Ocular Surface Microbiota in Contact Lens Users and Contact-Lens-Associated Bacterial Keratitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Conjunctival DNA-Isolation, PCR and 16S rRNA Gene Amplicon Sequencing
2.4. Cultivation of Samples of the Corneal Infiltrate
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. 16S rRNA Gene Amplicon Sequencing
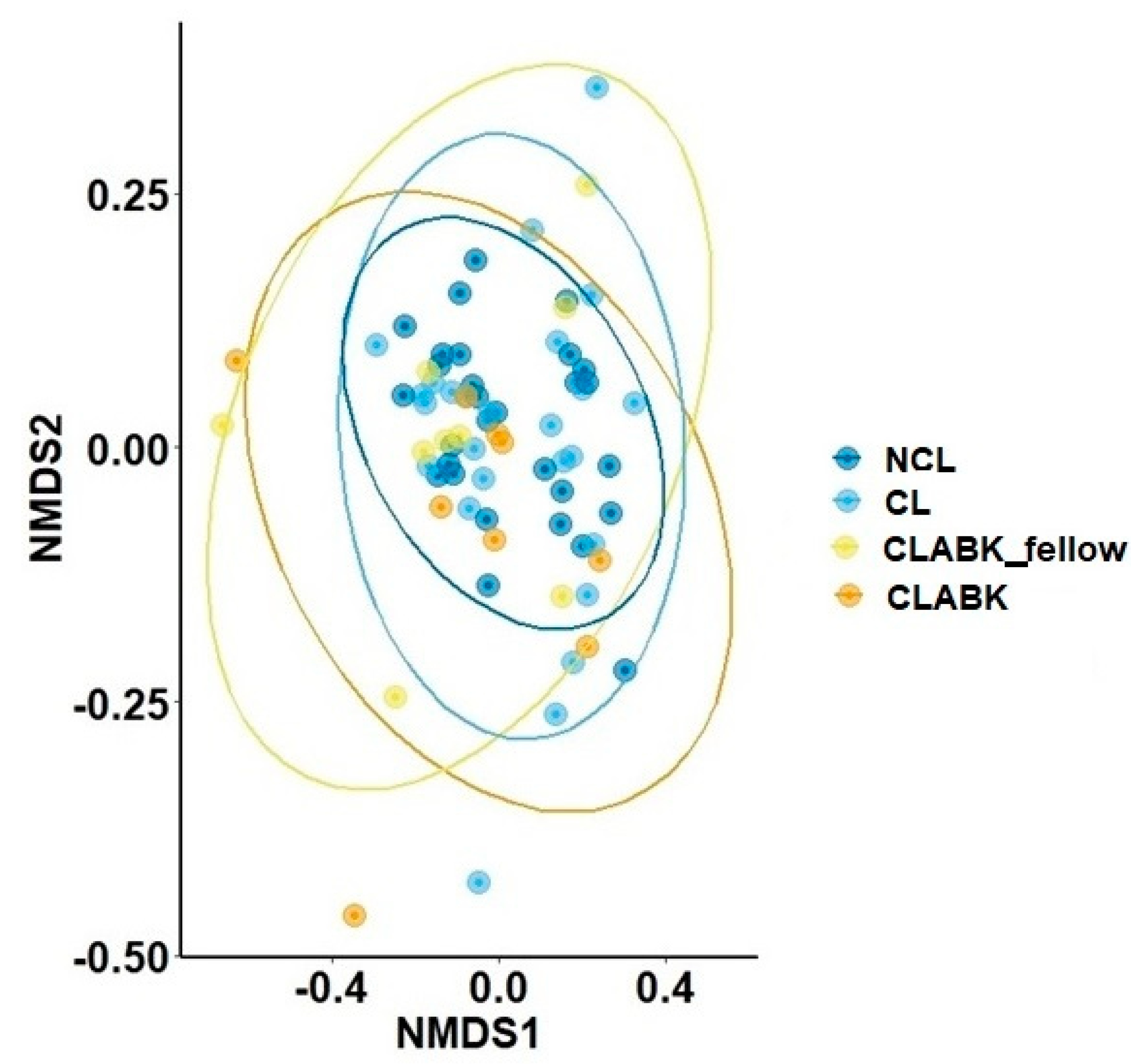
3.1.1. Bacterial Diversity and Composition of Ocular Surface Microbiota for NCL versus CL/CLABK_fellow
3.1.2. Bacterial Diversity and Composition of Ocular Surface Microbiota for NCL/CL versus CLABK
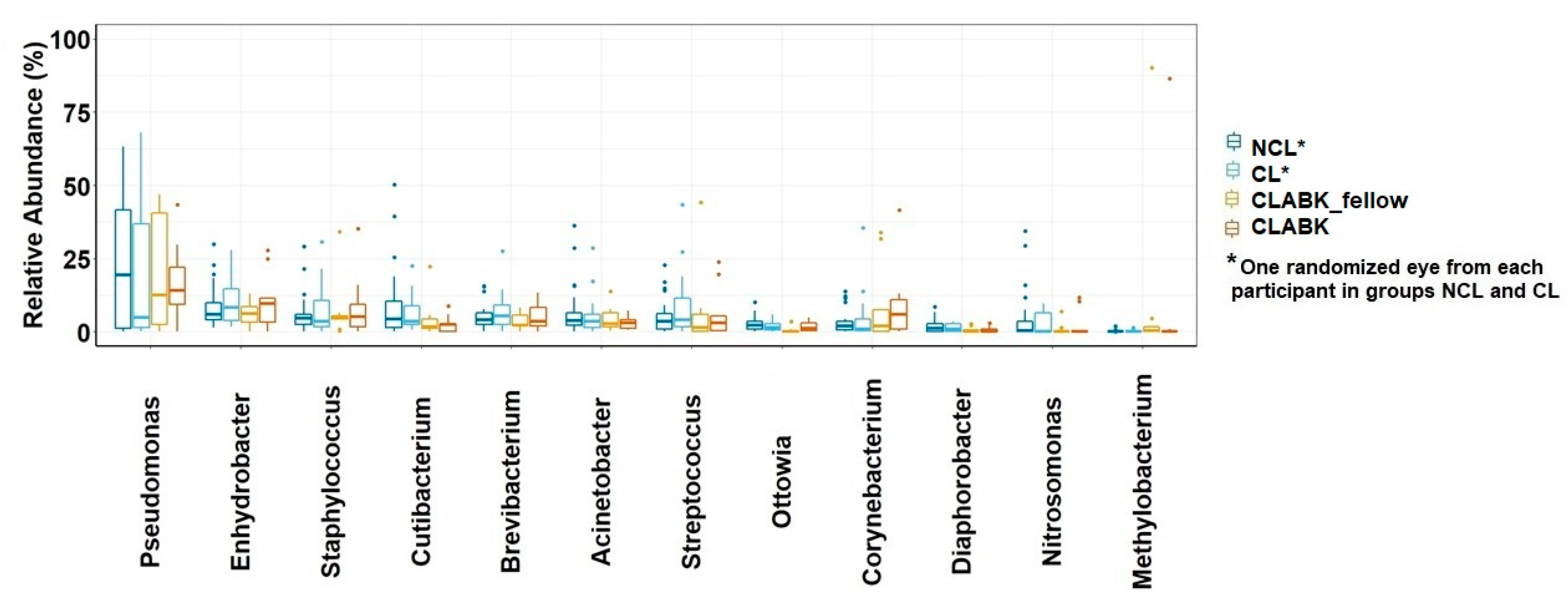
3.1.3. Bacterial Taxonomy of Ocular Surface Microbiota from the Whole Study Population
3.1.4. Several Genera Were Present in the Ocular Surface Microbiota from CLABK_fellow and CLABK of the Same Patient
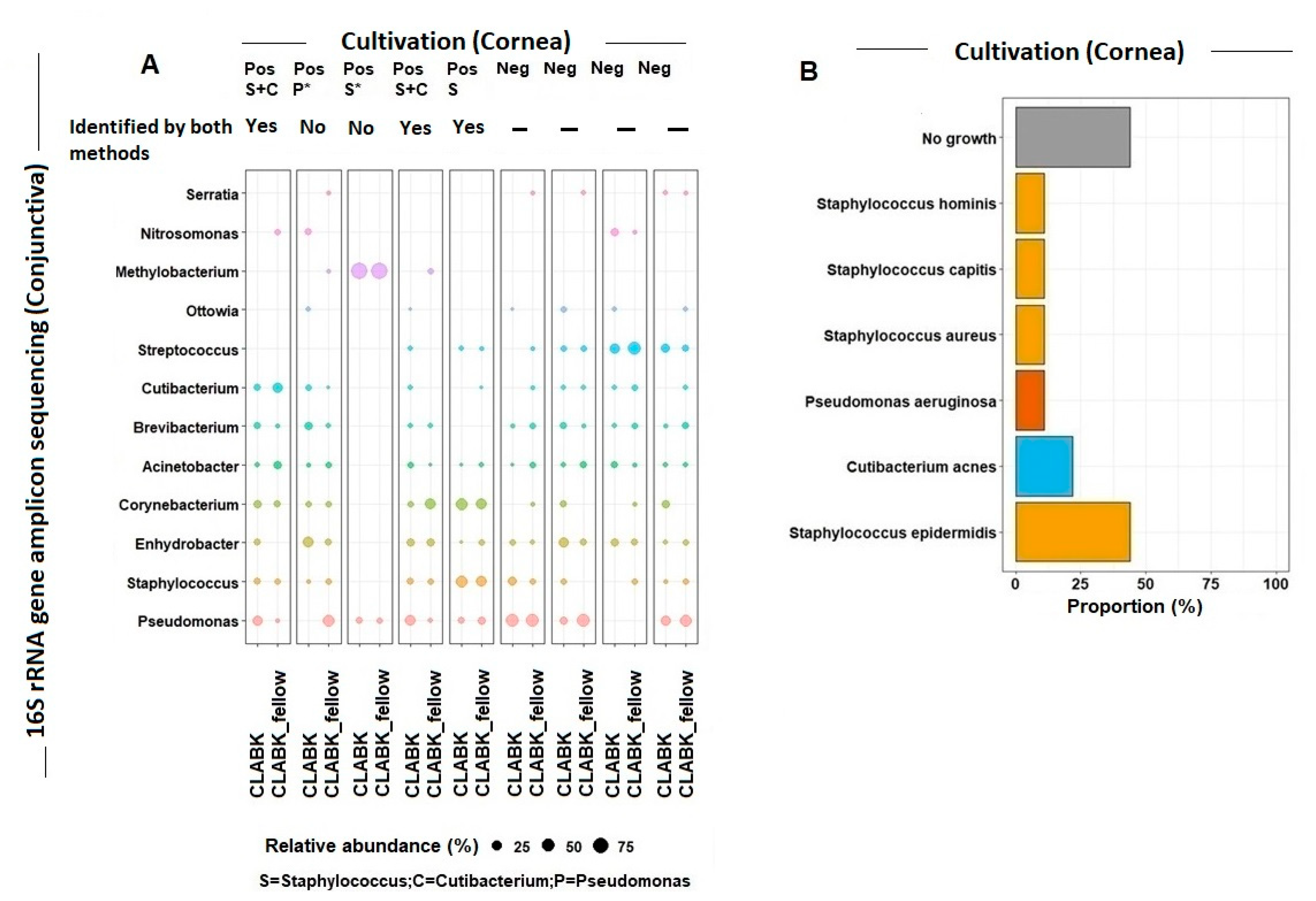
3.2. Cultivation versus 16S rRNA Gene Amplicon Sequencing of Ocular Surface Microbiota
Bacteria Identified in the Cornea and Conjunctiva of Patients with CLABK
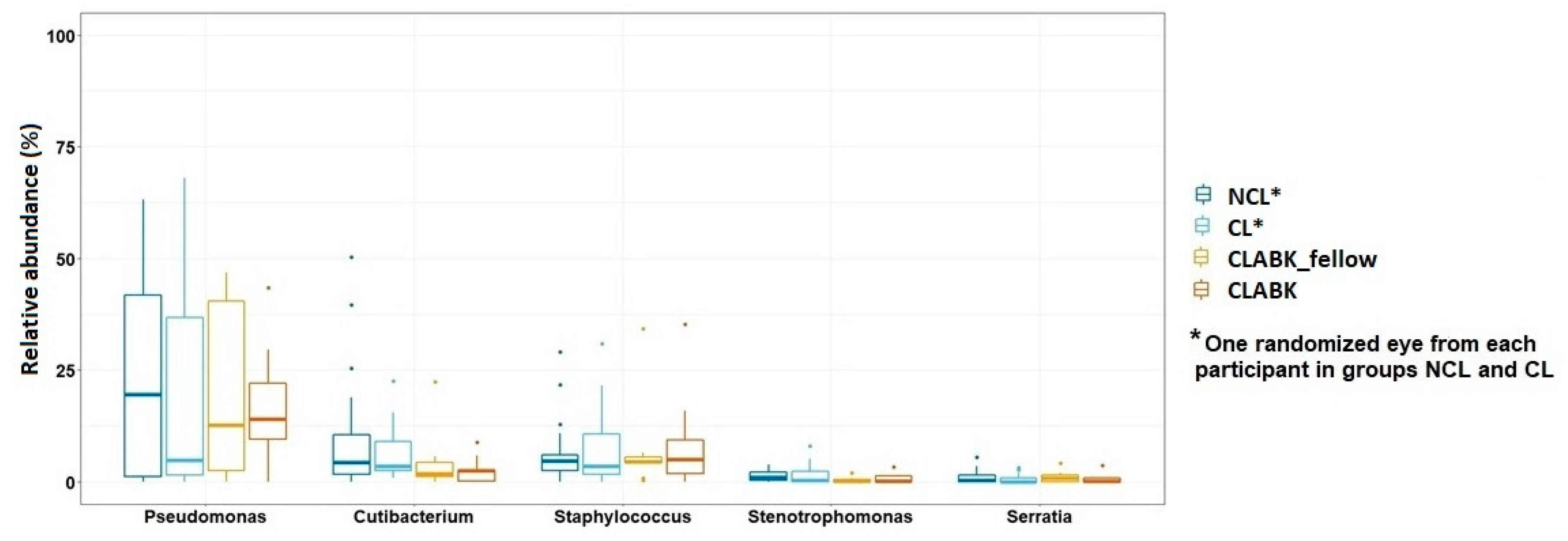
3.3. Opportunistic Pathogens in Conjunctival Microbiota of the Whole Study Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Thomas, F.; Borderie, V.; Chaumeil, C.; Laroche, L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003, 87, 834–838. [Google Scholar] [CrossRef]
- Galdiero, M.; Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; et al. Current evidence on the ocular surface microbiota and related diseases. Microorganisms 2020, 8, 1–13. [Google Scholar]
- Shin, H.; Price, K.; Albert, L.; Dodick, J.; Park, L.; Dominguez-Belloa, M.G. Changes in the eye microbiota associated with contact lens wearing. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, F.; Hutchinson, D.S.; Sun, W.; Ajami, N.J.; Lai, S.; Wong, M.C.; Petrosino, J.F.; Fang, J.; Jiang, J.; et al. Conjunctival microbiome changes associated with soft contact lens and orthokeratology lens wearing. Investig. Ophthalmol. Vis. Sci. 2017, 58, 128–136. [Google Scholar] [CrossRef]
- Kugadas, A.; Christiansen, S.H.; Sankaranarayanan, S.; Surana, N.K.; Gauguet, S.; Kunz, R.; Fichorova, R.; Vorup-Jensen, T.; Gadjeva, M. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 2016, 12. [Google Scholar] [CrossRef]
- Ge, C.; Wei, C.; Yang, B.X.; Cheng, J.; Huang, Y. Sen Conjunctival microbiome changes associated with fungal keratitis: Metagenomic analysis. Int. J. Ophthalmol. 2019, 12, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Dart, J. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br. J. Ophthalmol. 1995, 79, 864–865. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Pearlman, E.; Ghannoum, M. Microbial contamination of contact lenses, lens care solutions, and their accessories: A literature review. Eye Contact Lens 2010, 36, 116–129. [Google Scholar] [CrossRef]
- McLaughlin-Borlace, L.; Stapleton, F.; Matheson, M.; Dart, J.K.G. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 1998, 84, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Behlau, I.; Gilmore, M.S. Microbial biofilms in ophthalmology and infectious disease. Arch. Ophthalmol. 2008, 126, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Fleiszig, S.M.J.; Evans, D.J. Pathogenesis of Contact Lens-associated microbial keratitis. Optom. Vis. Sci. 2010, 87, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Elhanan, M.M.; Nabi, A. Bacterial Keratitis Risk Factors, Pathogens and Antibiotic Susceptibilities: A 5- Year Review of Cases at Dubai hospital, Dubai. J. Clin. Exp. Ophthalmol. 2016, 7. [Google Scholar] [CrossRef]
- Karaca, I.; Barut Selver, O.; Palamar, M.; Egrilmez, S.; Aydemir, S.; Yagci, A. Contact Lens–Associated Microbial Keratitis in a Tertiary Eye Care Center in Turkey. Eye Contact Lens Sci. Clin. Pract. 2019, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bartimote, C.; Foster, J.; Watson, S. The Spectrum of Microbial Keratitis: An Updated Review. Open Ophthalmol. J. 2020, 13, 100–130. [Google Scholar] [CrossRef]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Li, Z.H.; Gong, Y.; Chen, S.Z.; Li, S.Q.; Zhang, Y.; Zhong, H.M.; Wang, Z.C.; Chen, Y.F.; Deng, Q.X.; Jiang, Y.T.; et al. Comparative portrayal of ocular surface microbe with and without dry eye. J. Microbiol. 2019, 57, 1025–1032. [Google Scholar] [CrossRef]
- Wen, X.; Miao, L.; Deng, Y.; Bible, P.W.; Hu, X.; Zou, Y.; Liu, Y.; Guo, S.; Liang, J.; Chen, T.; et al. The influence of age and sex on ocular surface microbiota in healthy adults. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6030–6037. [Google Scholar] [CrossRef]
- Doan, T.; Akileswaran, L.; Andersen, D.; Johnson, B.; Ko, N.; Shrestha, A.; Shestopalov, V.; Lee, C.S.; Lee, A.Y.; Van Gelder, R.N. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5116–5126. [Google Scholar] [CrossRef]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Revanna, K.V.; Gao, X.; Antonopoulos, D.A.; Slepak, V.Z.; et al. Diversity of bacteria at healthy human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5408–5413. [Google Scholar] [CrossRef]
- Chao, C.; Akileswaran, L.; Bailey, J.N.C.; Willcox, M.; Van Gelder, R.; Lakkis, C.; Stapleton, F.; Richdale, K. Potential role of ocular microbiome, host genotype, tear cytokines, and environmental factors in corneal infiltrative events in contact lens wearers. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5752–5761. [Google Scholar] [CrossRef]
- Kam, K.W.; Yung, W.; Li, G.K.H.; Chen, L.J.; Young, A.L. Infectious keratitis and orthokeratology lens use: A systematic review. Infection 2017, 45, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Na, K.S.; Joo, C.K. Clinical Features of Infectious Keratitis Caused by Propionibacterium Acnes. Eye Contact Lens 2017, 43, 330–333. [Google Scholar] [CrossRef]
- Ovodenko, B.; Seedor, J.A.; Ritterband, D.C.; Shah, M.; Yang, R.; Koplin, R.S. The prevalence and pathogenicity of propionibacterium acnes keratitis. Cornea 2009, 28, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Holland, M.J.; Makalo, P.; Joof, H.; Roberts, C.H.; Mabey, D.C.W.; Bailey, R.L.; Burton, M.J.; Weinstock, G.M.; Burr, S.E. The conjunctival microbiome in health and trachomatous disease: A case control study. Genome Med. 2014, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.S.G.; Hayes, V.E.A.; Dartt, D.A.; Downes, C.S.; Moore, T.C.B. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef]
- Andersson, J.; Vogt, J.K.; Dalgaard, M.D.; Pedersen, O.; Holmgaard, K.; Heegaard, S. Ocular surface microbiota in patients with aqueous deficient dry-eye. Ocul. Surf. 2020, 19, 210–217. [Google Scholar] [CrossRef]
- Wieser, A.; Schneider, L.; Jung, J.; Schubert, S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 2012, 93, 965–974. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.S. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 2016, 8, 352–359. [Google Scholar] [CrossRef]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-Cran R; R Core Team: Vienna, Austria, 2019; Volume 1, p. 2. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- St. Leger, A.J.; Desai, J.V.; Drummond, R.A.; Kugadas, A.; Almaghrabi, F.; Silver, P.; Raychaudhuri, K.; Gadjeva, M.; Iwakura, Y.; Lionakis, M.S.; et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells. Immunity 2017, 47, 148–158.e5. [Google Scholar] [CrossRef] [PubMed]
- Bispo, P.J.M.; Haas, W.; Gilmore, M.S. Biofilms in infections of the eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.B.; Nixon, A.D.; Rueff, E.M. Contact lens associated microbial keratitis: Practical considerations for the optometrist. Clin. Optom. 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pachigolla, G.; Blomquist, P.; Cavanagh, H.D. Microbial keratitis pathogens and antibiotic susceptibilities: A 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens 2007, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
| NCL | CL | CLABK_fellow and CLABK | p-Values | |
|---|---|---|---|---|
| Number of participants | 28 | 26 | 9 | - |
| Age (years) median (IQR) | 32 (28–35) | 33 (26–38) | 44 (32–52) | 2.2 × 10−16 1 |
| Sex (%) (F/M) | 50%/50% | 61%/39% | 56%/44% | - |
| Number of samples | 56 (left and right eye) | 52 (left and right eye) | 18 (n = 9 eye CLABK_fellow and n = 9 eye CLABK) | - |
| Number of positive cultures from corneal swabs | NA | NA | 5 (56%) | - |
| Shannon diversity index Median (IQR) * | 4.6 (4.3–5.0) | 4.5 (4.1–5.0) | CLABK_fellow 3.9 (3.8–4.5) CLABK 4.8 (3.6–5.2) | 0.48 2 0.48 3 |
| Observed richness for ASV level Median (IQR) * | 297 (247–335) | 281 (220–337) | CLABK_fellow 251 (174–281) CLABK 314 (206–359) | 0.56 2 0.40 3 |
| Observed richness for genus level Median (IQR) * | 30 (24–33) | 25 (23–30) | CLABK_fellow 27 (19–29) CLABK 28 (19–34) | 0.52 2 0.45 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, J.; Vogt, J.K.; Dalgaard, M.D.; Pedersen, O.; Holmgaard, K.; Heegaard, S. Ocular Surface Microbiota in Contact Lens Users and Contact-Lens-Associated Bacterial Keratitis. Vision 2021, 5, 27. https://doi.org/10.3390/vision5020027
Andersson J, Vogt JK, Dalgaard MD, Pedersen O, Holmgaard K, Heegaard S. Ocular Surface Microbiota in Contact Lens Users and Contact-Lens-Associated Bacterial Keratitis. Vision. 2021; 5(2):27. https://doi.org/10.3390/vision5020027
Chicago/Turabian StyleAndersson, Jasmine, Josef K. Vogt, Marlene D. Dalgaard, Oluf Pedersen, Kim Holmgaard, and Steffen Heegaard. 2021. "Ocular Surface Microbiota in Contact Lens Users and Contact-Lens-Associated Bacterial Keratitis" Vision 5, no. 2: 27. https://doi.org/10.3390/vision5020027
APA StyleAndersson, J., Vogt, J. K., Dalgaard, M. D., Pedersen, O., Holmgaard, K., & Heegaard, S. (2021). Ocular Surface Microbiota in Contact Lens Users and Contact-Lens-Associated Bacterial Keratitis. Vision, 5(2), 27. https://doi.org/10.3390/vision5020027





