scenario, in which multiple mechanisms, processes and brain regions can be involved, in series or in parallel, in giving rise to learning [8,11,29,30,31] (see Stimulus box in Figure 1). A better understanding of VPL mechanisms led to the realization that features earlier reported, such as learning specificity, might be consequences of tasks and stimuli used in classic VPL studies rather than defining characteristics of it [6]. Crucially, generalization of learning from a limited set of stimuli to everyday life visual needs would improve VPL’s translational value, opening up possibilities for its use in visual rehabilitation and clinical interventions [32,33]. There is evidence of positive VPL results in treating some clinical pathologies such as myopia [34], presbyopia [26], and amblyopia [35], although the results are slightly less encouraging for more serious visual pathologies such as central vision loss due to macular degeneration [33,36,37,38].
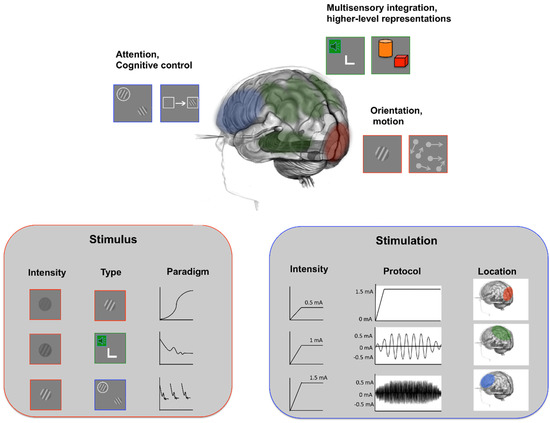
Figure 1.
Stimulation and training features contributing to learning outcomes. Our current understanding of both VPL and tES indicates that several elements concur in generating the behavioral improvements reported in both literature studies, and optimizing these aspects can help boost learning outcomes. Characteristics of the visual stimulus can engage different sensory, attentional and cognitive areas, while characteristics of the stimulation can boost or inhibit perceptual and learning outcomes.
1.2. Improvements through Electric Brain Stimulation
The last few years have witnessed an upsurge in studies describing the effects of different types of brain stimulation on modulating brain cortical activity and enhancing sensory performances [39,40] (see Stimulation box in Figure 1). Different types of non-invasive brain simulation techniques fall under the umbrella term of transcranial electric stimulation (tES) [41].
Direct current: A form of tES that has found large use in vision sciences is transcranial Direct Current Stimulation (tDCS) [42,43,44,45,46]. In this protocol, a weak electric current (1–2 mA) passes through two electrodes placed on the participant’s scalp, referred to as anodal (+) and cathodal (-) electrodes. This current is able to modulate the frequency rate of firing of the neural populations underneath by inducing polarity-specific changes in neural excitability [45]. Unlike other brain stimulation techniques such as transcranial magnetic stimulation (TMS) or deep brain stimulation (DBS), the intensity of the current used for tES is not sufficient to induce neuronal activation per se, but can cause alterations of resting membrane potential [47]. The effects of tES have been initially studied in the motor cortex, where they are well characterized. Specifically, anodal tDCS increases and cathodal tDCS decreases cortical excitability (as measured by TMS-induced motor-evoked potential (MEP) thresholds [45,46,48,49,50]). Furthermore, anodal tDCS over the motor cortex on five consecutive days during motor task sessions significantly enhanced the learning of the complex motor skill task when compared to a no stimulation (sham) condition [51].
Conversely, tES use in the visual cortex has a more recent history [52,53,54,55,56,57,58] and shows a higher degree of variability. While there is evidence of increases in visual cortex excitability via anodal tDCS and decreases via cathodal tDCS [43,44,52], other studies found conflicting results in visual-evoked potential (VEP) component modulation. Anodal stimulation increased and cathodal stimulation decreased the amplitude of the N70 component [59], while the opposite has been observed for the P100 component [52]. The authors in [60] showed that anodal tDCS transiently and significantly increased VEP amplitudes and contrast sensitivity (CS) in amblyopic eyes, while cathodal tDCS decreased VEP amplitude and CS. However, some other studies failed to observe any amplitude modulation after tDCS [61,62]. Behaviorally, Antal and colleagues [53] reported significant decreases in static and dynamic CS after cathodal stimulation, whereas anodal stimulation did not produce significant CS changes, a result corroborated by Chaieb and colleagues [55]. Conversely, other studies reported significant effects of anodal stimulation on CS, in particular for high spatial frequencies in healthy participants [54,63,64,65] and individuals suffering from amblyopia [58] and no effect of cathodal stimulation [63]. Reinhart and colleagues [64] showed that anodal stimulation improved Vernier acuity and increased VEPs amplitude, while cathodal stimulation had the opposite effects on both. Effects of anodal tDCS have also been reported to reduce surround suppression [66].
Alternate current: Alongside anodal and cathodal tDCS, the two other most common tES techniques seem to be transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS), in which the current flow direction, and consequently the role of the electrodes, change over time. Specifically, during tACS, the current is delivered with an alternated sinusoidal pattern at a single specific frequency, while during tRNS, the current is delivered in a range of frequencies (usually between 0.1 and 1000 Hz), although the current is often referred to as hf-tRNS for frequencies starting from above 600 Hz and lf-tRNS for frequencies below 100 Hz, see [57,67]. Both protocols have been successfully used to boost visual functions such as CS [54], crowding [68], motion discrimination [69], and orientation discrimination [57].
There is evidence of tES-related behavioral effects on visual tasks for stimulation of areas beyond the early visual cortex. Olma and colleagues [70] observed improvement in motion perception, a function attributed to the extrastriate area MT, after occipital anodal tDCS, while Battaglini and colleagues [68] reported improvements in visual crowding reduction following parietal cortex stimulation.
2. Perceptual Learning Combined with Different Types of tES
As mentioned earlier, VPL can improve sensory processing and in turn perceptual abilities. These improvements, however, often come with some constraints. For example, the majority of classic perceptual learning studies reported high degrees of learning specificity (i.e., lack of transfer of training effects to different tasks, stimuli, retinal location, etc.) and a large number of training sessions/hours of practice necessary to observe significant results [3] (however, see [71]).
As a first step toward understanding whether electric brain stimulation can alleviate some of these constraints, Fertonani, Pirulli and Miniussi [57] compared the effects of different brain stimulation protocols, specifically anodal tDCS, cathodal tDCS and tRNS, while participants were engaged in an orientation discrimination task. Results showed significant between-blocks improvements when electric brain stimulation, specifically anodal tDCS and tRNS, was delivered on the occipital cortex of participants when compared to sham stimulation (control condition). Additionally, the authors reported larger learning rates for tRNS with respect to anodal tDCS, as the stimulation protocol was coupled with training. Further studies using anodal tDCS and tRNS showed their effectiveness in promoting visual functions in healthy participants, both in fovea when paired with orientation and contrast detection tasks [72,73] and in peripheral vision when paired with crowding reduction training [74].
A series of studies have used tACS in combination with PL to benefit from tACS’s ability to transiently modulate brain oscillations at specific frequencies [75,76,77,78] some of which, i.e., those in the alpha band (8–12 Hz), are associated with learning and consolidation [79]. He and colleagues [80] showed that occipital tACS at 10 Hz, but not at 20 Hz or 40 Hz, increased both learning rate and performance improvement during an orientation discrimination task. Crucially, tES appears to boost both the early (within session [57]) and late (between sessions/days [81,82]) components of VPL, and its behavioral effects seem to be long-lasting [83,84] (however, see [74]). There is also evidence that tRNS coupled with training induces larger transfer of learning with respect to behavioral training or brain stimulation alone [82,85].
Taken together, results from this literature study suggests that combining electric brain stimulation and VPL can boost the learning rate of a task and reduce the time/number of sessions/trials needed to observe significant training improvements.
Importantly, behavioral effects of electric brain stimulation and perceptual learning on visual tasks can be observed for targeted regions beyond occipital, sensory areas. Contò and colleagues [86] applied tRNS over the intraparietal sulci during attentional training and found behavioral improvements and an increase in resting-state functional connectivity within the dorsal and ventral attention networks. This opens up the possibility to use brain stimulation to modulate several aspects of learning beyond low-level sensory enhancement, including, but not limited to, attention, oculomotor control, expectations, task and instruction comprehension [6].
3. Time Course of Different tES Protocols
A crucial aspect of tES, both when applied by itself and when combined with VPL, is its effect in relation to the time of delivery. The interaction between timing of stimulation and learning has been initially explored in the context of motor learning [87,88,89], with studies showing that anodal tDCS increased the rate of learning when applied ‘online’ (during the execution of the task) [88,89] but not ‘offline’ (before the task) [89]. Conversely, in the visual domain, Pirulli, Fertonani and Miniussi [72] reported significant improvement in performance when anodal tDCS was applied offline but not when it was applied during training, while the opposite was observed for tRNS. The evidence that the same tES protocol led to different behavioral outcomes suggests that the neural modulation effects induced by tES differ depending on the excitability levels of the stimulated neurons at the time of stimulation application [72]. Other studies showed that the time interval between consecutive stimulation periods influences the neural modulation effect. Fricke and colleagues [90] showed that 5 min of anodal tDCS increased neuronal excitability for approximately 5 min; however, when a second 5 min anodal tDCS period follows after 3 min from the end of the previous session, the second session has the opposite effect, thus leading to a decrease in cortical excitability. Similarly, Monte-Silva and collaborators [91] showed that a second period of stimulation during the after-effects of a first period initially causes a decrease in motor cortex excitability, which then turns into an increase in excitability. While Pirulli, Fertonani and Miniussi [72] reported offline effects of anodal tDCS when it was delivered before training on orientation discrimination, Yang, He and Fang [73] more recently showed that anodal tDCS delivered after the training session, during a texture discrimination task, led to larger learning effects than the sham, suggesting that anodal tDCS might help consolidate learning across sessions, consistent with what was observed in the motor learning [51].
Additionally, there is evidence that cortical modulation effects induced by tES extend beyond the stimulation window [47], possibly involving long-term potentiation-like mechanisms [92]. Kasten, Dowsett and Herrmann [93] reported a sustained enhancement of alpha power 70 min after occipital tACS at individual alpha frequency. Similarly, occipital tRNS exhibited consistent excitability (decrease in phosphene threshold), lasting 60 min post-stimulation [94], similar to what was reported for motor cortex stimulation [56].
4. Mechanisms of tES
The effectiveness of tES, alone or when paired with training, has often led, especially early on, to an overlook of the mechanisms involved in the observed phenomena, although the recent literature has offered an excellent overview of mechanisms and models (e.g., [41,95]). The exact mechanisms governing tES effects are still elusive, and animal models are lacking; thus, hypotheses on the mechanisms in the human brain are speculative. Concerning tDCS, one of the most common interpretations of its effects is that the weak current that travels from one electrode (anode) to another (cathode) induces polarity-specific effects on the cortical excitability of regions traversed by the current. Specifically, regions under cathodal stimulation experience membrane hyperpolarization, which has been shown to decrease cerebral excitability, while conversely, regions under anodal stimulation undergo depolarization, which leads to increased cortical excitability. However, prolonged delivery of anodal tDCS might cause sustained depolarization [96]; thus, an initial facilitatory effect might reverse into inhibition in case of longer stimulation periods. These saturation mechanisms may not occur when the stimulation is delivered before the task [72].
It has been suggested that anodal and cathodal tDCS might modulate distinct neuronal populations. Recent studies showed that anodal tDCS reduces intracortical inhibition [89,97] through a reduction in GABA concentration [98,99,100,101,102]. GABA-mediated inhibition has been linked to a number of suppressive neural interactions within the visual cortex such as those underlying surround suppression [103,104]. Consistently, Spiegel and collaborators [66] observed significant reductions in psychophysically measured surround suppression following anodal tDCS. GABA-mediated inhibition is one of the proposed mechanisms regulating cortical plasticity in rodent models of deprivation amblyopia [105], and behavioral results in humans show CS improvements in adults with amblyopia [60]. Moreover, cortical inhibition mediated by GABA seems to cast a constraint on brain plasticity, especially in the visual cortex [106,107,108,109], and some visual pathologies such as amblyopia appear related with abnormally higher cortical inhibition. A previous study has shown that inhibiting monoamine reuptake enhances the duration of the aftereffects of anodal tDCS, and that both anodal and cathodal after-effects were reduced by a β-adrenergic receptor blocker [110]. This is consistent with the suggestion that tDCS might ‘substitute’ neuromodulatory effects normally associated with neurobiological systems, in particular that of the norepinephrine (NE) circuit [111].
Conversely, cathodal tDCS does not seem to affect GABA-mediated inhibitory interactions. For example, administering a GABA-antagonist does not affect the reduction of motor cortex excitability induced by cathodal tDCS [112], while it blocked the reduction of intracortical inhibition normally observed with anodal tDCS. This is consistent with behavioral data showing no effect of cathodal tDCS on surround suppression [66]. By contrast, Stagg and colleagues [101] observed modulation of glutamate levels following cathodal tDCS. A functional MRI study also suggested that anodal and cathodal stimulations modulate distinct systems-level networks within the active motor system [113].
Regarding tACS, evidence suggests that the alternated current, delivered at a specific frequency, can entrain cortical oscillations [78,114]. A possible alternative interpretation is that tACS might induce long-lasting plasticity-like changes, which have been observed both in the motor [115] and the visual cortex [116].
Concerning tRNS, Terney and colleagues [56] suggested that the repeated stimulation of tRNS might allow Ca2+ and Na+ channels to rapidly reopen [117]. This repeated subthreshold stimulation could induce an increase in the sodium inflow and a consequent prolonged depolarization and induction of long-term potentiation-like phenomena [56,57,117]. Pirulli, Fertonani and Miniussi [72] suggested that the larger effects observed for online tRNS with respect to offline anodal tDCS on learning rate might be explained by temporal properties of this protocol and its interaction with task-induced activity. The repetitive and random-wave shape of tRNS would lead to temporal summation of stimulus-induced activity, thus boosting neural activity in the process. The authors further suggested that the high frequency of tRNS (600–1000 Hz) may interact optimally with neural activity because it approaches the time constant of the cell body and dendrites, between 1 and 10 ms [118]. Conversely, if the stimulated neural population is not involved in the task execution, no task-related neuronal activity should occur, and the effects of tRNS should be null, which is consistent with experimental data with offline tRNS [57].
In the context of tES effects on the visual system, a model proposed by Miniussi and colleagues addresses the interaction between stimulation intensity and cortical activity elicited by a visual stimulus. This stochastic resonance framework of brain stimulation [119] combines the interaction between external noise coming from the tES, internal baseline activity and stimulus-driven activity, providing predictions on the perceptual outcome. In particular, in case of a low intensity stimulus, external noise would enhance the signal above the perceptual threshold, increasing the signal-to-noise ratio; while in case of a higher intensity stimulus, the external noise would disruptively boost the non-signal spontaneous internal activity, thus reducing the signal-to-noise ratio [120,121].
5. Perceptual Learning, tES and Clinical Populations
As mentioned earlier, VPL has been used in pathologies in which the optical or cortical aberrations are relatively mild and can be counteracted by neural plasticity to achieve total restoration of foveal functions (e.g., [26,34,35]). VPL has the potential of becoming the treatment of election for treating sensory pathologies [32,33]; however, some of its drawbacks, in particular the large number of sessions required to observe significant results and the high degree of learning specificity often reported, still limit its large-scale use in clinical populations. Adding tES to VPL holds the promise of addressing such limitations. There is evidence of larger training effects [57,74] and transfer [81,82,122] when electric brain stimulation was coupled with VPL with respect to VPL alone. Specifically, Camilleri and colleagues [81] used VPL in combination with tRNS in myopic patients. The results showed that 2 weeks of training with this protocol led to improvements in visual acuity (VA) comparable to those observed after 8 weeks with a purely behavioral training, with additional improvement in CS that was not observed in the behavioral-only training.
Similarly, Campana and collaborators [82] showed that the combination of tRNS and contrast detection training substantially improved VA and CS of amblyopic patients after eight sessions of training, with effect sizes comparable to those reported in a previous behavioral study with 30 to 80 training sessions [26]. More recently, Herpich and colleagues [84] used tDCS and tRNS coupled with VPL to train cortical blindness (CB) patients on motion perception in their blind field for 10 days. The results showed fast and long-lasting (6-month follow up) improvements in motion perception for the three CB patients in the tRNS group, while tDCS showed no advantage over the training-only group and the control region stimulation group.
6. Considerations on the Use of tES with VPL
tES and VPL are both valuable tools for inducing performance-enhancing neural plasticity. With the right understanding of their combined use, the outcome could be extremely valuable, from a theoretical and clinical perspective. However, both fields present complexities that are often overlooked, while conflicting results are sometimes left unaddressed. For example, while some of the disagreements between motor and visual tES studies may be explained by structural [123,124] and functional [125,126] differences between neural regions, the observation opens a larger point on which elements concur in producing the tES effects observed in different studies. The fact that multiple aspects most likely concur in the effects reported by brain stimulation studies has been acknowledged by several authors (e.g., [41,95,127]). Similarly, several authors in the field of PL are working on new and more refined models to describe the mechanisms governing learning and plasticity and to address relatively recent evidence in apparent contradiction with earlier (and simpler) models (e.g., [11,13]). We here identify four aspects that we consider crucial in the understanding behavioral effects of tES and PL.
Anatomy. As mentioned earlier, anatomical and functional characteristics of the stimulated area play a role in the brain stimulation effects. For example, stronger occipital tES effects would be expected for higher spatial frequencies because the current is most intense the closest to the surface of the cortex [128,129], where V1 neurons are tuned toward higher spatial frequencies [130,131]. Similarly, neurons located away from the occipital poles have peripheral receptive fields; thus, stimuli presented in the parafovea might not be affected as strongly by tES with respect to foveal stimuli [63]. Furthermore, the orientation of the stimulated units might play a role in the effect of tDCS [95], with evidence showing that neurons with axons parallel to the electric field are activated by anodal and are inhibited by cathodal tDCS [47,132], while the opposite has been observed for non-apical dendrites [133]. Finally, the spatial extent of tDCS-induced cortical modulation can reach beyond the targeted regions to structures connected to them [134].
Baseline performance. Sensory systems are ‘tuned’ to preferred features of the sensory stimulation, which determine the baseline performance of the system and in turn modulate tES effects. For example the visual system has higher sensitivity for stimuli oriented along cardinal orientations [135,136], thus potentially reducing room tES-mediated improvements in sensitivity along those axes, but allowing room for tES inhibitory effects (i.e., cathodal tDCS). Conversely, oblique stimuli may be more responsive to the facilitatory effects of anodal tDCS [65]. In the context of cognitive and memory studies, it has been shown that the effect of the stimulation correlates with the baseline performance of the participant [137,138].
Stimulus intensity and type. Evidence from perceptual studies show that the type of stimulus can selectively engage different cortical regions, while occipital tDCS studies show opposite phase-dependent modulation of the same VEP component when participants were tested using different stimuli (checkerboard pattern-reversal in [52] vs. stripe pattern-onset in [43]), thus suggesting an equally relevant role of stimulus type in both contexts. Similarly, Antal and colleagues [59] showed that cathodal tDCS over the occipital cortex improved or impaired motion perception depending on the type of motion stimulus.
Stimulus intensity affects the perceptual outcome by modifying the signal-to-noise ratio between the target and distractors/background. Consequently, low stimulus signal might be enhanced by tES, while high signal might be disturbed [119]. In the context of VPL, it then becomes important to calculate performance level across training days to estimate the optimal signal intensity. Studies utilizing low signal-to-noise perceptual threshold tasks detected significant anodal tDCS effects on CS [63,139], while a similar study identified no stimulation effects of anodal tDCS when supra-threshold stimuli were used [53]. Similarly, Antal and collaborators [43] reported phase-dependent modulation of N70 component amplitude with occipital cathodal and anodal stimulation, but only with presentation of low contrast stimuli. Higher contrast stimuli might lead to optimal activation of visual areas responding to the stimuli, such that the subthreshold (excitatory) modulation effect of the tDCS would not be able to modify the VEP amplitude. Stimulus intensity also seems crucial in eliciting transfer of learning, with training with para-threshold stimuli usually leading to better learning and transfer effects with respect to supra-threshold stimuli in healthy participants [3,140,141] and transfer [26] as well as in some clinical populations [142].
Stimulation intensity and type. Mirroring the previous point, intensity and type play a crucial role for stimulation as well. Changes in stimulation intensity can give rise to opposite outcomes for identical stimulation protocols. Pavan and colleagues [67] showed that tRNS modulated performance in a global motion perception task as a function of the stimulus intensity, with low (1.5 mA) stimulation leading to optimal performance and high (2.5 mA) stimulation leading to impairment of motion discrimination, while evidence from tACS studies at 140 Hz suggests that the excitatory effects observed at 1 mA [143] might be reversed at lower (0.4 mA) stimulation intensities [144]. Some studies reported an increase in cortical excitability proportional to stimulation intensity in the motor cortex [145], while others reported a less straightforward relationship [146].
Similarly, stimulation type can lead to different outcomes, with anodal tDCS generally enhancing and cathodal tDCS generally inhibiting cortical excitability, while, in the context of tACS and tRNS, aspects such as stimulation frequency can produce different results. He et al. (2021), using tACS, reported that training improvements when stimulation was delivered at 10 Hz, but not 20 Hz or 40 Hz, lead to improvements. Similarly, Fertonani, Pirulli and Miniussi [57] showed that high-frequency (600–1000 Hz) but not low-frequency (0.1–100 Hz) tRNS increased learning effects with respect to the sham, while Moret, Donato, Nucci, Cona, and Campana [147] showed that offline tRNS cortical modulation in the motor cortex was observed only when the range of stimulation frequencies was large (100 Hz–700 Hz), but not when it was only high (400–700 Hz) or low (100–400 Hz). Of note, some studies reported changes in polarity-specific effects as a function of distance between the electrode and the targeted area. Anodal tDCS increased and cathodal tDCS decreased cortical activation in units close to the electrode in mice studies [89], while the opposite (facilitation after cathodal tDCS and inhibition after anodal tDCS) was observed for units in deeper cortical layers [148].
To sum up, differences between the simple relationship between type of stimulation and measurable effects on the motor cortex vs. the complex effects in the visual cortex can be at least partially explained in terms of anatomy, baseline performance, underlying system activity, stimulus, and stimulation intensity and type [149]. Recent models of tES in the visual cortex take into account features of the stimulation, baseline activity of the visual system and stimulus-driven activity [119], which seem consistent with behavioral results using visual tasks and occipital stimulation (e.g., [67]).
Concerning the stimulus, different cortical activations in sensory areas and performance levels are expected for different intensity levels, often subject to large inter-individual differences. Different types of stimuli can selectively engage different areas. For example, simple gratings would maximally engage the early visual cortex and audio–visual stimulation would tap into multisensory integration regions in the parietal lobe, while attentional manipulation can engage prefrontal, cognitive regions as well as perceptual areas. In terms of paradigm, differences in procedures (constant stimuli vs. long adaptive staircase vs. short adaptive staircase) can lead to differences in learning and transfer (e.g., Hung and Seitz, 2014). Concerning the stimulation, the right intensity level seems to be crucial in observing positive, rather than disruptive, perceptual effects. Similarly, different protocols seem to affect the targeted populations differently, with some general effects (e.g., anodal tDCS increasing and cathodal tDCS decreasing cortical excitability) and some specific to parameters (e.g., tRNS is mostly effective in boosting learning for a large range of frequencies [147]; anodal tDCS appears to boost learning only when delivered before the training session [72]; tACS enhances learning effects at 10 Hz but not at 20 and 40 Hz [80], etc.). Finally, the targeted location plays a role in the behavioral effects, depending on whether it is directly engaged in the visual task and depending on which subcomponent of the training procedure (i.e., task vs. stimulus component) the targeted area subserves.
7. Perspectives on the Combined Use of tES and VPL
We here present a series of observations on these techniques that we hope can guide future studies on this topic.
- -
- Learning is a complex and dynamic process involving low-level, perceptual regions as well as higher-level, cognitive and attentional areas [6,8,31,150]; moreover, multiple mechanisms, acting in series or in parallel [10] underlie learning, potentially resulting in modifications of the functional specialization of cortical areas [151,152]. Our current understanding of brain mechanisms involved in learning conveys an image of higher sophistication than the earlier studies led us to believe. Multiple mechanisms, parallel or serial, are involved. For example, Jing and colleagues [8], using monkey electrophysiology, reported that improvements in a global form detection task were accompanied by parallel neural changes in both sensory and prefrontal areas, which exhibited different time courses within each area as the training progressed. Specifically, stimulus- and task-dependent changes emerged earlier in sensory areas (V4) than in prefrontal areas (ventrolateral prefrontal cortex) and exhibited high specificity for task and target features, while behavioral-related changes followed the opposite pattern, emerging earlier in prefrontal than in sensory areas and exhibiting larger generalization to untrained configurations. Similarly, Shibata and collaborators [10], using human neuroimaging, showed evidence for task- and stimulus-related plasticity, taking place in different regions of the occipital cortex and intraparietal sulcus, following motion-detection training. There is also evidence of different time-specific learning mechanisms. Itthipuripat and colleagues [153], using electrophysiology, suggested that learning has an initial phase dominated by an increase in attentional gain, later replaced by noise reduction mechanisms. Moreover, neuroimaging evidence suggests that VPL is characterized by dissociable neural and functional changes in the visual cortex over time [151,152,154].
Chang and colleagues [151] showed that TMS over the posterior parietal cortex disrupted the extraction of depth cues in noise, but only before participants were trained in a fine-depth discrimination task. Similarly, Chen and colleagues [152] found that inhibiting the activity of MT+ would affect extracting motion direction from noise, but only before motion discrimination training. Similar results have been reported in studies with non-human primates [155,156].
Taken together, these results suggest that multiple brain regions and time-dependent dynamics dominate learning, which could result in changes in systems that are read-out to solve a given visual task. Multiple brain regions involved in learning mean that both multiple possible sites of stimulation and multiple sites can be selectively engaged by accurate task design (see Figure 2). Studies using brain stimulation and VPL should be cognizant of such dynamics and complexities and use our understanding of learning mechanisms from VPL literature to optimize the selection of stimulus, protocols, and target regions of stimulation.
- -
- tES modulates cortical excitability beyond sensory and motor areas. While the majority of tES studies has focused on motor or sensory brain regions, multiple pieces of evidence suggest that tES can modulate cortical excitability in brain regions preferentially involved in higher-level processes implicated in vision such as attention and cognition. Arif and colleagues [157] showed that occipital anodal tDCS has a polarity-dependent effect on the neural oscillations subserving attentional reorientation in adults and that such effects may be related to altered concentrations of GABA within neural networks involved in attentional reorientation. Contò and colleagues [86] showed improvements in behavior and functional connectivity between nodes of the dorsal and ventral attention network when tRNS was delivered over the intraparietal sulci during attentional training. Furthermore, long-term effects of multi-session tRNS have been reported for dorsolateral prefrontal cortex, resulting in a boost in mental arithmetic performances 6 months post-stimulation, which also correlated with an increase in activity within the stimulated area [158].
- -
- Select the stimulation protocol to optimize the behavioral outcome. Different protocols can be used to achieve different results. tRNS seems to be more effective in improving learning rate [57,74] and generalization [81,82,122] when used during training, while anodal tDCS boosts both perceptual performance and learning consolidation when used before [72] or after [73] behavioral sessions, respectively, rather than online. Similar to tRNS, tACS seems to be effective in modulating cortical excitability mainly when used online [159].
- -
- Optimize stimulus and stimulation intensity. Brain stimulation effects on behavior are dependent upon the intensity of the stimulation [67]. The optimal stimulation intensity might in turn depend on the targeted cortical region(s), the task at hand, and the participants’ individual threshold. Additionally, the size of the affected cortical and the current density are dependent on both the stimulation intensity and the size of the electrode [160]. Additionally, there is evidence of non-linear effects involving timing and dosage. Mosayebi-Samani conducted a systematic exploration of the effects of parameters manipulation on tDCS over the motor cortex [161]. The results showed non-linear effects of stimulation intensity and duration, in particular intensities of 1 and 3 mA reduced cortical excitability, while 2 mA increased it. Additionally, 1 and 3 mA stimulation for 15 min induced long-term depotentiation-like plasticity, while on the contrary, 20 min of 2 mA stimulation induced long-term potentiation-like plasticity. Agboada and colleagues (2020) compared a 15 min session of motor cortex anodal tDCS at 1 mA with a 20 min session at 3 mA [162]. When cortical excitability was measured after a single session, both protocols showed a 30 min aftereffect when compared to the sham. When a second session was delivered after a 20 min interval, the aftereffect of the 3 mA protocol lasted 2 h, while that of the 1 mA was still present after 24 h. When the second session was instead delivered 3 h after the first, no increase in cortical excitability was observed for the 3 mA, and only a minor increase was observed for the 1 mA intensity. This once again points toward non-linear effects of the numerous parameters involved in brain stimulation. Importantly, such systematic studies have not yet been conducted in the visual cortex. Further evidence supports intensity-dependent effects in tACS as well. Specifically, Johnson and colleagues (2020), using monkey single-cell recording, showed that tACS-induced modulation, in the form of phase entrainment, and increase in spike frequency, was proportional to the current intensity, with more units exhibiting modulation for higher intensities of stimulation [163]. Similarly, VPL effects are dependent upon the intensity of the stimulus; training too close to the threshold might disrupt transfer of learning [164], while providing a variety of stimulation might prevent sensory habituation [30]. In the context of stochastic resonance, both stimulus and stimulation intensity combine to produce the final behavioral outcome. To optimize such an outcome, one should carefully select and control for both, from choosing the size of the electrodes (and possibly, the electrode configuration montage; see [52,59]) and the current intensity to the experimental paradigm and features of the stimuli, such as size, orientation, contrast, speed, etc.
- -
- Understand the time course and washout of tES to optimize learning consolidation. While brain stimulation is commonly used before or during training to increase cortical excitability and to boost neural plasticity, post-stimulation effects are somehow overlooked. There is evidence of post-stimulation washout effects of tDCS [70], tACS [93] and tRNS [94,165] extending beyond the window of stimulation for over 1 h, which could potentially disrupt some of the learning gain. Thus, promoting consolidation by means of brain stimulation might produce more robust learning. Reis and colleagues [51], and more recently Yang, He and Fang [73], showed that anodal tDCS delivered after motor and visual training, respectively, led to larger learning effects than the sham.
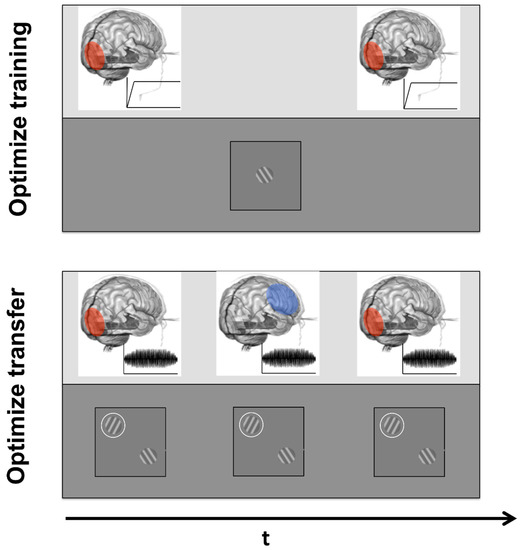
Figure 2.
Examples of combined use of brain stimulation and perceptual learning to optimize learning (above) and transfer (below). Above: VPL can benefit from the help of tES to boost both neural plasticity before training (e.g., [72]) and consolidation after training [73]. Below: Having a task that engages both perceptual and attentional mechanisms and a stimulation protocol that targets both low- and high-level cortical regions might lead to larger generalization effects at both perceptual and cognitive levels.
Effective learning needs both plasticity and consolidation (see Figure 2); however, no study thus far investigated the possibility of using brain stimulation to both boost and consolidate learning.
- -
- Individual differences. Both brain stimulation and VPL are sensitive to inter-individual variability [55,166,167,168]. Several factors pertaining to subjective characteristics might play a role in the training effects, with evidence from both VPL and brain stimulation literature showing that anatomy, sex, age, and initial performance/cortical excitability baseline modulate training effects. For example, Kasten and colleagues showed a correlation between individual electric field variability and the modulatory effects of occipital tACS in increasing alpha power [169]. Similarly, Mosayebi-Samani and colleagues addressed observed inter-individual differences in tDCS-induced motor cortex excitability by estimating individual electric fields and anatomy [170]. The results showed that anatomical factors such as electrode-to-cortex distance and cortico-spinal fluid thickness negatively correlated with individual electric fields, which in turn correlated positively with tDCS cortical effects. Chaieb and colleagues [55] reported larger anodal (but not cathodal) tDCS effects in females with respect to male participants, suggesting that brain stimulation might interact with hormonal cycles. Some of the variability can be reduced following stimulation guidelines (e.g., [171]) by collecting a larger set of assessment tasks and individual difference measurements (i.e., questionnaires) and by using more sophisticated statistical models that include mediators and moderators of the effects we observe.
- -
- Understand the limitations of tES. While it is a powerful tool, tES still presents some constraints, mostly of technical nature. It is limited by coarse spatial and temporal resolution, which prevents small structures from being accurately and selectively targeted, and its shallow depth is not ideal for reaching inner structures. Moreover, despite attempts at reducing some of its adverse physical effects [172], some participants might still find it unpleasant, thus affecting compliance. Finally, it is of paramount importance to follow strict safety guidelines [171].
8. Open Questions
The relative novelty of this combined approach and the consequent size of the literature are bound to leave several open questions. Here, we list some of these questions.
- -
- Lack of systematic comparison of transfer and training effects across stimulation types. Few studies looked at training effects of different stimulation protocols [57,173], or their ideal onset of stimulation with respect to training sessions [72]; however, no comparison of transfer effects has been conducted, except for studies looking at cognitive/arithmetical abilities [173] or targeting higher-level cortical regions [174]. Crucially, transfer of learning is a more relevant measure of the translational value of a technique when it comes to its rehabilitative application.
- -
- Lack of multi-site stimulation effects on learning. Particularly, electric brain stimulation delivered beyond sensory areas. This might be in part due to the use of ‘local’ and ‘sensory’ frameworks of VPL, which interpret learning effects as a product of neural plasticity changes at the early stages of sensory processing, i.e., sensory areas. However, recent results [11,13,71] and models [6] suggest a more complex scenario in which several regions, including those associated with attention, memory and cognition, can be involved in VPL.
- -
- While VPL and tES have been used to treat mild optical conditions (e.g., myopia, presbyopia) or some visual pathologies of cortical nature (e.g., amblyopia), no study thus far has investigated tES effects in severe retinal pathologies, such as those leading to loss of central vision. For central vision loss following macular degeneration, basic intervention with behavioral paradigms might not be sufficient [36,37,175]. The loss of central vision in MD forces these patients to use a peripheral retinal spot to replace the fovea; thus, any intervention in MD should consider the need for this clinical population not only to improve the detail resolution of their peripheral vision, but also to reroute their oculomotor reference and attentional system toward a peripheral region that must be repurposed to accomplish this feat.
While results in simulated central vision loss seem to suggest that characteristics of oculomotor training could help MD participants develop PRL more rapidly [176,177,178,179,180], brain stimulation might provide a necessary boost to promote larger-scale cortical reorganization. Evidence of significantly larger transfer [122] and training effects in peripheral vision of healthy participants [74] is an encouraging result toward using this technique to improve peripheral vision in MD.
- -
- Lack of follow-up studies to quantify and evaluate long-term effects of tES. Unlike the vast literature on the long-term effects of the use of VPL paradigms alone [3,26,175], studies looking at the lasting effects of tES and VPL together are limited (see Table 1, last column). The few studies that did conduct follow-up tests of training effects are encouraging, suggesting that the learning and transfer gains observed by the end of the training are preserved at least 3 to 6 months after the end of the studies [81,84,122] (however, see [74]), in which a subgroup of participants trained with tRNS and VPL showed lack of long-term effect at 3-month follow up.
 Table 1. Overview of PL studies using tES.
Table 1. Overview of PL studies using tES.
Finally, we cannot exclude that our understanding of the effects of tES in improving perception, alone or paired with behavioral training, is at least partially limited by the tendency to publish statistically significant results showing advantage of tES vs. sham, often overlooking null or negative results, which would be rather informative in understanding optimal parameters for the maximization of training effects.
9. Closing Remarks
One of the most remarkable human features is the ability to improve in perceptual tasks after repeated practice. A body of work and techniques falling under the umbrella term of perceptual learning has studied this skill for close to 200 years, with the first studies dating back to the mid-XIX century. Early evidence and models of perceptual learning, especially in the visual domain (visual perceptual learning, VPL), suggest that this phenomenon is characterized by learning specificity (meaning that the improvements observed after practice are limited to the stimulus features and task used during training but disappear when learning is tested for different stimulus features or tasks) and finds its neural substrates in early sensory areas. Both VPL and brain stimulation lead to behavioral improvements due to neural plasticity, most likely in early sensory cortex.
Similar to VPL, brain stimulation effects are often discussed in the form of local and anatomically defined changes, often at the level of changes in neuron membrane potential within the confines of the stimulated region.
In the last few years, VPL studies have used concomitant brain stimulation to boost the learning effect, showing improved effects with respect to each of the two techniques used in isolation. This is often taken as evidence that the two techniques affect the same cortical regions and possible mechanisms. However, recent studies in the field of VPL have suggested that the local nature of its effects may be a methodological artifact due to classic studies in the field, which had little to no changes in stimulus and task features. Thus, location specificity might not be a property of VPL but rather a consequence of the way it has been studied. Later studies showed a transfer of learning after manipulations of classic paradigms and new and more refined models of VPL that tried to capture this newfound complexity.
Combining VPL and electric brain stimulation has led to promising results that can pave the way for important clinical applications of this protocol; however, the use of brain stimulation has not yet followed this new understanding of VPL. Evidence of additional complexity of brain stimulation effects on learning and interaction with VPL further suggests a need for a more thorough investigation of all the possible variables involved in these studies, with the goal of developing testable theoretical frameworks. Recent steps in the direction of a more integrative have been proposed, and this understanding appears crucial in moving the field forward [181].
In order for brain stimulation to unlock its full potential when coupled with VPL, time course, loci and type of stimulation should be carefully selected according to the mechanisms of VPL as we now better understand them. Such an approach holds the promise for more effective training paradigms, which could benefit both healthy and clinical populations.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
As this is a perspective paper which reviews current literature, it does not contain new data or involves informed consent from participants.
Conflicts of Interest
The author declares no conflict of interest.
References
- Gibson, E.J. Principles of Perceptual Learning and Development; Appleton-Century Crofts: New York, NY, USA, 1969. [Google Scholar]
- Zaghi, S.; Acar, M.; Hultgren, B.; Boggio, P.S.; Fregni, F. Noninvasive brain stimulation with low-intensity electrical currents: Putative mechanisms of action for direct and alternating current stimulation. Neuroscientist 2010, 16, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Sagi, D. Perceptual learning in Vision Research. Vis. Res. 2011, 51, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Ahissar, M.; Hochstein, S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn. Sci. 2004, 8, 457–464. [Google Scholar] [CrossRef]
- Dosher, B.A.; Lu, Z.L. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc. Natl. Acad. Sci. USA 1998, 95, 13988–13993. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, M.; Seitz, A.R. Towards a whole brain model of Perceptual Learning. Curr. Opin. Behav. Sci. 2018, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Fahle, M. Perceptual learning: A case for early selection. J. Vis. 2004, 4, 879–890. [Google Scholar] [CrossRef]
- Jing, R.; Yang, C.; Huang, X.; Li, W. Perceptual learning as a result of concerted changes in prefrontal and visual cortex. Curr. Biol. 2021, 31, 4521–4533.e3. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nanez, J.E.; Watanabe, T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 2010, 11, 53–60. [Google Scholar] [CrossRef]
- Shibata, K.; Sasaki, Y.; Kawato, M.; Watanabe, T. Neuroimaging Evidence for 2 Types of Plasticity in Association with Visual Perceptual Learning. Cereb. Cortex 2016, 26, 3681–3689. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Zhang, J.-Y.; Xie, X.-Y.; Yang, Y.-X.; Luo, S.-H.; Yu, C.; Li, W. Perceptual learning at a conceptual level. J. Neurosci. 2016, 36, 2238–2246. [Google Scholar] [CrossRef]
- Wright, B.A.; Sabin, A.T.; Zhang, Y.; Marrone, N.; Fitzgerald, M.B. Enhancing perceptual learning by combining practice with periods of additional sensory stimulation. J. Neurosci. 2010, 30, 12868–12877. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-Q.; Zhang, J.-Y.; Wang, R.; Klein, S.A.; Levi, D.M.; Yu, C. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr. Biol. 2008, 18, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- De Valois, K.K. Spatial frequency adaptation can enhance contrast sensitivity. Vis. Res. 1977, 17, 1057–1065. [Google Scholar] [CrossRef]
- Fiorentini, A.; Berardi, N. Perceptual learning specific for orientation and spatial frequency. Nature 1980, 287, 43–44. [Google Scholar] [CrossRef]
- Mayer, M.J. Practice improves adults’ sensitivity to diagonals. Vis. Res. 1983, 23, 547–550. [Google Scholar] [CrossRef]
- Ball, K.; Sekuler, R.; Machamer, J. Detection and identification of moving targets. Vis. Res. 1983, 23, 229–238. [Google Scholar] [CrossRef]
- Ball, K.; Sekuler, R. A specific and enduring improvement in visual motion discrimination. Science 1982, 218, 697–698. [Google Scholar] [CrossRef]
- Ball, K.; Sekuler, R. Direction-specific improvement in motion discrimination. Vis. Res. 1987, 27, 953–965. [Google Scholar] [CrossRef]
- Ahissar, M.; Hochstein, S. Learning pop-out detection: Specificities to stimulus characteristics. Vis. Res. 1996, 36, 3487–3500. [Google Scholar] [CrossRef]
- Ellison, A.; Walsh, V. Perceptual learning in visual search: Some evidence of specificities. Vis. Res. 1998, 38, 333–345. [Google Scholar] [CrossRef]
- Sireteanu, R.; Rettenbach, R. Perceptual learning in visual search: Fast, enduring, but non-specific. Vis. Res. 1995, 35, 2037–2043. [Google Scholar] [CrossRef][Green Version]
- Karni, A.; Sagi, D. Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proc. Natl. Acad. Sci. USA 1991, 88, 4966–4970. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Sagi, D. The time course of learning a visual skill. Nature 1993, 365, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Schoups, A.; Vogels, R.; Qian, N.; Orban, G. Practising orientation identification improves orientation coding in V1 neurons. Nature 2001, 412, 549–553. [Google Scholar] [CrossRef]
- Polat, U. Making perceptual learning practical to improve visual functions. Vis. Res. 2009, 49, 2566–2573. [Google Scholar] [CrossRef]
- Pourtois, G.; Rauss, K.S.; Vuilleumier, P.; Schwartz, S. Effects of perceptual learning on primary visual cortex activity in humans. Vis. Res. 2008, 48, 55–62. [Google Scholar] [CrossRef]
- Saarinen, J.; Levi, D.M. Perceptual learning in vernier acuity: What is learned? Vis. Res. 1995, 35, 519–527. [Google Scholar] [CrossRef][Green Version]
- Green, C.S.; Kattner, F.; Siegel, M.H.; Kersten, D.; Schrater, P.R. Differences in perceptual learning transfer as a function of training task. J. Vis. 2015, 15, 5. [Google Scholar] [CrossRef]
- Harris, H.; Gliksberg, M.; Sagi, D. Generalized perceptual learning in the absence of sensory adaptation. Curr. Biol. 2012, 22, 1813–1817. [Google Scholar] [CrossRef]
- Szpiro, S.F.A.; Carrasco, M. Exogenous attention enables perceptual learning. Psychol. Sci. 2015, 26, 1854–1862. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Lin, Z.; Dosher, B.A. Translating Perceptual Learning from the Laboratory to Applications. Trends Cogn. Sci. 2016, 20, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, M.; Cottereau, B.R.; Soler, V.; Trotter, Y. Rehabilitation approaches in macular degeneration patients. Front. Syst. Neurosci. 2016, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.H.; Fong, A. Efficacy of neural vision therapy to enhance contrast sensitivity function and visual acuity in low myopia. J. Cataract Refract. Surg. 2008, 34, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.M.; Li, R.W. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vis. Res. 2009, 49, 2535–2549. [Google Scholar] [CrossRef]
- Chung, S.T.L. Improving reading speed for people with central vision loss through perceptual learning. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1164–1170. [Google Scholar] [CrossRef]
- Maniglia, M.; Soler, V.; Trotter, Y. Combining fixation and lateral masking training enhances perceptual learning effects in patients with macular degeneration. J. Vis. 2020, 20, 19. [Google Scholar] [CrossRef]
- Plank, T.; Rosengarth, K.; Schmalhofer, C.; Goldhacker, M.; Brandl-Rühle, S.; Greenlee, M.W. Perceptual learning in patients with macular degeneration. Front. Psychol. 2014, 5, 1189. [Google Scholar] [CrossRef]
- Dubljević, V.; Saigle, V.; Racine, E. The rising tide of tDCS in the media and academic literature. Neuron 2014, 82, 731–736. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Transcranial direct current stimulation--update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef]
- Fertonani, A.; Miniussi, C. Transcranial electrical stimulation: What we know and do not know about mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef]
- Angelakis, E.; Liouta, E. Transcranial Electrical Stimulation: Methodology and Applications. J. Neurother. 2011, 15, 337–357. [Google Scholar] [CrossRef]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Paulus, W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp. Brain Res. 2003, 150, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Paulus, W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia 2003, 41, 1802–1807. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef]
- Furubayashi, T.; Terao, Y.; Arai, N.; Okabe, S.; Mochizuki, H.; Hanajima, R.; Hamada, M.; Yugeta, A.; Inomata-Terada, S.; Ugawa, Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp. Brain Res. 2008, 185, 279–286. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Nitsche, M.A.; Tergau, F.; Paulus, W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci. Lett. 2000, 296, 61–63. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Accornero, N.; Li Voti, P.; La Riccia, M.; Gregori, B. Visual evoked potentials modulation during direct current cortical polarization. Exp. Brain Res. 2007, 178, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Nitsche, M.A.; Paulus, W. External modulation of visual perception in humans. Neuroreport 2001, 12, 3553–3555. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, L.; Contemori, G.; Penzo, S.; Maniglia, M. tRNS effects on visual contrast detection. Neurosci. Lett. 2020, 717, 134696. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Antal, A.; Paulus, W. Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Vis. Neurosci. 2008, 25, 77–81. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Fertonani, A.; Pirulli, C.; Miniussi, C. Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 2011, 31, 15416–15423. [Google Scholar] [CrossRef]
- Spiegel, D.P.; Byblow, W.D.; Hess, R.F.; Thompson, B. Anodal transcranial direct current stimulation transiently improves contrast sensitivity and normalizes visual cortex activation in individuals with amblyopia. Neurorehabil. Neural Repair 2013, 27, 760–769. [Google Scholar] [CrossRef]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Bartfai, O.; Paulus, W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: Direct electrophysiological evidence. Investig. Ophthalmol. Vis. Sci. 2004, 45, 702. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Spiegel, D.P.; Chen, Z.; Chan, L.; Luo, G.; Yuan, J.; Deng, D.; Yu, M.; Thompson, B. The effect of transcranial direct current stimulation on contrast sensitivity and visual evoked potential amplitude in adults with amblyopia. Sci. Rep. 2016, 6, 19280. [Google Scholar] [CrossRef]
- Strigaro, G.; Mayer, I.; Chen, J.-C.; Cantello, R.; Rothwell, J.C. Transcranial direct current stimulation effects on single and paired flash visual evoked potentials. Clin. EEG Neurosci. 2015, 46, 208–213. [Google Scholar] [CrossRef]
- Viganò, A.; D’Elia, T.S.; Sava, S.L.; Auvé, M.; De Pasqua, V.; Colosimo, A.; Di Piero, V.; Schoenen, J.; Magis, D. Transcranial Direct Current Stimulation (tDCS) of the visual cortex: A proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J. Headache Pain 2013, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.; Roehmel, J.; Olma, M.C.; Schmidt, S.; Irlbacher, K.; Brandt, S.A. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp. Brain Res. 2010, 207, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, R.M.G.; Xiao, W.; McClenahan, L.J.; Woodman, G.F. Electrical stimulation of visual cortex can immediately improve spatial vision. Curr. Biol. 2016, 26, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.; Johnson, A.P.; Thompson, B.; Hansen, B.C. The Effects of tDCS Across the Spatial Frequencies and Orientations that Comprise the Contrast Sensitivity Function. Front. Psychol. 2015, 6, 1784. [Google Scholar] [CrossRef]
- Spiegel, D.P.; Hansen, B.C.; Byblow, W.D.; Thompson, B. Anodal transcranial direct current stimulation reduces psychophysically measured surround suppression in the human visual cortex. PLoS ONE 2012, 7, e36220. [Google Scholar] [CrossRef]
- Pavan, A.; Ghin, F.; Contillo, A.; Milesi, C.; Campana, G.; Mather, G. Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS): A combined stochastic resonance and equivalent noise approach. Brain Stimulat. 2019, 12, 967–977. [Google Scholar] [CrossRef]
- Battaglini, L.; Ghiani, A.; Casco, C.; Ronconi, L. Parietal tACS at beta frequency improves vision in a crowding regime. Neuroimage 2020, 208, 116451. [Google Scholar] [CrossRef]
- Kar, K.; Krekelberg, B. Transcranial alternating current stimulation attenuates visual motion adaptation. J. Neurosci. 2014, 34, 7334–7340. [Google Scholar] [CrossRef]
- Olma, M.C.; Dargie, R.A.; Behrens, J.R.; Kraft, A.; Irlbacher, K.; Fahle, M.; Brandt, S.A. Long-Term Effects of Serial Anodal tDCS on Motion Perception in Subjects with Occipital Stroke Measured in the Unaffected Visual Hemifield. Front. Hum. Neurosci. 2013, 7, 314. [Google Scholar] [CrossRef]
- Amar-Halpert, R.; Laor-Maayany, R.; Nemni, S.; Rosenblatt, J.D.; Censor, N. Memory reactivation improves visual perception. Nat. Neurosci. 2017, 20, 1325–1328. [Google Scholar] [CrossRef]
- Pirulli, C.; Fertonani, A.; Miniussi, C. The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimulat. 2013, 6, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; He, Q.; Fang, F. Transcranial direct current stimulation over the visual cortex facilitates awake consolidation of visual perceptual learning. Brain Stimulat. 2022, 15, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Contemori, G.; Trotter, Y.; Cottereau, B.R.; Maniglia, M. tRNS boosts perceptual learning in peripheral vision. Neuropsychologia 2019, 125, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, F.; McCormick, D.A. Endogenous electric fields may guide neocortical network activity. Neuron 2010, 67, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef]
- Ozen, S.; Sirota, A.; Belluscio, M.A.; Anastassiou, C.A.; Stark, E.; Koch, C.; Buzsáki, G. Transcranial electric stimulation entrains cortical neuronal populations in rats. J. Neurosci. 2010, 30, 11476–11485. [Google Scholar] [CrossRef]
- Reato, D.; Rahman, A.; Bikson, M.; Parra, L.C. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front. Hum. Neurosci. 2013, 7, 687. [Google Scholar] [CrossRef]
- Freyer, F.; Becker, R.; Dinse, H.R.; Ritter, P. State-dependent perceptual learning. J. Neurosci. 2013, 33, 2900–2907. [Google Scholar] [CrossRef]
- He, Q.; Gong, B.; Bi, K.; Fang, F. The causal role of transcranial alternating current stimulation at alpha frequency in boosting visual perceptual learning. bioRxiv 2021. [Google Scholar] [CrossRef]
- Camilleri, R.; Pavan, A.; Ghin, F.; Battaglini, L.; Campana, G. Improvement of uncorrected visual acuity and contrast sensitivity with perceptual learning and transcranial random noise stimulation in individuals with mild myopia. Front. Psychol. 2014, 5, 1234. [Google Scholar] [CrossRef]
- Campana, G.; Camilleri, R.; Pavan, A.; Veronese, A.; Lo Giudice, G. Improving visual functions in adult amblyopia with combined perceptual training and transcranial random noise stimulation (tRNS): A pilot study. Front. Psychol. 2014, 5, 1402. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Gessaroli, E.; Hithersay, R.; Mitolo, M.; Didino, D.; Kanai, R.; Cohen Kadosh, R.; Walsh, V. Transfer of cognitive training across magnitude dimensions achieved with concurrent brain stimulation of the parietal lobe. J. Neurosci. 2013, 33, 14899–14907. [Google Scholar] [CrossRef] [PubMed]
- Herpich, F.; Melnick, M.D.; Agosta, S.; Huxlin, K.R.; Tadin, D.; Battelli, L. Boosting Learning Efficacy with Noninvasive Brain Stimulation in Intact and Brain-Damaged Humans. J. Neurosci. 2019, 39, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, R.; Pavan, A.; Campana, G. The application of online transcranial random noise stimulation and perceptual learning in the improvement of visual functions in mild myopia. Neuropsychologia 2016, 89, 225–231. [Google Scholar] [CrossRef]
- Contò, F.; Edwards, G.; Tyler, S.; Parrott, D.; Grossman, E.; Battelli, L. Attention network modulation via tRNS correlates with attention gain. eLife 2021, 10, e63782. [Google Scholar] [CrossRef]
- Kuo, M.-F.; Paulus, W.; Nitsche, M.A. Sex differences in cortical neuroplasticity in humans. Neuroreport 2006, 17, 1703–1707. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Fricke, K.; Seeber, A.A.; Thirugnanasambandam, N.; Paulus, W.; Nitsche, M.A.; Rothwell, J.C. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2011, 105, 1141–1149. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulat. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Dowsett, J.; Herrmann, C.S. Sustained Aftereffect of α-tACS Lasts Up to 70 min after Stimulation. Front. Hum. Neurosci. 2016, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Herpich, F.; Contò, F.; van Koningsbruggen, M.; Battelli, L. Modulating the excitability of the visual cortex using a stimulation priming paradigm. Neuropsychologia 2018, 119, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Holland, P.; Frens, M.A.; Donchin, O. Impact of transcranial direct current stimulation (tdcs) on neuronal functions. Front. Neurosci. 2016, 10, 550. [Google Scholar] [CrossRef]
- Messer, W.S. The Neuron: Cell And Molecular Biology. Irwin B. Levitan, Leonard K. Kaczmarek. Q. Rev. Biol. 1998, 73, 107. [Google Scholar] [CrossRef]
- Boros, K.; Poreisz, C.; Münchau, A.; Paulus, W.; Nitsche, M.A. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur. J. Neurosci. 2008, 27, 1292–1300. [Google Scholar] [CrossRef]
- Bachtiar, V.; Near, J.; Johansen-Berg, H.; Stagg, C.J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife 2015, 4, e08789. [Google Scholar] [CrossRef]
- Baroncelli, L.; Braschi, C.; Spolidoro, M.; Begenisic, T.; Maffei, L.; Sale, A. Brain plasticity and disease: A matter of inhibition. Neural. Plast. 2011, 2011, 286073. [Google Scholar] [CrossRef]
- Kim, S.; Stephenson, M.C.; Morris, P.G.; Jackson, S.R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7 T magnetic resonance spectroscopy study. Neuroimage 2014, 99, 237–243. [Google Scholar] [CrossRef]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, X.S.; Wang, Y.C.; Zhang, J.; Liang, Z.; Zhou, Y.F.; Ma, Y.Y. The effects of aging on the strength of surround suppression of receptive field of V1 cells in monkeys. Neuroscience 2010, 169, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Maddock, R.J.; Rokem, A.; Silver, M.A.; Minzenberg, M.J.; Ragland, J.D.; Carter, C.S. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 2010, 30, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.; Berardi, N.; Spolidoro, M.; Baroncelli, L.; Maffei, L. GABAergic inhibition in visual cortical plasticity. Front. Cell. Neurosci. 2010, 4, 10. [Google Scholar] [CrossRef]
- Bavelier, D.; Levi, D.M.; Li, R.W.; Dan, Y.; Hensch, T.K. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J. Neurosci. 2010, 30, 14964–14971. [Google Scholar] [CrossRef]
- Huang, S.; Gu, Y.; Quinlan, E.M.; Kirkwood, A. A refractory period for rejuvenating GABAergic synaptic transmission and ocular dominance plasticity with dark exposure. J. Neurosci. 2010, 30, 16636–16642. [Google Scholar] [CrossRef]
- Sale, A.; Maya Vetencourt, J.F.; Medini, P.; Cenni, M.C.; Baroncelli, L.; De Pasquale, R.; Maffei, L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 2007, 10, 679–681. [Google Scholar] [CrossRef]
- Maya Vetencourt, J.F.; Sale, A.; Viegi, A.; Baroncelli, L.; De Pasquale, R.; O’Leary, O.F.; Castrén, E.; Maffei, L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008, 320, 385–388. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Grundey, J.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb. Cortex 2004, 14, 1240–1245. [Google Scholar] [CrossRef]
- Adelhöfer, N.; Mückschel, M.; Teufert, B.; Ziemssen, T.; Beste, C. Anodal tDCS affects neuromodulatory effects of the norepinephrine system on superior frontal theta activity during response inhibition. Brain Struct. Funct. 2019, 224, 1291–1300. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Schlitterlau, A.; Henschke, U.; Fricke, K.; Frommann, K.; Lang, N.; Henning, S.; Paulus, W.; Tergau, F. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur. J. Neurosci. 2004, 19, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; O’Shea, J.; Kincses, Z.T.; Woolrich, M.; Matthews, P.M.; Johansen-Berg, H. Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur. J. Neurosci. 2009, 30, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Miniussi, C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. 2009, 13, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimulat. 2008, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Vossen, A.; Gross, J.; Thut, G. Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (α-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimulat. 2015, 8, 499–508. [Google Scholar] [CrossRef]
- Schoen, I.; Fromherz, P. Extracellular stimulation of mammalian neurons through repetitive activation of Na+ channels by weak capacitive currents on a silicon chip. J. Neurophysiol. 2008, 100, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Principles of Neural Science, 4th ed.; McGraw-Hill Companies: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Miniussi, C.; Harris, J.A.; Ruzzoli, M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Biobehav. Rev. 2013, 37, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- van der Groen, O.; Wenderoth, N. Transcranial random noise stimulation of visual cortex: Stochastic resonance enhances central mechanisms of perception. J. Neurosci. 2016, 36, 5289–5298. [Google Scholar] [CrossRef]
- van der Groen, O.; Wenderoth, N. Random noise stimulation of the cortex: Stochastic resonance enhances central mechanisms of perception. Brain Stimulat. 2017, 10, e4. [Google Scholar] [CrossRef]
- Moret, B.; Camilleri, R.; Pavan, A.; Lo Giudice, G.; Veronese, A.; Rizzo, R.; Campana, G. Differential effects of high-frequency transcranial random noise stimulation (hf-tRNS) on contrast sensitivity and visual acuity when combined with a short perceptual training in adults with amblyopia. Neuropsychologia 2018, 114, 125–133. [Google Scholar] [CrossRef]
- Brodal, A. Neurological Anatomy in Relation To Clinical Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1981. [Google Scholar]
- Stuart, G.; Spruston, N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J. Neurosci. 1998, 18, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Radman, T.; Ramos, R.L.; Brumberg, J.C.; Bikson, M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimulat. 2009, 2, 215–228.e3. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Weiskrantz, L. Impaired discrimination following polarisation of the striate cortex. Exp. Brain Res. 1969, 9, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef]
- Miranda, P.C.; Lomarev, M.; Hallett, M. Modeling the current distribution during transcranial direct current stimulation. Clin. Neurophysiol. 2006, 117, 1623–1629. [Google Scholar] [CrossRef]
- Rahman, A.; Reato, D.; Arlotti, M.; Gasca, F.; Datta, A.; Parra, L.C.; Bikson, M. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J. Physiol. 2013, 591, 2563–2578. [Google Scholar] [CrossRef]
- De Valois, R.L.; Albrecht, D.G.; Thorell, L.G. Spatial frequency selectivity of cells in macaque visual cortex. Vis. Res. 1982, 22, 545–559. [Google Scholar] [CrossRef]
- Tootell, R.B.; Silverman, M.S.; De Valois, R.L. Spatial frequency columns in primary visual cortex. Science 1981, 214, 813–815. [Google Scholar] [CrossRef]
- Chan, C.Y.; Nicholson, C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J. Physiol. 1986, 371, 89–114. [Google Scholar] [CrossRef]
- Chan, C.Y.; Hounsgaard, J.; Nicholson, C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J. Physiol. 1988, 402, 751–771. [Google Scholar] [CrossRef]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.W.; Kulikowski, J.J.; Levinson, J. The effect of orientation on the visual resolution of gratings. J. Physiol. 1966, 187, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Essock, E.A. The influence of stimulus length on the oblique effect of contrast sensitivity. Vis. Res. 1990, 30, 1243–1246. [Google Scholar] [CrossRef]
- Berryhill, M.E.; Jones, K.T. tDCS selectively improves working memory in older adults with more education. Neurosci. Lett. 2012, 521, 148–151. [Google Scholar] [CrossRef]
- Gill, J.; Shah-Basak, P.P.; Hamilton, R. It’s the thought that counts: Examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimulat. 2015, 8, 253–259. [Google Scholar] [CrossRef]
- Olma, M.C.; Kraft, A.; Roehmel, J.; Irlbacher, K.; Brandt, S.A. Excitability changes in the visual cortex quantified with signal detection analysis. Restor. Neurol. Neurosci. 2011, 29, 453–461. [Google Scholar] [CrossRef]
- Seitz, A.; Watanabe, T. A unified model for perceptual learning. Trends Cogn. Sci. 2005, 9, 329–334. [Google Scholar] [CrossRef]
- Tsodyks, M.; Gilbert, C. Neural networks and perceptual learning. Nature 2004, 431, 775–781. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; Brent, M.H.; Steinbach, M.J.; Markowitz, S.N.; González, E.G. Reading training with threshold stimuli in people with central vision loss: A feasibility study. Optom. Vis. Sci. 2014, 91, 86–96. [Google Scholar] [CrossRef]
- Moliadze, V.; Antal, A.; Paulus, W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin. Neurophysiol. 2010, 121, 2165–2171. [Google Scholar] [CrossRef]
- Moliadze, V.; Atalay, D.; Antal, A.; Paulus, W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimulat. 2012, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimulat. 2018, 11, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Moret, B.; Donato, R.; Nucci, M.; Cona, G.; Campana, G. Transcranial random noise stimulation (tRNS): A wide range of frequencies is needed for increasing cortical excitability. Sci. Rep. 2019, 9, 15150. [Google Scholar] [CrossRef]
- Purpura, D.P.; Mcmurtry, J.G. Intracellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol. 1965, 28, 166–185. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhang, G.-L.; Xiao, L.-Q.; Klein, S.A.; Levi, D.M.; Yu, C. Rule-based learning explains visual perceptual learning and its specificity and transfer. J. Neurosci. 2010, 30, 12323–12328. [Google Scholar] [CrossRef]
- Chang, D.H.F.; Mevorach, C.; Kourtzi, Z.; Welchman, A.E. Training transfers the limits on perception from parietal to ventral cortex. Curr. Biol. 2014, 24, 2445–2450. [Google Scholar] [CrossRef]
- Chen, N.; Cai, P.; Zhou, T.; Thompson, B.; Fang, F. Perceptual learning modifies the functional specializations of visual cortical areas. Proc. Natl. Acad. Sci. USA 2016, 113, 5724–5729. [Google Scholar] [CrossRef]
- Itthipuripat, S.; Cha, K.; Byers, A.; Serences, J.T. Two different mechanisms support selective attention at different phases of training. PLoS Biol. 2017, 15, e2001724. [Google Scholar] [CrossRef]
- Yotsumoto, Y.; Watanabe, T.; Sasaki, Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 2008, 57, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.A.; DeAngelis, G.C. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron 2008, 60, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.D.; Pack, C.C. The contribution of area MT to visual motion perception depends on training. Neuron 2017, 95, 436–446.e3. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Spooner, R.K.; Heinrichs-Graham, E.; Wilson, T.W. High-definition transcranial direct current stimulation modulates performance and alpha/beta parieto-frontal connectivity serving fluid intelligence. J. Physiol. 2021, 599, 5451–5463. [Google Scholar] [CrossRef]
- Snowball, A.; Tachtsidis, I.; Popescu, T.; Thompson, J.; Delazer, M.; Zamarian, L.; Zhu, T.; Cohen Kadosh, R. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr. Biol. 2013, 23, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Pozdniakov, I.; Vorobiova, A.N.; Galli, G.; Rossi, S.; Feurra, M. Online and offline effects of transcranial alternating current stimulation of the primary motor cortex. Sci. Rep. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial direct current stimulation (tdcs): A beginner’s guide for design and implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Mosayebi Samani, M.; Agboada, D.; Jamil, A.; Kuo, M.-F.; Nitsche, M.A. Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 2019, 119, 350–361. [Google Scholar] [CrossRef]
- Agboada, D.; Mosayebi-Samani, M.; Kuo, M.-F.; Nitsche, M.A. Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation—Better effects with intensified protocols? Brain Stimulat. 2020, 13, 987–997. [Google Scholar] [CrossRef]
- Johnson, L.; Alekseichuk, I.; Krieg, J.; Doyle, A.; Yu, Y.; Vitek, J.; Johnson, M.; Opitz, A. Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates. Sci. Adv. 2020, 6, eaaz2747. [Google Scholar] [CrossRef]
- Hung, S.-C.; Seitz, A.R. Prolonged training at threshold promotes robust retinotopic specificity in perceptual learning. J. Neurosci. 2014, 34, 8423–8431. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Paulus, W.; Antal, A. Evaluating aftereffects of short-duration transcranial random noise stimulation on cortical excitability. Neural Plast. 2011, 2011, 105927. [Google Scholar] [CrossRef] [PubMed]
- Dale, G.; Cochrane, A.; Green, C.S. Individual difference predictors of learning and generalization in perceptual learning. Atten. Percept. Psychophys. 2021, 83, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Fahle, M.; Daum, I. Visual learning and memory as functions of age. Neuropsychologia 1997, 35, 1583–1589. [Google Scholar] [CrossRef]
- Fahle, M.; Henke-Fahle, S. Interobserver variance in perceptual performance and learning. Investig. Ophthalmol. Vis. Sci. 1996, 37, 869–877. [Google Scholar]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef]
- Mosayebi-Samani, M.; Jamil, A.; Salvador, R.; Ruffini, G.; Haueisen, J.; Nitsche, M.A. The impact of individual electrical fields and anatomical factors on the neurophysiological outcomes of tDCS: A TMS-MEP and MRI study. Brain Stimulat. 2021, 14, 316–326. [Google Scholar] [CrossRef]
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (tDCS): Clinical Applications and Safety Concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef]
- Mosbacher, J.A.; Halverscheid, S.; Pustelnik, K.; Danner, M.; Prassl, C.; Brunner, C.; Vogel, S.E.; Nitsche, M.A.; Grabner, R.H. Theta band transcranial alternating current stimulation enhances arithmetic learning: A systematic comparison of different direct and alternating current stimulations. Neuroscience 2021, 477, 89–105. [Google Scholar] [CrossRef]
- Ghafoor, U.; Yang, D.; Hong, K.-S. Neuromodulatory Effects of HD-tACS/tDCS on the Prefrontal Cortex: A Resting-State fNIRS-EEG Study. IEEE J. Biomed. Health Inform. 2022, 26, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, M.; Pavan, A.; Sato, G.; Contemori, G.; Montemurro, S.; Battaglini, L.; Casco, C. Perceptual learning leads to long lasting visual improvement in patients with central vision loss. Restor. Neurol. Neurosci. 2016, 34, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Barraza-Bernal, M.J.; Rifai, K.; Wahl, S. A preferred retinal location of fixation can be induced when systematic stimulus relocations are applied. J. Vis. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, M.; Nandy, A.S.; Tjan, B.S. Rapid and persistent adaptability of human oculomotor control in response to simulated central vision loss. Curr. Biol. 2013, 23, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, M.; Jogin, R.; Visscher, K.M.; Seitz, A.R. We don’t all look the same; detailed examination of peripheral looking strategies after simulated central vision loss. J. Vis. 2020, 20, 5. [Google Scholar] [CrossRef]
- Maniglia, M.; Visscher, K.M.; Seitz, A.R. A method to characterize compensatory oculomotor strategies following simulated central vision loss. J. Vis. 2020, 20, 15. [Google Scholar] [CrossRef]
- Walsh, D.V.; Liu, L. Adaptation to a simulated central scotoma during visual search training. Vis. Res. 2014, 96, 75–86. [Google Scholar] [CrossRef]
- Beliaeva, V.; Savvateev, I.; Zerbi, V.; Polania, R. Toward integrative approaches to study the causal role of neural oscillations via transcranial electrical stimulation. Nat. Commun. 2021, 12, 2243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).