The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing
Abstract
1. Introduction
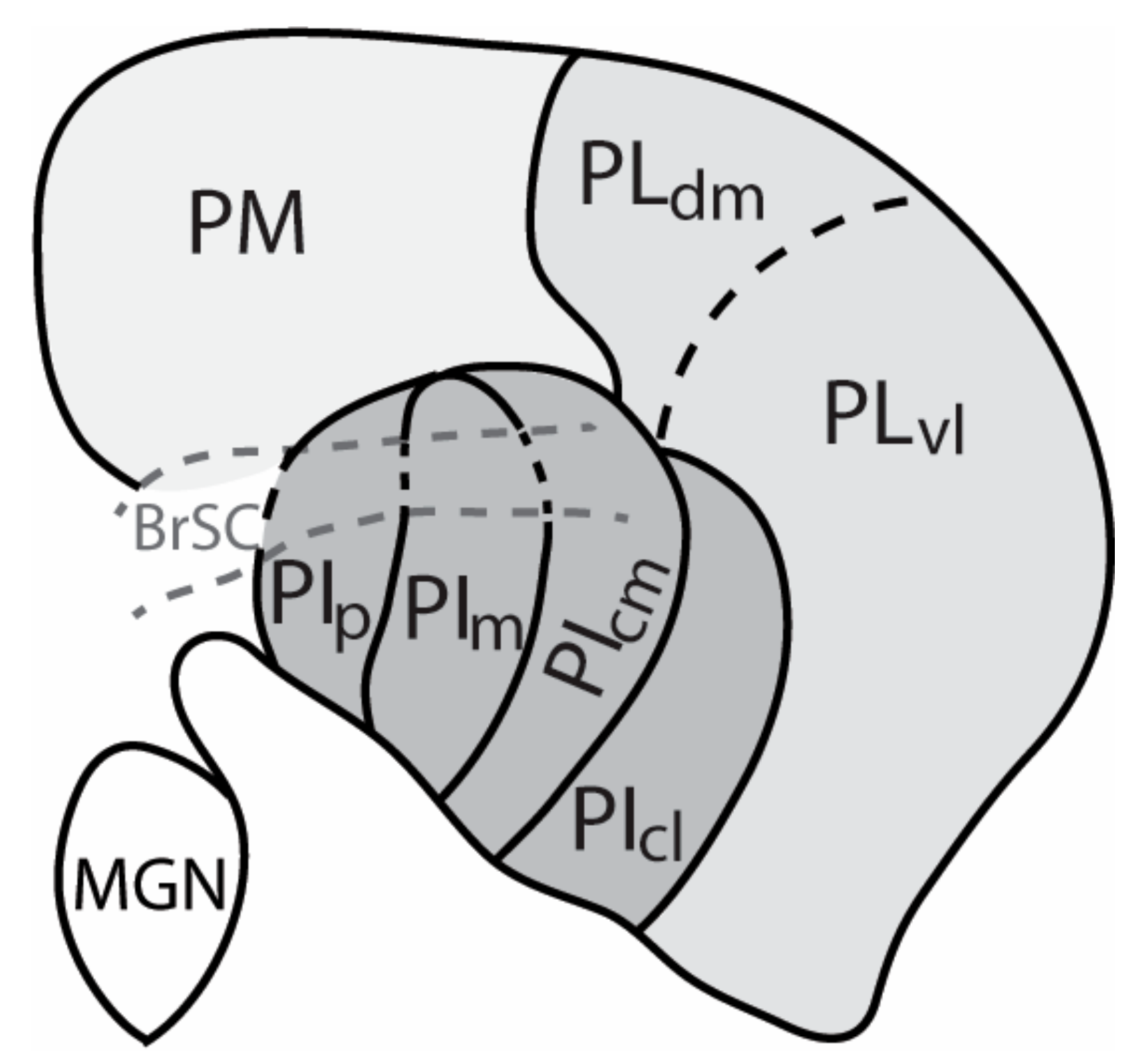
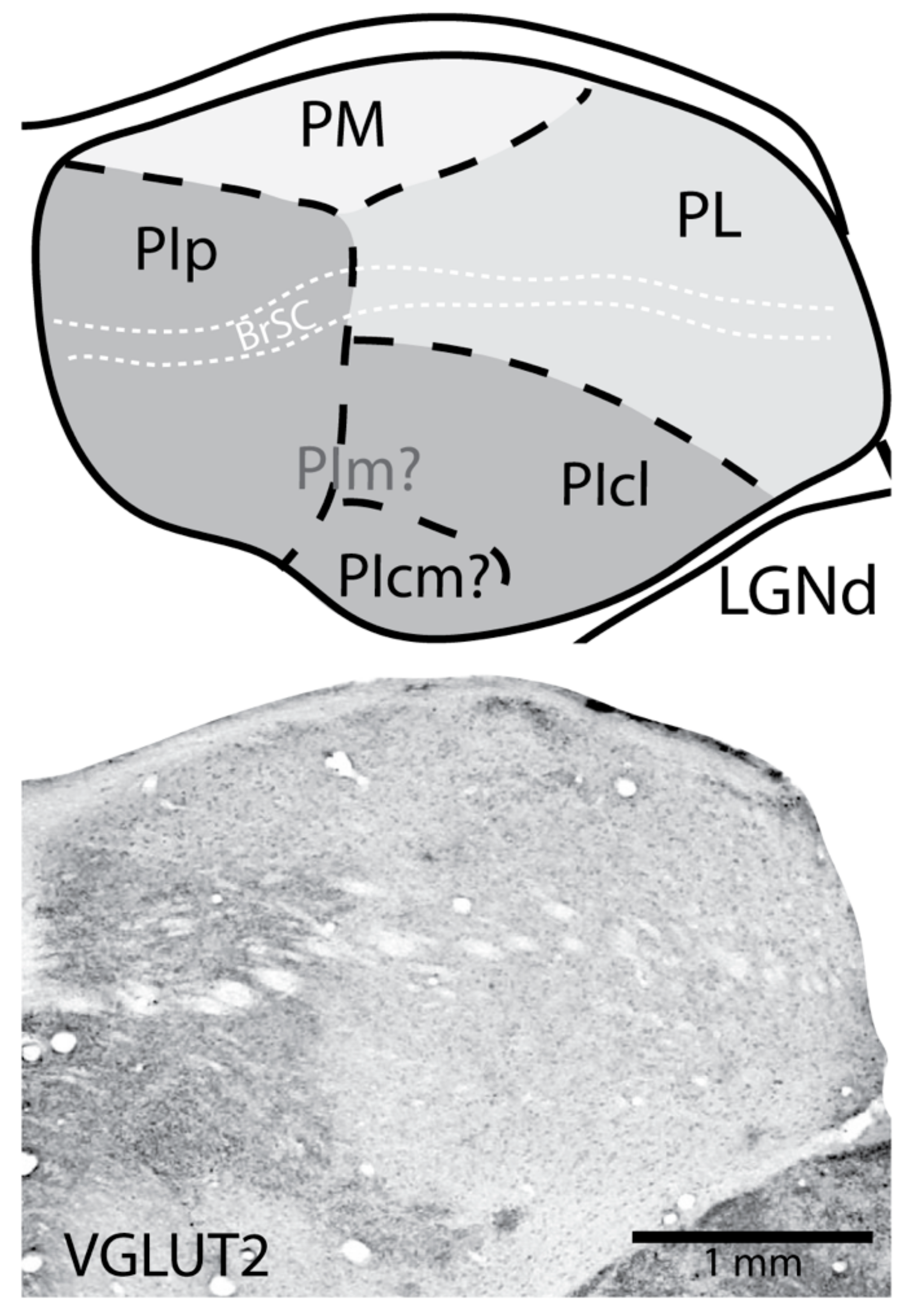
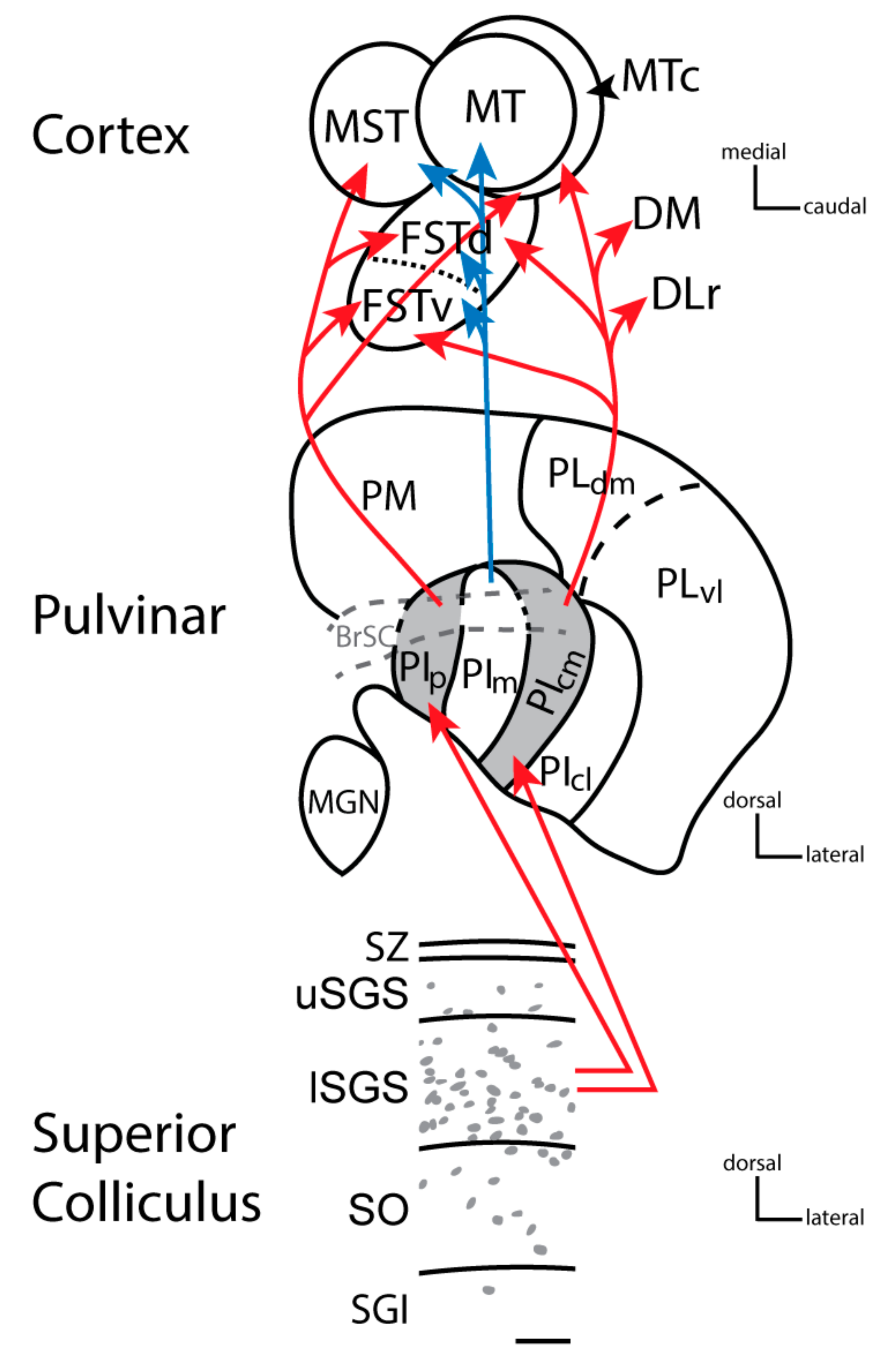
2. The Nuclei of the Primate Pulvinar and Their Connections
3. The Dorsal and Ventral Streams of Visual Processing in Primates
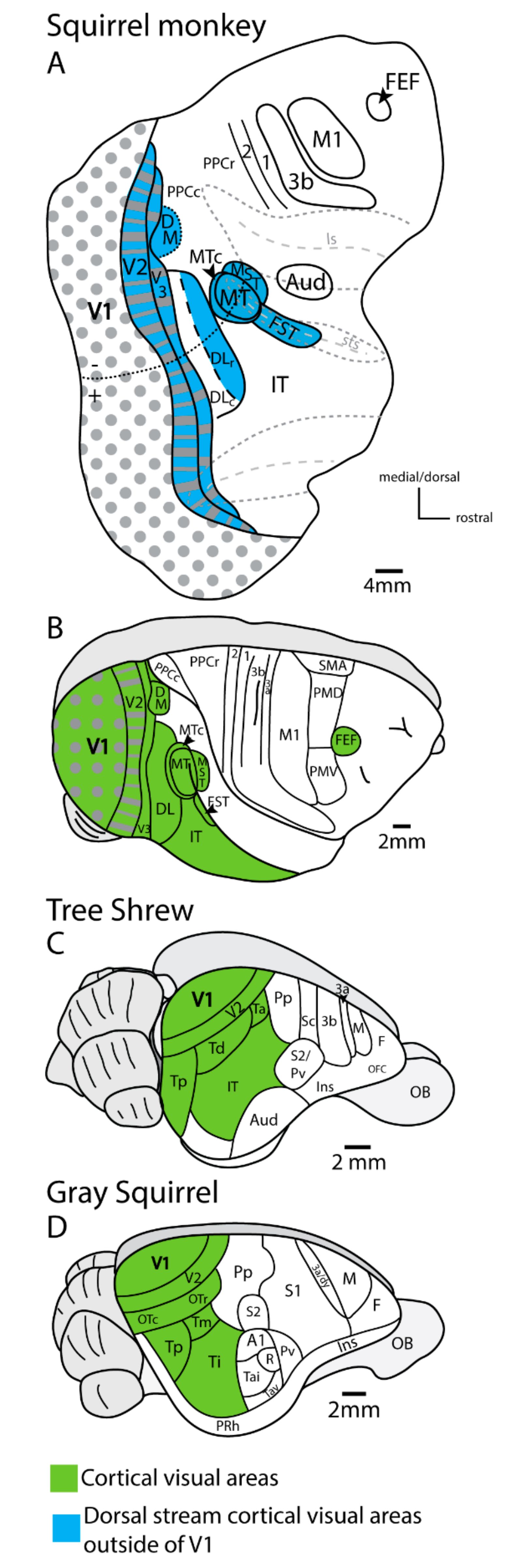
4. Lesions of Primary Visual Cortex (V1) in Rodents and Tree Shrews Have Less Impact on Visual Behavior Than in Primates
5. Blindsight and the Co-Evolution of PIm and MT
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Ungerleider, L.G.; Mishkin, M. Two cortical visual systems. In Analysis of Visual Behavior; Ingle, D.J., Goodale, M.A., Mansfield, R.J.W., Eds.; MIT Press: Cambridge, MA, USA, 1982; pp. 549–586. [Google Scholar]
- Mishkin, M.; Ungerleider, L.G.; Macko, K.A. Object vision and spatial vision: Two cortical pathways. TINS 1983, 6, 414–417. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Baizer, J.S.; Ungerleider, L.G.; Desimone, R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci. 1991, 11, 168–190. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Bullier, J. Anatomical segregation of two cortical visual pathways in the macaque monkey. Vis. Neurosci. 1990, 4, 555–578. [Google Scholar] [CrossRef]
- Kaas, J.H.; Morel, A. Connections of visual areas of the upper temporal lobe of owl monkeys: The MT crescent and dorsal and ventral subdivisions of FST. J. Neurosci. 1993, 13, 534–546. [Google Scholar] [CrossRef]
- Livingstone, M.; Hubel, D. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 1988, 240, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.S.; Hubel, D.H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci. 1987, 7, 3416–3468. [Google Scholar] [CrossRef]
- Casagrande, V.A.; Khaytin, I.; Boyde, J. The evolution of parallel visual pathways in the brains of primates. In Evolution of the Nervous Systems; Preuss, T.M., Kaas, J.H., Eds.; Academic Press: London, UK, 2007; Volume 4, pp. 87–108. [Google Scholar]
- Diamond, I.T.; Hall, W.T. Evolution of neocortex. Science 1969, 164, 251–262. [Google Scholar] [CrossRef]
- Kaas, J.H.; Preuss, T.M. Archonten affinities as reflected in the visual system. In Mammal Phylogeny; Placentals; Szalay, F.S., Novacek, M.J., McKenna, M.C., Eds.; Springer: New York, NY, USA, 1993; pp. 115–128. [Google Scholar]
- Allman, J.M.; Kaas, J.H. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res. 1971, 31, 85–105. [Google Scholar] [CrossRef]
- Krubitzer, L.; Kaas, J. Convergence of processing channels in the extrastriate cortex of monkeys. Vis. Neurosci. 1990, 5, 609–613. [Google Scholar] [CrossRef]
- Baldwin, M.K.L.; Balaram, P.; Kaas, J.H. The evolution and functions of nuclei of the visual pulvinar in primates. J. Comp. Neurol. 2017, 525, 3207–3226. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.G. The Thalamus; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lin, C.S.; Kaas, J.H. Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex. Neuroscience 1980, 5, 2219–2228. [Google Scholar] [CrossRef]
- Lin, C.S.; Kaas, J.H. The inferior pulvinar complex in owl monkeys: Architectonic subdivisions and patterns of input from the superior colliculus and subdivisions of visual cortex. J. Comp. Neurol. 1979, 187, 655–678. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Hof, P.R.; Gattass, R.; Webster, M.J.; Ungerleider, L.G. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J. Comp. Neurol. 2000, 419, 377–393. [Google Scholar] [CrossRef]
- Gutierrez, C.; Yaun, A.; Cusick, C.G. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J. Comp. Neurol. 1995, 363, 545–562. [Google Scholar] [CrossRef]
- Stepniewska, I.; Kaas, J.H. Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 1997, 14, 1043–1060. [Google Scholar] [CrossRef]
- Mundinano, I.C.; Kwan, W.C.; Bourne, J.A. Retinotopic specializations of cortical and thalamic inputs to area MT. Proc. Natl. Acad. Sci. USA 2019, in press. [Google Scholar] [CrossRef]
- Allman, J.M.; Kaas, J.H.; Lane, R.H.; Miezin, F.M. A representation of the visual field in the inferior nucleus of the pulvinar in the owl monkey (Aotus trivirgatus). Brain Res. 1972, 40, 291–302. [Google Scholar] [CrossRef]
- Bender, D.B. Retinotopic organization of macaque pulvinar. J. Neurophysiol. 1981, 46, 672–693. [Google Scholar] [CrossRef]
- Gattass, R.; Oswaldo-Cruz, E.; Sousa, A.P. Visuotopic organization of the cebus pulvinar: A double representation the contralateral hemifield. Brain Res. 1978, 152, 1–16. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Galkin, T.W.; Mishkin, M. Visuotopic organization of projections from striate cortex to inferior and lateral pulvinar in rhesus monkey. J. Comp. Neurol. 1983, 217, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.; Kagan, I.; Andersen, R.A. Effects of pulvinar inactivation on spatial decision-making between equal and asymmetric reward options. J. Cogn. Neurosci. 2013, 25, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.; Gutierrez, C.; Cusick, C.G. Neurochemical organization of inferior pulvinar complex in squirrel monkeys and macaques revealed by acetylcholinesterase histochemistry, calbindin and Cat-301 immunostaining, and Wisteria floribunda agglutinin binding. J. Comp. Neurol. 1999, 409, 452–468. [Google Scholar] [CrossRef]
- Jameson, N.M.; Hou, Z.C.; Sterner, K.N.; Weckle, A.; Goodman, M.; Steiper, M.E.; Wildman, D.E. Genomic data reject the hypothesis of a prosimian primate clade. J. Hum. Evol. 2011, 61, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Hendrickson, A.; Kaas, J.H. Overview of the visual system of Tarsius. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 287, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Collins, C.E.; Kaas, J.H. Overview of Sensory Systems of Tarsius. Int. J. Primatol. 2010, 31, 1002–1031. [Google Scholar] [CrossRef]
- Symonds, L.L.; Kaas, J.H. Connections of striate cortex in the prosimian, Galago senegalensis. J. Comp. Neurol. 1978, 181, 477–512. [Google Scholar] [CrossRef]
- Saraf, M.P.; Balaram, P.; Pifferi, F.; Kennedy, H.; Kaas, J.H. The sensory thalamus and visual midbrain in mouse lemurs. J. Comp. Neurol. 2019, 527, 2599–2611. [Google Scholar] [CrossRef]
- Balaram, P.; Hackett, T.; Kaas, J.H. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. J. Chem. Neuroanat. 2013, 50–51, 21–38. [Google Scholar] [CrossRef]
- Balaram, P.; Takahata, T.; Kaas, J.H. VGLUT2 mRNA and protein expression in the visual thalamus and midbrain of prosimian galagos (Otolemur garnetti). Eye Brain 2011, 2011, 5–15. [Google Scholar] [CrossRef]
- Baldwin, M.K.; Balaram, P.; Kaas, J.H. Projections of the superior colliculus to the pulvinar in prosimian galagos (Otolemur garnettii) and VGLUT2 staining of the visual pulvinar. J. Comp. Neurol. 2013, 521, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Collins, C.E.; Baldwin, M.K.; Kaas, J.H. Cortical connections of the visual pulvinar complex in prosimian galagos (Otolemur garnetti). J. Comp. Neurol. 2009, 517, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.K.L.; Bourne, J.A. The Evolution of Subcortical Pathways to the Extrastriate Cortex. In Evolution of Nervous Systems, 2nd ed.; Kaas, J.H., Krubitzer, L.A., Eds.; Elsevier: Oxford, UK, 2017; Volume 3, pp. 165–185. [Google Scholar]
- Sherman, S.M.; Guillery, R.W. Exploring the Thalamus and its Role in Cortical Function; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Rovo, Z.; Ulbert, I.; Acsady, L. Drivers of the primate thalamus. J. Neurosci. 2012, 32, 17894–17908. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.C.; Mundinano, I.C.; de Souza, M.J.; Lee, S.C.S.; Martin, P.R.; Grunert, U.; Bourne, J.A. Unravelling the subcortical and retinal circuitry of the primate inferior pulvinar. J. Com. Neurol. 2019, 527, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Stepniewska, I.; Qi, H.X.; Kaas, J.H. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur. J. Neurosci. 1999, 11, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.; Koch, C. Constraints on cortical and thalamic projections: The no-strong-loops hypothesis. Nature 1998, 391, 245–250. [Google Scholar] [CrossRef]
- Warner, C.E.; Goldshmit, Y.; Bourne, J.A. Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Front. Neuroanat. 2010, 4, 8. [Google Scholar] [CrossRef]
- Collins, C.E.; Xu, X.; Khaytin, I.; Kaskan, P.M.; Casagrande, V.A.; Kaas, J.H. Optical imaging of visually evoked responses in the middle temporal area after deactivation of primary visual cortex in adult primates. Proc. Natl. Acad. Sci. USA 2005, 102, 5594–5599. [Google Scholar] [CrossRef]
- Kaas, J.H.; Krubitzer, L.A. Area 17 lesions deactivate area MT in owl monkeys. Vis. Neurosci. 1992, 9, 399–407. [Google Scholar] [CrossRef]
- Rodman, H.R.; Gross, C.G.; Albright, T.D. Afferent basis of visual response properties in area MT of the macaque. II. Effects of superior colliculus removal. J. Neurosci. 1990, 10, 1154–1164. [Google Scholar] [CrossRef]
- Zenon, A.; Krauzlis, R.J. Attention deficits without cortical neuronal deficits. Nature 2012, 489, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Bogadhi, A.R.; Katz, L.N.; Bollimunta, A.; Leopold, D.A.; Krauzlis, R.J. Midbrain activity supports high-level visual properties in primate temporal cortex. bioRxiv 2019. [Google Scholar] [CrossRef]
- Beltramo, R.; Scanziani, M. A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 2019, 363, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Lyon, D.C. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res. Rev. 2007, 55, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.K.L.; Krubitzer, L. Architectonic characteristics of the visual thalamus and superior colliculus in titi monkeys. J. Comp. Neurol. 2018, 526, 1760–1776. [Google Scholar] [CrossRef] [PubMed]
- Stepniewska, I.; Qi, H.X.; Kaas, J.H. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 2000, 17, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.K.; Kaskan, P.M.; Zhang, B.; Chino, Y.M.; Kaas, J.H. Cortical connections of V1 and V2 in early postnatal macaque monkeys. J. Comp. Neurol. 2012, 520, 544–569. [Google Scholar] [CrossRef]
- Li, K.; Patel, J.; Purushothaman, G.; Marion, R.T.; Casagrande, V.A. Retinotopic maps in the pulvinar of bush baby (Otolemur garnettii). J. Comp. Neurol. 2013, 521, 3432–3450. [Google Scholar] [CrossRef]
- Arcaro, M.J.; Pinsk, M.A.; Kastner, S. The Anatomical and Functional Organization of the Human Visual Pulvinar. J. Neurosci. 2015, 35, 9848–9871. [Google Scholar] [CrossRef]
- Bender, D.B. Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain Res. 1983, 279, 258–261. [Google Scholar] [CrossRef]
- Bender, D.B. Receptive-field properties of neurons in the macaque inferior pulvinar. J. Neurophysiol. 1982, 48, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Wurtz, R.H. Use of an extraretinal signal by monkey superior colliculus neurons to distinguish real from self-induced stimulus movement. J. Neurophysiol. 1976, 39, 852–870. [Google Scholar] [CrossRef]
- Moore, B.; Li, K.; Kaas, J.H.; Liao, C.C.; Boal, A.M.; Mavity-Hudson, J.; Casagrande, V. Cortical projections to the two retinotopic maps of primate pulvinar are distinct. J. Comp. Neurol. 2019, 527, 577–588. [Google Scholar] [CrossRef]
- Purushothaman, G.; Marion, R.; Li, K.; Casagrande, V.A. Gating and control of primary visual cortex by pulvinar. Nat. Neurosci. 2012, 15, 905–912. [Google Scholar] [CrossRef]
- Harting, J.K.; Huerta, M.F.; Hashikawa, T.; van Lieshout, D.P. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: Organization of tectogeniculate pathways in nineteen species. J. Comp. Neurol. 1991, 304, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Ahmadlou, M.; Zweifel, L.S.; Heimel, J.A. Functional modulation of primary visual cortex by the superior colliculus in the mouse. Nat. Commun. 2018, 9, 3895. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A. Visuomotor modules in the vertebrate brain. Can. J. Physiol. Pharmacol. 1996, 74, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.; Freiwald, W.A.; Tsao, D.Y. Patches with links: A unified system for processing faces in the macaque temporal lobe. Science 2008, 320, 1355–1359. [Google Scholar] [CrossRef]
- Tsao, D.Y.; Livingstone, M.S. Mechanisms of face perception. Annu. Rev. Neurosci. 2008, 31, 411–437. [Google Scholar] [CrossRef]
- Viskontas, I.V.; Quiroga, R.Q.; Fried, I. Human medial temporal lobe neurons respond preferentially to personally relevant images. Proc. Natl. Acad. Sci. USA 2009, 106, 21329–21334. [Google Scholar] [CrossRef]
- Baldwin, M.K.L.; Cooke, D.F.; Goldring, A.B.; Krubitzer, L. Representations of Fine Digit Movements in Posterior and Anterior Parietal Cortex Revealed Using Long-Train Intracortical Microstimulation in Macaque Monkeys. Cereb. Cortex 2018, 28, 4244–4263. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.F.; Taylor, C.S.; Moore, T.; Graziano, M.S. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc. Natl. Acad. Sci. USA 2003, 100, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.S.; Taylor, C.S.; Moore, T. Complex movements evoked by microstimulation of precentral cortex. Neuron 2002, 34, 841–851. [Google Scholar] [CrossRef]
- Kaas, J.H.; Stepniewska, I. Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates. J. Comp. Neurol. 2016, 524, 595–608. [Google Scholar] [CrossRef]
- Stepniewska, I.; Fang, P.C.; Kaas, J.H. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc. Natl. Acad. Sci. USA 2005, 102, 4878–4883. [Google Scholar] [CrossRef]
- Cooke, D.F.; Stepniewska, I.; Miller, D.J.; Kaas, J.H.; Krubitzer, L. Reversible Deactivation of Motor Cortex Reveals Functional Connectivity with Posterior Parietal Cortex in the Prosimian Galago (Otolemur garnettii). J. Neurosci. 2015, 35, 14406–14422. [Google Scholar] [CrossRef][Green Version]
- Stepniewska, I.; Gharbawie, O.A.; Burish, M.J.; Kaas, J.H. Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation. J. Neurophysiol. 2014, 111, 1100–1119. [Google Scholar] [CrossRef]
- Kanwisher, N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc. Natl. Acad. Sci. USA 2010, 107, 11163–11170. [Google Scholar] [CrossRef]
- Gross, C.G.; Rocha-Miranda, C.E.; Bender, D.B. Visual properties of neurons in inferotemporal cortex of the Macaque. J. Neurophysiol. 1972, 35, 96–111. [Google Scholar] [CrossRef]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011, 12, 217–230. [Google Scholar] [CrossRef]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Ungerleider, L.G.; Mishskin, M. The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends Cog. Sci. 2013, 17, 26–49. [Google Scholar] [CrossRef]
- Schneider, G.E. Two visual systems. Science 1969, 163, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H. The organization of neocortex in early mammals. In Evolution of Nervous Systems, 2nd ed.; Kaas, J.H., Herculano-Houzel, S., Eds.; Elsevier: London, UK, 2017; Volume 2, pp. 87–101. [Google Scholar]
- Homman-Ludiye, J.; Bourne, J.A. The medial pulvinar: Function, origin and association with neurodevelopmental disorders. J. Anat. 2019, 235, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Cowey, A. The blindsight saga. Exp. Brain Res. 2010, 200, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Cowey, A.; Stoerig, P. Visual detection in monkeys with blindsight. Neuropsychologia 1997, 35, 929–939. [Google Scholar] [CrossRef]
- Stoerig, P.; Cowey, A. Blindsight in man and monkey. Brain 1997, 120 Pt 3, 535–559. [Google Scholar] [CrossRef]
- Keating, E.G. Impaired orientation after primate tectal lesions. Brain Res. 1974, 67, 538–541. [Google Scholar] [CrossRef]
- Petry, H.M.; Bickford, M.E. The Second Visual System of The Tree Shrew. J. Comp. Neurol. 2019, 527, 679–693. [Google Scholar] [CrossRef]
- Killackey, H.; Snyder, M.; Diamond, I.T. Function of striate and temporal cortex in the tree shrew. J. Comp. Physiol. Psychol. 1971, 74 (Suppl. S2), 1–29. [Google Scholar] [CrossRef]
- Snyder, M.; Diamond, I.T. The organization and function of the visual cortex in tree shrews. Brain Behav. Evol. 1968, 1, 244–288. [Google Scholar] [CrossRef]
- Ward, J.P.; Masterton, B. Encephalization and visual cortex in the Tree Shrew (Tupaia glis). Brain Behav. Evol. 1970, 3, 421–469. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.B.; Casagrande, V.A.; Diamond, I.T. Does the acuity of the tree shrew suffer from removal of striate cortex? A commentary on the paper by ward and Masterton. Brain Behav. Evol. 1972, 5, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, V.A.; Harting, J.K.; Hall, W.C.; Diamond, I.T.; Martin, G.F. Superior colliculus of the tree shrew: A structural and functional subdivision into superficial and deep layers. Science 1972, 177, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, V.A.; Diamond, I.T. Ablation study of the superior colliculus in the tree shrew (Tupaia glis). J. Comp. Neurol. 1974, 156, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Wagor, E. Pattern vision in the grey squirrel after visual cortex ablation. Behav. Biol. 1978, 22, 1–22. [Google Scholar] [CrossRef]
- Levey, N.H.; Harris, J.; Jane, J.A. Effects of visual cortical ablation on pattern discrimination in the ground squirrel (Citellus tridecemlineatus). Exp. Neurol. 1973, 39, 270–276. [Google Scholar] [CrossRef]
- Lewellyn, D.; Lowes, G.; Isaacson, R.L. Visually mediated behaviors following neocortical destruction in the rat. J. Comp. Physiol. Psychol. 1969, 69, 25–32. [Google Scholar] [CrossRef]
- Mize, R.R.; Wetzel, A.B.; Thompson, V.E. Contour discrimination in the rat following removal of posterior neocortex. Physiol. Behav. 1971, 6, 241–246. [Google Scholar] [CrossRef]
- Chalupa, L.M.; Thompson, I. Retinal ganglion cell projections to the superior colliculus of the hamster demonstrated by the horseradish peroxidase technique. Neurosci. Lett. 1980, 19, 13–19. [Google Scholar] [CrossRef]
- Hofbauer, A.; Dräger, U.C. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J. Comp. Neurol. 1985, 234, 465–474. [Google Scholar] [CrossRef]
- Linden, R.; Perry, V.H. Massive retinotectal projection in rats. Brain Res. 1983, 272, 145–149. [Google Scholar] [CrossRef]
- Albano, J.E.; Humphrey, A.L.; Norton, T.T. Laminar organization of receptive-field properties in tree shrew superior colliculus. J. Neurophysiol. 1978, 41, 1140–1164. [Google Scholar] [CrossRef]
- Tigges, J. Ein experimenteller Beitrag zum subkortikalen optischen System von Tupaia glis. Folia Primat. 1966, 4, 173–216. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.K.; Wong, P.; Reed, J.L.; Kaas, J.H. Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): Evidence for four subdivisions within the pulvinar complex. J. Comp. Neurol. 2011, 519, 1071–1094. [Google Scholar] [CrossRef] [PubMed]
- Chomsung, R.D.; Petry, H.M.; Bickford, M.E. Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. J. Comp. Neurol. 2008, 510, 24–46. [Google Scholar] [CrossRef]
- Perry, V.H.; Cowey, A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 1984, 12, 1125–1137. [Google Scholar] [CrossRef]
- Perry, V.H.; Oehler, R.; Cowey, A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience 1984, 12, 1101–1123. [Google Scholar] [CrossRef]
- Weller, R.E.; Kaas, J.H. Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates. Vis. Neurosci. 1989, 3, 327–349. [Google Scholar] [CrossRef]
- Sherman, S.M.; Wilson, J.R.; Kaas, J.H.; Webb, S.V. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). Science 1976, 192, 475–477. [Google Scholar] [CrossRef]
- Kaas, J.H.; Heurta, M.F.; Weber, J.T.; Harting, J.K. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J. Comp. Neurol. 1978, 182, 517–554. [Google Scholar] [CrossRef]
- Chomsung, R.D.; Wei, H.; Day-Brown, J.D.; Petry, H.M.; Bickford, M.E. Synaptic organization of connections between the temporal cortex and pulvinar nucleus of the tree shrew. Cereb. Cortex 2010, 20, 997–1011. [Google Scholar] [CrossRef]
- Harting, J.K.; Diamond, I.T.; Hall, W.C. Anterograde degeneration study of the cortical projections of the lateral geniculate and pulvinar nuclei in the tree shrew (Tupaia glis). J. Comp. Neurol. 1973, 150, 393–439. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.C.; Jain, N.; Kaas, J.H. The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. J. Comp. Neurol. 2003, 467, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Sesma, M.A.; Casagrande, V.A.; Kaas, J.H. Cortical connections of area 17 in tree shrews. J. Comp. Neurol. 1984, 230, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Gharbawie, O.A.; Luethke, L.E.; Kaas, J.H. Thalamic connections of architectonic subdivisions of temporal cortex in grey squirrels (Sciurus carolinensis). J. Comp. Neurol. 2008, 510, 440–461. [Google Scholar] [CrossRef]
- Kaas, J.H.; Krubitzer, L.A.; Johanson, K.L. Cortical connections of areas 17 (V-I) and 18 (V-II) of squirrels. J. Comp. Neurol. 1989, 281, 426–446. [Google Scholar] [CrossRef]
- Negwer, M.; Liu, Y.J.; Schubert, D.; Lyon, D.C. V1 connections reveal a series of elongated higher visual areas in the California ground squirrel, Otospermophilus beecheyi. J. Comp. Neurol. 2017, 525, 1909–1921. [Google Scholar] [CrossRef]
- Gale, S.D.; Murphy, G.J. Distinct cell types in the superficial superior colliculus project to the dorsal lateral geniculate and lateral posterior thalamic nuclei. J. Neurophysiol. 2018, 120, 1286–1292. [Google Scholar] [CrossRef]
- Zhou, N.A.; Maire, P.S.; Masterson, S.P.; Bickford, M.E. The mouse pulvinar nucleus: Organization of the tectorecipient zones. Vis. Neurosci. 2017, 34, E011. [Google Scholar] [CrossRef]
- Masterson, S.P.; Li, J.; Bickford, M.E. Synaptic organization of the tectorecipient zone of the rat lateral posterior nucleus. J. Comp. Neurol. 2009, 515, 647–663. [Google Scholar] [CrossRef]
- Nakamura, H.; Hioki, H.; Furuta, T.; Kaneko, T. Different cortical projections from three subdivisions of the rat lateral posterior thalamic nucleus: A single-neuron tracing study with viral vectors. Eur. J. Neurosci. 2015, 41, 1294–1310. [Google Scholar] [CrossRef]
- Bennett, C.; Gale, S.D.; Garrett, M.E.; Newton, M.L.; Callaway, E.M.; Murphy, G.J.; Olsen, S.R. Higher-order thalamic neurons channel parallel streams of visual information in mice. Neuron 2019, 102, 477–492. [Google Scholar] [CrossRef]
- Kaas, J.H. Reconstructing the organization of neocortex of the first mammals and subsequent modifications. In Evolution of Nervous Systems in Mammals; Kaas, J.H., Krubitzer, L.A., Eds.; Academic Press: Oxford, UK, 2006; pp. 27–48. [Google Scholar]
- Ebbesson, S.O. The parcellation theory and its relation to interspecific variability in brain organization, evolutionary and ontogenetic development, and neuronal plasticity. Cell Tissue Res. 1980, 213, 179–212. [Google Scholar] [CrossRef]
- Krubitzer, L. The organization of neocortex in mammals: Are species differences really so different? Trends Neurosci. 1995, 18, 408–417. [Google Scholar] [CrossRef]
- Grove, E.A.; Fukuchi-Shimogori, T. Generating the cerebral cortical area map. Annu. Rev. Neurosci. 2003, 26, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Mundinano, I.C.; Fox, D.M.; Kwan, W.C.; Vidaurre, D.; Teo, L.; Homman-Ludiye, J.; Goodale, M.A.; Leopold, D.A.; Bourne, J.A. Transient visual pathway critical for normal development of primate grasping behavior. Proc. Natl. Acad. Sci. USA 2018, 115, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Warner, C.E.; Kwan, W.C.; Bourne, J.A. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J. Neurosci. 2012, 32, 17073–17085. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.A.; Rosa, M.G. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: Early maturation of the middle temporal area (MT). Cereb. Cortex 2006, 16, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ortega, J.A.; Hayhow, W.R.; Cluver, P.F. A note on the problem of retinal projections to the inferior pulvinar nucleus of primates. Brain Res. 1970, 22, 126–130. [Google Scholar] [CrossRef]
- Cowey, A.; Stoerig, P.; Bannister, M. Retinal ganglion cells labelled from the pulvinar nucleus in macaque monkeys. Neuroscience 1994, 61, 691–705. [Google Scholar] [CrossRef]
- O’Brien, B.J.; Abel, P.L.; Olavarria, J.F. The retinal input to calbindin-D28k-defined subdivisions in macaque inferior pulvinar. Neurosci. Lett. 2001, 312, 145–148. [Google Scholar] [CrossRef]
- Kahn, D.M.; Krubitzer, L. Retinofugal projections in the short-tailed opossum (Monodelphis domestica). J. Comp. Neurol. 2002, 447, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Bickford, M.E. Comparison of the ultrastructure of cortical and retinal terminals in the rat dorsal lateral geniculate and lateral posterior nuclei. J. Comp. Neurol. 2003, 460, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Major, D.E.; Luksch, H.; Karten, H.J. Bottlebrush dendritic endings and large dendritic fields: Motion-detecting neurons in the mammalian tectum. J. Comp. Neurol. 2000, 423, 243–260. [Google Scholar] [CrossRef]
- Ohno, T.; Misgeld, U.; Kitai, S.T.; Wagner, A. Organization of the visual afferents into the LGd and the pulvinar of the tree shrew Tupaia glis. Brain Res. 1975, 90, 153–158. [Google Scholar] [CrossRef]
- Somogyi, G.; Hajdu, F.; Hassler, R.; Wagner, A. An experimental electron microscopical study of a direct retino-pulvinar pathway in the tree shrew. Exp. Brain Res. 1981, 43, 447–450. [Google Scholar] [CrossRef]
- Cusick, C.G.; Kaas, J.H. Retinal projections in adult and newborn grey squirrels. Brain Res. 1982, 256, 275–284. [Google Scholar] [CrossRef]
- Lane, R.H.; Allman, J.M.; Kaas, J.H.; Miezin, F.M. The visuotopic organization of the superior colliculus of the owl monkey (Aotus trivirgatus) and the bush baby (Galago senegalensis). Brain Res. 1973, 60, 335–349. [Google Scholar] [CrossRef]
- Gross, C.G.; Moore, T.; Rodman, H.R. Visually guided behavior after V1 lesions in young and adult monkeys and its relation to blindsight in humans. Prog. Brain Res. 2004, 144, 279–294. [Google Scholar] [PubMed]
- Moore, T.; Rodman, H.R.; Repp, A.B.; Gross, C.G.; Mezrich, R.S. Greater residual vision in monkeys after striate cortex damage in infancy. J. Neurophysiol. 1996, 76, 3928–3933. [Google Scholar] [CrossRef] [PubMed]
- Warner, C.E.; Kwan, W.C.; Wright, D.; Johnston, L.A.; Egan, G.F.; Bourne, J.A. Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Curr. Biol. 2015, 25, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.C.; Mrowka, S.W.; Turchi, J.; Saunders, R.C.; Wilke, M.; Peters, A.J.; Ye, F.Q.; Leopold, D.A. Blindsight depends on the lateral geniculate nucleus. Nature 2010, 466, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Reed, J.L.; Qi, H.X.; Sawyer, E.K.; Kaas, J.H. Second-order spinal cord pathway contributes to cortical responses after long recoveries from dorsal column injury in squirrel monkeys. Proc. Natl. Acad. Sci. USA 2018, 115, 4258–4263. [Google Scholar] [CrossRef] [PubMed]
- Balaram, P.; Isaamullah, M.; Petry, H.M.; Bickford, M.E.; Kaas, J.H. Distributions of vesicular glutamate transporters 1 and 2 in the visual system of tree shrews (Tupaia belangeri). J. Comp. Neurol. 2015, 523, 1792–1808. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaas, J.H.; Baldwin, M.K.L. The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing. Vision 2020, 4, 3. https://doi.org/10.3390/vision4010003
Kaas JH, Baldwin MKL. The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing. Vision. 2020; 4(1):3. https://doi.org/10.3390/vision4010003
Chicago/Turabian StyleKaas, Jon H., and Mary K. L. Baldwin. 2020. "The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing" Vision 4, no. 1: 3. https://doi.org/10.3390/vision4010003
APA StyleKaas, J. H., & Baldwin, M. K. L. (2020). The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing. Vision, 4(1), 3. https://doi.org/10.3390/vision4010003




