Illusional Perspective across Humans and Bees
Abstract
1. Introduction
2. Bee Perspective
3. Do Bees Experience Visual Illusions?
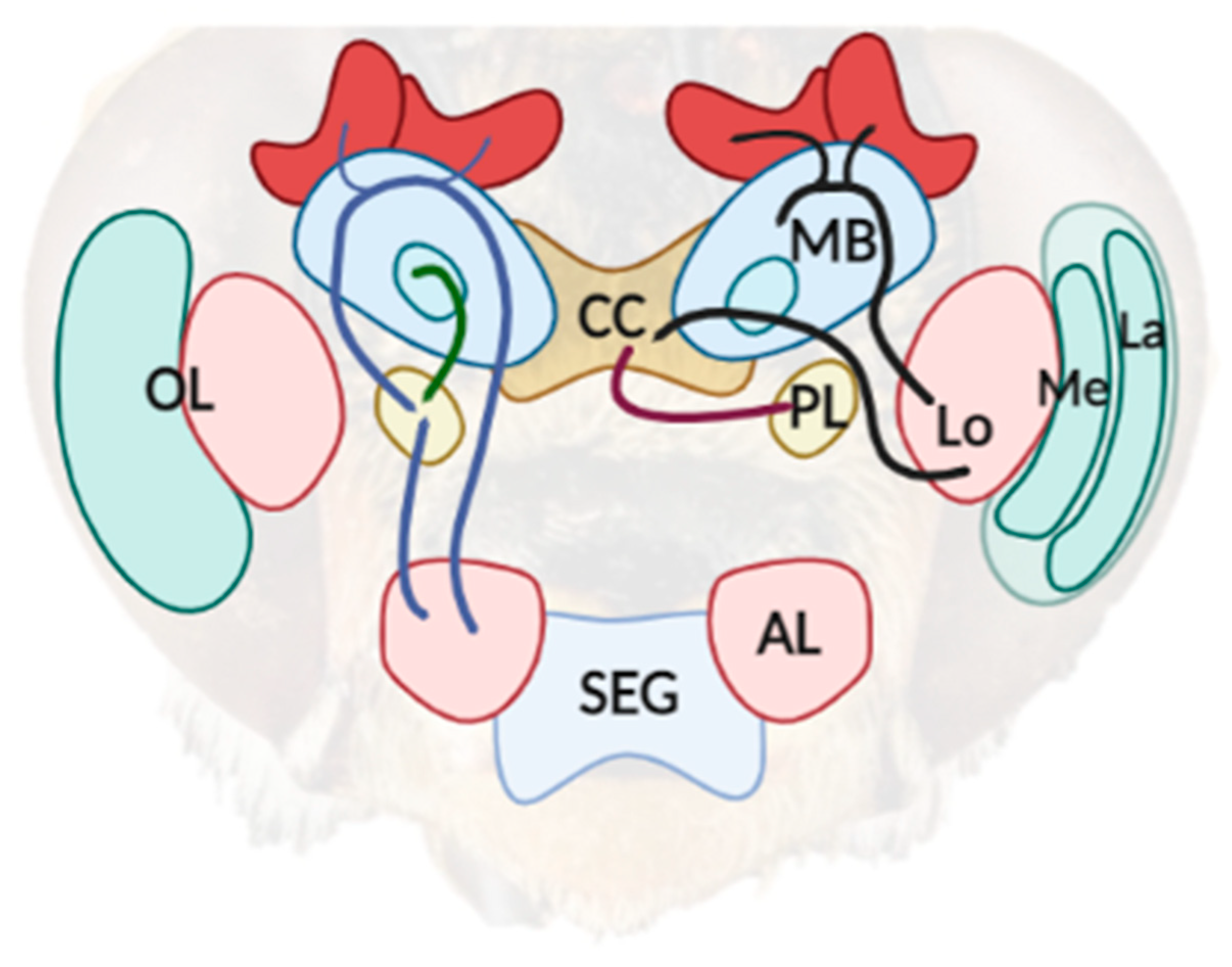
4. The Neural Root of Illusory Misperception
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro, A.G.; Todorovic, D. The Oxford Compendium of Visual Illusions; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Shams, L.; Kim, R. Crossmodal influences on visual perception. Phys. Life Rev. 2010, 7, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.L. Visual illusions classified. Trends Cogn. Sci. 1997, 1, 190–194. [Google Scholar] [CrossRef]
- Eagleman, D.M. Visual illusions and neurobiology. Nat. Rev. Neurosci. 2001, 2, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Santacà, M.; Pecunioso, A.; Miletto Petrazzini, M.E. Everything is subjective under water surface, too: Visual illusions in fish. Anim. Cogn. 2020, 23, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Eysel, U.T. Illusions and perceived images in the primate brain. Science 2003, 302, 789–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelley, L.A.; Kelley, J.L. Animal visual illusion and confusion: The importance of a perceptual perspective. Behav. Ecol. 2014, 25, 450–463. [Google Scholar] [CrossRef]
- Fujita, K. Seeing what is not there: Illusion, completion, and spaciotemporal boundary formation in comparative perspective. In The Oxfor Handbook of Comparative Cognition; Zentall, T.R., Wasserman, E.A., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 27–47. [Google Scholar]
- Lowe, C.J.; Clarke, D.N.; Medeiros, D.M.; Rokhsar, D.S.; Gerhart, J. The deuterostome context of chordate origins. Nature 2015, 520, 456–465. [Google Scholar] [CrossRef]
- Denes, A.S.; Jékely, G.; Steinmetz, P.R.; Raible, F.; Snyman, H.; Prud’homme, B.; Ferrier, D.E.K.; Balavoine, G.; Arendt, D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 2007, 129, 277–288. [Google Scholar] [CrossRef]
- Brown, F.D.; Prendergast, A.; Swalla, B.J. Man is but a worm: Chordate origins. Genesis 2008, 46, 605–613. [Google Scholar] [CrossRef]
- Freeman, R.; Ikuta, T.; Wu, M.; Koyanagi, R.; Kawashima, T.; Tagawa, K.; Humphreys, T.; Fang, G.-C.; Fujiyama, A.; Saiga, H.; et al. Identical genomic organization of two hemichordate hox clusters. Curr. Biol. 2012, 22, 2053–2058. [Google Scholar] [CrossRef]
- Giurfa, M.; Zhang, S.; Jenett, A.; Menzel, R.; Srinivasan, M.V. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 2001, 410, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Avarguès-Weber, A.; Garcia, J.E.; Greentree, A.D.; Dyer, A.G. Numerical ordering of zero in honey bees. Science 2018, 360, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Avarguès-Weber, A.; d’Amaro, D.; Metzler, M.; Finke, V.; Baracchi, D.; Dyer, A.G. Does holistic processing require a large brain? Insights from honeybees and wasps in fine visual recognition tasks. Front. Psychol. 2018, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Land, M.F. The optics of animal eyes. Contemp. Phys. 1988, 29, 435–455. [Google Scholar] [CrossRef]
- Chittka, L.; Skorupski, P. Information processing in miniature brains. Proc. R. Soc. B-Biol. Sci. 2011, 278, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Collett, M.; Chittka, L.; Collett, T.S. Spatial memory in insect navigation. Curr. Biol. 2013, 23, 789–800. [Google Scholar] [CrossRef]
- Nilsson, D.E. The diversity of eyes and vision. Annu. Rev. Vis. Sci. 2021, 7, 19–41. [Google Scholar] [CrossRef]
- MaBouDi, H.; Barron, A.B.; Li, S.; Honkanen, M.; Loukola, O.J.; Peng, F.; Li, W.; Marshall, J.A.R.; Cope, A.; Vasilaki, E.; et al. Non-numerical strategies used by bees to solve numerical cognition tasks. Proc. R. Soc. B-Biol. Sci. 2021, 288, 20202711. [Google Scholar] [CrossRef]
- MaBouDi, H.; Galpayage Dona, H.S.; Gatto, E.; Loukola, O.J.; Buckley, E.; Onoufriou, P.D.; Skorupski, P.; Chittka, L. Bumblebees use sequential scanning of countable items in visual patterns to solve numerosity tasks. Integr. Comp. Biol. 2020, 60, 929–942. [Google Scholar] [CrossRef]
- Salem, W.; Cellini, B.; Frye, M.A.; Mongeau, J.M. Fly eyes are not still: A motion illusion in Drosophila flight supports parallel visual processing. J. Exp. Biol. 2020, 223, jeb212316. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Lin, A.; Witvliet, D.; Hernandez-Nunez, L.; Linderman, S.W.; Samuel, A.D.; Venkatachalam, V. Imaging whole-brain activity to understand behaviour. Nat. Rev. Phys. 2022, 4, 292–305. [Google Scholar] [CrossRef]
- Brandt, R.; Rohlfing, T.; Rybak, J.; Krofczik, S.; Maye, A.; Westerhoff, M.; Hege, H.-C.; Menzel, R. A three-dimensional average-shape atlas of the honeybee brain and its applications. J. Comp. Neurol. 2005, 492, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J.; Kuss, A.; Lamecker, H.; Zachow, S.; Hege, H.; Lienhard, M.; Singer, J.; Neubert, K.; Menzel, R. The digital bee brain: Integrating and managing neurons in a common 3D reference system. Front. Syst. Neurosci. 2010, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Chittka, L.; Menzel, R. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J. Comp. Physiol. A 1992, 171, 171–181. [Google Scholar] [CrossRef]
- Peitsch, D.; Fietz, A.; Hertel, H.; de Souza, J.; Ventura, D.F.; Menzel, R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 1992, 170, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Wakakuwa, M.; Kurasawa, M.; Giurfa, M.; Arikawa, K. Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften 2005, 92, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Ribi, W.A. The first optic ganglion of the bee. Cell Tissue Res. 1975, 165, 103–111. [Google Scholar] [CrossRef]
- Ribi, W.A.; Scheel, M. The second and third optic ganglia of the worker bee. Cell Tissue Res. 1981, 221, 17–43. [Google Scholar] [CrossRef]
- von Hadeln, J.; Hensgen, R.; Bockhorst, T.; Rosner, R.; Heidasch, R.; Pegel, U.; Pérez, M.Q.; Homberg, U. Neuroarchitecture of the central complex of the desert locust: Tangential neurons. J. Comp. Neurol. 2020, 528, 906–934. [Google Scholar] [CrossRef]
- Hensgen, R.; England, L.; Homberg, U.; Pfeiffer, K. Neuroarchitecture of the central complex in the brain of the honeybee: Neuronal cell types. J. Comp. Neurol. 2021, 529, 159–186. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Homberg, U. Organization and functional roles of the central complex in the insect brain. Annu. Rev. Entomol. 2014, 59, 165–184. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.E.; Le, P.T.; Davis, R.L. The role of Drosophila mushroom body signaling in olfactory memory. Science 2001, 293, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.; Schnaitmann, C.; Dylla, K.V.; Knapek, S.; Aso, Y.; Rubin, G.M.; Tanimoto, H. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife 2014, 3, e02395. [Google Scholar] [CrossRef] [PubMed]
- Plath, J.A.; Entler, B.V.; Kirkerud, N.H.; Schlegel, U.; Galizia, C.G.; Barron, A.B. Different roles for honey bee mushroom bodies and central complex in visual learning of colored lights in an aversive conditioning assay. Front. Behav. Neurosci. 2017, 11, 98. [Google Scholar] [CrossRef]
- Brembs, B.; Heisenberg, M. The operant and the classical in conditioned orientation of Drosophila melanogaster at the flight simulator. Learn. Mem. 2000, 7, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.V.; Dvorak, D.R. The waterfall illusion in an insect visual system. Vis. Res. 1979, 19, 1435–1437. [Google Scholar] [CrossRef]
- Ginsburg, A.P. Is the illusory triangle physical or imaginary? Nature 1975, 257, 219–220. [Google Scholar] [CrossRef]
- Petry, S.; Meyer, G.E. The Perception of Illusory Contours; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Van Hateren, J.H.; Srinivasan, M.V.; Wait, P.B. Pattern recognition in bees: Orientation discrimination. J. Comp. Physiol. A 1990, 167, 649–654. [Google Scholar] [CrossRef]
- Horridge, G.A.; Zhang, S.W.; O’Carroll, D. Insect perception of illusory contours. Phil. Trans. R. Soc. B 1992, 337, 59–64. [Google Scholar] [CrossRef]
- Srinivasan, M.; Lehrer, M.; Wehner, R. Bees perceive illusory colours induced by movement. Vis. Res. 1987, 27, 1285–1289. [Google Scholar] [CrossRef]
- Davey, M.P.; Maddess, T.; Srinivasan, M.V. Temporal analysis of the Craik-O’Brien-Cornsweet effect. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2127. [Google Scholar]
- Avargues-Weber, A.; Dyer, A.G.; Ferrah, N.; Giurfa, M. The forest or the trees: Preference for global over local image processing is reversed by prior experience in honeybees. Proc. R. Soc. B-Biol. Sci. 2015, 282, 20142384. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Avarguès-Weber, A.; Garcia, J.E.; Stuart-Fox, D.; Dyer, A.G. Perception of contextual size illusions by honeybees in restricted and unrestricted viewing conditions. Proc. R. Soc. B-Biol. Sci. 2017, 284, 20172278. [Google Scholar] [CrossRef] [PubMed]
- Kanizsa, G. Organization in Vision: Essays on Gestalt Perception; Praeger Publishers: Santa Barbara, CA, USA, 1979. [Google Scholar]
- Ratliff, F. Mach Bands: Quantitative Studies on Neural Networks in the Retina; Holden-Day: San Francisco, CA, USA, 1965. [Google Scholar]
- Menzel, R. Spectral sensitivity and color vision in invertebrates. In Comparative Physiology and Evolution of Vision in Invertebrates; Autrum, H., Ed.; Springer: Berlin, Germany, 1979; pp. 503–580. [Google Scholar]
- Navon, D. Forest before trees: The precedence of global features in visual perception. Cogn. Psychol. 1977, 9, 353–383. [Google Scholar] [CrossRef]
- Navon, D. The forest revisited: More on global precedence. Psychol. Res. 1981, 43, 1–32. [Google Scholar] [CrossRef]
- Goto, K.; Wills, A.J.; Lea, S.E. Global-feature classification can be acquired more rapidly than local-feature classification in both humans and pigeons. Anim. Cogn. 2004, 7, 109–113. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Washburn, D.A. Matching visual stimuli on the basis of global and local features by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta). Anim. Cogn. 2002, 5, 27–31. [Google Scholar] [CrossRef]
- Deruelle, C.; Fagot, J. Visual search for global/local stimulus features in humans and baboons. Psychon. B Rev. 1998, 5, 476–481. [Google Scholar] [CrossRef]
- Cavoto, K.K.; Cook, R.G. Cognitive precedence for local information in hierarchical stimulus processing by pigeons. J. Exp. Psychol. Anim. 2001, 27, 3. [Google Scholar] [CrossRef]
- Pahl, M.; Hong, Z.; Jürgen, T.; Zhang, S. Large scale homing in honeybees. PLoS ONE 2011, 6, e19669. [Google Scholar] [CrossRef]
- Wystrach, A.; Beugnon, G.; Cheng, K. Landmarks or panoramas: What do navigating ants attend to for guidance? Front. Zool. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Komatsu, H. Neural representation of the luminance and brightness of a uniform surface in the macaque primary visual cortex. J. Neurophysiol. 2001, 86, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.W.; Fritsches, K.; Pettigrew, J.D. Optical imaging of functional organization of V1 and V2 in marmoset visual cortex. Anat. Rec. Part A 2005, 287, 1213–1225. [Google Scholar] [CrossRef]
- Anzai, A.; Peng, X.; Van Essen, D.C. Neurons in monkey visual area V2 encode combinations of orientations. Nat. Neurosci. 2007, 10, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A. Seeing more than meets the eye: Processing of illusory contours in animals. J. Comp. Physiol. A 2002, 188, 249–260. [Google Scholar] [CrossRef]
- Shinomiya, K.; Huang, G.; Lu, Z.; Parag, T.; Xu, C.S.; Aniceto, R.; Ansari, N.; Cheatham, N.; Lauchie, S.; Neace, E.; et al. Comparisons between the ON-and OFF-edge motion pathways in the Drosophila brain. Elife 2019, 8, e40025. [Google Scholar] [CrossRef]
- Agrochao, M.; Tanaka, R.; Salazar-Gatzimas, E.; Clark, D.A. Mechanism for analogous illusory motion perception in flies and humans. Proc. Natl. Acad. Sci. USA 2020, 117, 23044–23053. [Google Scholar] [CrossRef]
- Cardin, J.A.; Kumbhani, R.D.; Contreras, D.; Palmer, L.A. Cellular mechanisms of temporal sensitivity in visual cortex neurons. J. Neurosci. 2010, 30, 3652–3662. [Google Scholar] [CrossRef]
- Lennie, P.; Movshon, J.A. Coding of color and form in the geniculostriate visual pathway (invited review). J. Opt. Soc. Am. A 2005, 22, 2013–2033. [Google Scholar] [CrossRef]
- Livingstone, M.S.; Hubel, D.H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci. 1987, 7, 3416–3468. [Google Scholar] [CrossRef]
- Ammer, G.; Vieira, R.M.; Fendl, S.; Borst, A. Anatomical distribution and functional roles of electrical synapses in Drosophila. Curr. Biol. 2022, 32, 2022–2036.e4. [Google Scholar] [CrossRef]
- Giurfa, M.; Hammer, M.; Stach, S.; Stollhoff, N.; Müller-Deisig, N.I.N.A.; Mizyrycki, C. Pattern learning by honeybees: Conditioning procedure and recognition strategy. Anim. Behav. 1999, 57, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Giurfa, M.; Schubert, M.; Reisenman, C.; Gerber, B.; Lachnit, H. The effect of cumulative experience on the use of elemental and configural visual discrimination strategies in honeybees. Behav. Brain Res. 2003, 145, 161–169. [Google Scholar] [CrossRef]
- Stach, S.; Giurfa, M. The influence of training length on generalization of visual feature assemblies in honeybees. Behav. Brain Res. 2005, 161, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Waddington, K.D. Subjective evaluation and choice behavior by nectar-and pollen-collecting bees. In Cognitive Ecology of Pollination; Chittka, L., Thomson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 41–60. [Google Scholar]
- Dyer, A.G.; Neumeyer, C.; Chittka, L. Honeybee (Apis mellifera) vision can discriminate between and recognise images of human faces. J. Exp. Biol. 2005, 208, 4709–4714. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.J.; Tibbetts, E.A. Specialized face learning is associated with individual recognition in paper wasps. Science 2011, 334, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, E.A. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. B-Biol. Sci. 2002, 269, 1423–1428. [Google Scholar] [CrossRef]
- Baracchi, D.; Petrocelli, I.; Cusseau, G.; Pizzocaro, L.; Teseo, S.; Turillazzi, S. Facial markings in the hover wasps: Quality signals and familiar recognition cues in two species of Stenogastrinae. Anim. Behav. 2013, 85, 203–212. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Desjardins, E.; Kou, N.; Wellman, L. Social isolation prevents the development of individual face recognition in paper wasps. Anim. Behav. 2019, 152, 71–77. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Ortiz, C.C.; Auteri, G.G.; Simons, M.; Fearon, M.L.; Knowles, L.L. Individual recognition and individual identity signals in Polistes fuscatus wasps vary geographically. Anim. Behav. 2021, 176, 87–98. [Google Scholar] [CrossRef]
- Jernigan, C.M.; Zaba, N.C.; Sheehan, M.J. Age and social experience induced plasticity across brain regions of the paper wasp Polistes fuscatus. Biol. Lett. 2021, 17, 20210073. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Tibbetts, E. Insects as models for studying the evolution of animal cognition. Curr. Opin. Insect Sci. 2019, 34, 117–122. [Google Scholar] [CrossRef] [PubMed]
| Class of Illusion | Description | Illusory Stimulus |
|---|---|---|
| Illusory contours | For humans, a white rectangle is generated from the identification of an edge between the different ”Pac-Man” elements that create the scene. Bees showed similar susceptibility of those expressed by humans when presented with stimuli with high-contrast borders [42,43]. |  |
| Color Illusion | This class of illusion is generated from the contrasts between the pattern generated from moving elements (the Fechner color illusion) [44] or physical similarities (i.e., brightness and luminance) between elements (Cornsweet illusion) [45]. Humans and bees show behavioral similarities when presented with high-colored contrast stimuli. | The Fechner color |
The Craik–O’Brien–Cornsweet Illusion (Cornsweet Illusion) | ||
| Global Perception | The tendency to process the overall scene rather than a whole set of single elements which define it seems widespread from humans to bees [15,46]. |  |
| Contextual Illusion | Humans and bees are susceptible to the contextual illusion. The sizes of two identical squares are misperceived due to the background that surrounds each of them [47]. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, E.; Loukola, O.J.; Petrazzini, M.E.M.; Agrillo, C.; Cutini, S. Illusional Perspective across Humans and Bees. Vision 2022, 6, 28. https://doi.org/10.3390/vision6020028
Gatto E, Loukola OJ, Petrazzini MEM, Agrillo C, Cutini S. Illusional Perspective across Humans and Bees. Vision. 2022; 6(2):28. https://doi.org/10.3390/vision6020028
Chicago/Turabian StyleGatto, Elia, Olli J. Loukola, Maria Elena Miletto Petrazzini, Christian Agrillo, and Simone Cutini. 2022. "Illusional Perspective across Humans and Bees" Vision 6, no. 2: 28. https://doi.org/10.3390/vision6020028
APA StyleGatto, E., Loukola, O. J., Petrazzini, M. E. M., Agrillo, C., & Cutini, S. (2022). Illusional Perspective across Humans and Bees. Vision, 6(2), 28. https://doi.org/10.3390/vision6020028









