Eye Movements and Fixation-Related Potentials in Reading: A Review
Abstract
1. The Timing Conundrum of Eye Movements and Event-Related Potentials
2. A Tool to Discriminate between Theoretical Accounts
3. Phases of Co-Registration Research
3.1. Pioneering Co-Registration Studies
3.2. Separate Recording of Eye Movements and Event-Related Potentials
3.3. Simultaneous Recording of Eye Movements and Fixation-Related Potentials
4. Are FRP Components Reliable Correlates of Cognitive Processes in Reading?
5. Do Co-Registration Studies add Value to Our Understanding of Reading?
6. What is the Nature of the Relationship between Eye Movements and Fixation-Related Potentials?
7. Future Directions
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayner, K. Eye movements in reading and information processing: 20 Years of research. Psychol. Bulletin 1998, 124, 372–422. [Google Scholar] [CrossRef]
- Rayner, K. Eye movements and attention in reading, scene perception, and visual search. Q. J. Exp. Psychol. 2009, 62, 1457–1506. [Google Scholar] [CrossRef]
- Rayner, K.; Clifton, C., Jr. Language processing in reading and speech perception is fast and incremental: Implications for event-related potential research. Biol. Psychol. 2009, 80, 49. [Google Scholar] [CrossRef] [PubMed]
- Frazier, L.; Rayner, K. Making and correcting errors during sentence comprehension: Eye movements in the analysis of structurally ambiguous sentences. Cogn. Psychol. 1982, 14, 178–210. [Google Scholar] [CrossRef]
- Just, M.A.; Carpenter, P.A. A theory of reading: From eye fixations to comprehension. Psychol. Rev. 1980, 87, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Schotter, E.R.; Angele, B.; Rayner, K. Parafoveal processing in reading. Atten. Percept. Psychophys. 2012, 74, 5–35. [Google Scholar] [CrossRef]
- Liversedge, S.P.; Findlay, J.M. Saccadic eye movements and cognition. Trends Cogn. Sci. 2000, 4, 6–14. [Google Scholar] [CrossRef]
- Rayner, K. Eye movements in reading and information processing. Psychol. Bull. 1978, 85, 618–660. [Google Scholar] [CrossRef]
- Inhoff, A.W.; Rayner, K. Parafoveal word processing during eye fixations in reading: Effects of word frequency. Percept. Psychophys. 1986, 40, 431–439. [Google Scholar] [CrossRef]
- Rayner, K.; Duffy, S.A. Lexical complexity and fixation times in reading: Effects of word frequency, verb complexity, and lexical ambiguity. Mem. Cogn. 1986, 14, 191–201. [Google Scholar] [CrossRef]
- Ehrlich, S.F.; Rayner, K. Contextual effects on word perception and eye movements during reading. J. Verbal Learn. Verbal Behav. 1981, 20, 641–655. [Google Scholar] [CrossRef]
- Rayner, K.; Well, A.D. Effects of contextual constraint on eye movements in reading: A further examination. Psychon. Bull. Rev. 1996, 3, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Reichle, E.D.; Reingold, E.M. Neurophysiological constraints on the eye-mind link. Front. Hum. Neurosci. 2013, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Callaway, E.; Tueting, P.; Koslow, S.H. Event-Related Potentials in Man; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Luck, S.J.; Kappenman, E.S. The Oxford Handbook of Event-Related Potential Components; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Kutas, M.; Federmeier, K.D. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science 1980, 207, 203–205. [Google Scholar] [CrossRef]
- Kutas, M.; Lindamood, T.E.; Hillyard, S.A. Word expectancy and event-related brain potentials during sentence processing. In Preparatory States and Processes; Kornblum, S., Requin, J., Eds.; Erlbaum: Hillsdale, NJ, USA, 1984; pp. 217–237. [Google Scholar]
- Osterhout, L.; Holcomb, P.J. Event-related brain potentials elicited by syntactic anomaly. J. Mem. Lang. 1992, 31, 785–806. [Google Scholar] [CrossRef]
- DeLong, K.A.; Urbach, T.P.; Kutas, M. Probabilistic word pre-activation during language comprehension inferred from electrical brain activity. Nat. Neurosci. 2005, 8, 1117–1121. [Google Scholar] [CrossRef]
- Liversedge, S.P.; Paterson, K.B.; Pickering, M.J. Eye movements and measures of reading time. In Eye Guidance in Reading and Scene Perception; Underwood, G., Ed.; Elsevier Science Ltd.: Oxford, UK, 1998; pp. 55–75. [Google Scholar]
- Sereno, S.C.; Rayner, K. The when and where of reading in the brain. Brain Cogn. 2000, 42, 78–81. [Google Scholar] [CrossRef][Green Version]
- Sereno, S.C.; Rayner, K. Measuring word recognition in reading: Eye movements and event-related potentials. Trends Cogn. Sci. 2003, 7, 489–493. [Google Scholar] [CrossRef]
- Dimigen, O.; Sommer, W.; Hohlfeld, A.; Jacobs, A.M.; Kliegl, R. Coregistration of eye movements and EEG in natural reading: Analyses and review. J. Exp. Psychol. 2011, 140, 552–572. [Google Scholar] [CrossRef]
- Kliegl, R.; Dambacher, M.; Dimigen, O.; Jacobs, A.M.; Sommer, W. Eye movements and brain electric potentials during reading. Psychol. Res. 2012, 76, 145–158. [Google Scholar] [CrossRef]
- Rayner, K. The perceptual span and peripheral cues in reading. Cogn. Psychol. 1975, 7, 65–81. [Google Scholar] [CrossRef]
- McConkie, G.W.; Rayner, K. The span of the effective stimulus during a fixation in reading. Percept. Psychophys. 1975, 17, 578–586. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Event-related potentials to semantically inappropriate and surprisingly large words. Biol. Psychol. 1980, 11, 99–116. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Reading between the lines: Event-related brain potentials during natural sentence processing. Brain Lang. 1980, 11, 354–373. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Event-related brain potentials to grammatical errors and semantic anomalies. Mem. Cogn. 1983, 11, 539–550. [Google Scholar] [CrossRef]
- Marton, M.; Szirtes, J.; Breuer, P. Electrocortical signs of word categorization in saccade-related brain potentials and visual evoked potentials. Int. J. Psychophysiol. 1985, 3, 131–144. [Google Scholar] [CrossRef]
- Marton, M.; Szirtes, J. Context effects on saccade-related brain potentials to words during reading. Neuropsychologia 1988, 26, 453–463. [Google Scholar] [CrossRef]
- Marton, M.; Szirtes, J. Saccade-related brain potentials during reading correct and incorrect versions of proverbs. Int. J. Psychophysiol. 1988, 6, 273–280. [Google Scholar] [CrossRef]
- Barber, H.A.; Ben-Zvi, S.; Bentin, S.; Kutas, M. Parafoveal perception during sentence reading? An ERP paradigm using rapid serial visual presentation (RSVP) with flankers. Psychophysiology 2011, 48, 523–531. [Google Scholar] [CrossRef]
- Barber, H.A.; Doñamayor, N.; Kutas, M.; Münte, T. Parafoveal N400 effect during sentence reading. Neurosci. Lett. 2010, 479, 152–156. [Google Scholar] [CrossRef]
- Barber, H.A.; van der Meij, M.; Kutas, M. An electrophysiological analysis of contextual and temporal constraints on parafoveal word processing. Psychophysiology 2013, 50, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Niefind, F.; Wang, S.; Sommer, W.; Dimigen, O. Parafoveal processing in reading Chinese sentences: Evidence from event-related brain potentials. Psychophysiology 2015, 52, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Reichle, E.D.; Pollatsek, A.; Fisher, D.L.; Rayner, K. Toward a model of eye movement control in reading. Psychol. Rev. 1998, 105, 125. [Google Scholar] [CrossRef] [PubMed]
- Reichle, E.D.; Warren, T.; McConnell, K. Using EZ Reader to model the effects of higher level language processing on eye movements during reading. Psychon. Bull. Rev. 2009, 16, 1–21. [Google Scholar] [CrossRef]
- Reichle, E.D.; Liversedge, S.P.; Pollatsek, A.; Rayner, K. Encoding multiple words simultaneously in reading is implausible. Trends Cogn. Sci. 2009, 13, 115–119. [Google Scholar] [CrossRef]
- Schotter, E.R. Reading ahead by hedging our bets on seeing the future: Eye tracking and electrophysiology evidence for parafoveal lexical processing and saccadic control by partial word recognition. Psychol. Learn. Motiv. 2018, 68, 263–298. [Google Scholar]
- Schotter, E.R.; Reichle, E.D.; Rayner, K. Rethinking parafoveal processing in reading: Serial-attention models can explain semantic preview benefit and N+ 2 preview effects. Vis. Cogn. 2014, 22, 309–333. [Google Scholar] [CrossRef]
- Engbert, R.; Nuthmann, A.; Richter, E.M.; Kliegl, R. SWIFT: A dynamical model of saccade generation during reading. Psychol. Rev. 2005, 112, 777–813. [Google Scholar] [CrossRef]
- Schad, D.J.; Engbert, R. The zoom lens of attention: Simulating shuffled versus normal text reading using the SWIFT model. Vis. Cogn. 2012, 20, 391–421. [Google Scholar] [CrossRef]
- Snell, J.; van Leipsig, S.; Grainger, J.; Meeter, M. OB1-reader: A model of word recognition and eye movements in text reading. Psychol. Rev. 2018, 125, 969–984. [Google Scholar] [CrossRef]
- Zang, C. New Perspectives on Serialism and Parallelism in Oculomotor Control During Reading: The Multi-Constituent Unit Hypothesis. Vision 2019, 3, 50. [Google Scholar] [CrossRef]
- Evans, C.C. Spontaneous excitation of the visual cortex and association areas; lambda waves. Electroencephalogr. Clin. Neurophysiol. 1953, 5, 69–74. [Google Scholar] [CrossRef]
- Kurtzberg, D.; Vaughan, H.G., Jr. Electrophysiological observations on the visuomotor system and visual neurosensorium. In Visual Evoked Potentials in Man: New Developments; Desmedt, J.E., Ed.; Clarendon Press: Oxford, UK, 1977. [Google Scholar]
- Yagi, A. Saccade size and lambda complex in man. Physiol. Psychol. 1979, 7, 370–376. [Google Scholar] [CrossRef]
- Barlow, J.S.; Cigánek, L. Lambda responses in relation to visual evoked responses in man. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 183–192. [Google Scholar] [CrossRef]
- Becker, W.; Hoehne, O.; Iwase, K.; Kornhuber, H.H. Bereitschaftspotential, prfimotorische Positivierung und andere Hirnpotentiale bei sakkadischen Augenbewegungen. Vis. Res. 1972, 12, 421–436. [Google Scholar] [CrossRef]
- Kurtzberg, D.; Vaughan, H.G., Jr. Topographic analysis of human cortical potentials preceding self-initiated and visually triggered saccades. Brain Res. 1982, 243, 1–9. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Mastaglia, F.L. Presaccadic ‘spike’potential: Investigation of topography and source. Brain Res. 1985, 339, 271–280. [Google Scholar] [CrossRef]
- Csibra, G.; Johnson, M.H.; Tucker, L.A. Attention and oculomotor control: A high-density ERP study of the gap effect. Neuropsychologia 1997, 35, 855–865. [Google Scholar] [CrossRef]
- Richards, J.E. Cortical sources of event-related potentials in the prosaccade and antisaccade task. Psychophysiology 2003, 40, 878–894. [Google Scholar] [CrossRef]
- Everling, S.; Krappmann, P.; Flohr, H. Cortical potentials preceding pro-and antisaccades in man. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 356–362. [Google Scholar] [CrossRef]
- Wauschkuhn, B.; Verleger, R.; Wascher, E.; Klostermann, W.; Burk, M.; Heide, W.; Kömpf, D. Lateralized human cortical activity for shifting visuospatial attention and initiating saccades. J. Neurophysiol. 1998, 80, 2900–2910. [Google Scholar] [CrossRef][Green Version]
- Riggs, L.A.; Merton, P.A.; Morton, H.B. Suppression of visual phosphenes during saccadic eye-movements. Vis. Res. 1974, 14, 997–1011. [Google Scholar] [CrossRef]
- Marton, M.; Szirtes, J. Averaged lambda potential and visual information processing. Studia Psychol. 1982, 24, 165–170. [Google Scholar]
- Boylan, C.; Doig, H.R. Effect of saccade size on presaccadic spike potential amplitude. Investigative Ophthalmol. Vis. Sci. 1989, 30, 2521–2527. [Google Scholar]
- Riemslag, F.C.; Van der Heijde, G.L.; Van Dongen, M.M.; Ottenhoff, F. On the origin of the presaccadic spike potential. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 281–287. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Mastaglia, F.L. Presaccadic spike potential. Relation to eye movement direction. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 211–214. [Google Scholar] [CrossRef]
- Scott, D.F.; Moffett, A.; Bickford, R.G. Comparison of two types of visual evoked potentials: Pattern reversal and eye movement (lambda). Electroencephalogr. Clin. Neurophysiol. 1981, 52, 102–104. [Google Scholar] [CrossRef]
- Yagi, A. Averaged cortical potentials (lambda responses) time-locked to onset and offset of saccades. Physiol. Psychol. 1981, 9, 318–320. [Google Scholar] [CrossRef][Green Version]
- Thickbroom, G.W.; Knezevic, W.; Carroll, W.M.; Mastaglia, F.L. Saccade onset and offset lambda waves: Relation to pattern movement visually evoked potentials. Brain Res. 1991, 551, 150–156. [Google Scholar] [CrossRef]
- Gaarder, K.; Krauskopf, J.; Graf, V.; Kropfl, W.; Armington, J.C. Averaged brain activity following saccadic eye movement. Science 1964, 146, 1481–1483. [Google Scholar] [CrossRef]
- Kazai, K.; Yagi, A. Integrated effects of stimulation at fixation points on EFRP (eye-fixation related brain potentials). Int. J. Psychophysiol. 1999, 32, 193–203. [Google Scholar] [CrossRef]
- Kazai, K.; Yagi, A. Contrast dependence of lambda response. Int. Congr. Ser. 2005, 1278, 61–64. [Google Scholar] [CrossRef]
- Marton, M.; Szirtes, J.; Donauer, N.; Breuer, P. Sccade-related brain potentials in semantic categorization tasks. Biol. Psychol. 1985, 20, 163–184. [Google Scholar] [CrossRef]
- Barlow, J.S. Brain information processing during reading: Electrophysiological correlates. Dis. Nerv. Syst. 1971, 32, 668–672. [Google Scholar]
- Kutas, M.; Hillyard, S.A. Event-related potentials in cognitive science. In Handbook of Cognitive Neuroscience; Gazzaniga, M.S., Ed.; Plenum: New York, NY, USA, 1984. [Google Scholar]
- Joyce, C.A.; Gorodnitsky, I.F.; King, J.W.; Kutas, M. Tracking eye fixations with electroocular and electroencephalographic recordings. Psychophysiology 2002, 39, 607–618. [Google Scholar] [CrossRef]
- Takeda, Y.; Sugai, M.; Yagi, A. Eye fixation related potentials in a proof reading task. Int. J. Psychophysiol. 2001, 40, 181–186. [Google Scholar] [CrossRef]
- Reichle, E.D.; Tokowicz, N.; Liu, Y.; Perfetti, C.A. Testing an assumption of the E-Z Reader model of eye-movement control during reading: Using event-related potentials to examine the familiarity check. Psychophysiology 2011, 48, 993–1003. [Google Scholar] [CrossRef]
- Raney, G.E.; Rayner, K. Event-Related Brain Potentials, Eye Movements, and Reading. Psychol. Sci. 1993, 4, 283–286. [Google Scholar] [CrossRef]
- Dambacher, M.; Kliegl, R. Synchronizing timelines: Relations between fixation durations and N400 amplitudes during sentence reading. Brain Res. 2007, 1155, 147–162. [Google Scholar] [CrossRef][Green Version]
- Ashby, J.; Martin, A.E. Prosodic phonological representations early in visual word recognition. J. Exp. Psychol. 2008, 34, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Sereno, S.C.; Rayner, K.; Posner, M.I. Establishing a time-line of word recognition: Evidence from eye movements and event-related potentials. Neuroreport 1998, 9, 2195–2200. [Google Scholar] [CrossRef]
- Raney, G.E.; Rayner, K. The effects of word frequency during two readings of a text. Presented at the meeting of the Psychonomic Society, San Francisco, CA, USA, November 1991. [Google Scholar]
- Raney, G.E. Monitoring changes in cognitive load during reading: An event-related brain potential and reaction time analysis. J. Exp. Psychol. 1993, 19, 51–69. [Google Scholar] [CrossRef]
- Morris, R.K. Sentence context effects on lexical access. In Eye Movements and Visual Cognition: Scene Perception and Reading; Rayner, K., Ed.; Springer-Verlag: New York, NY, USA, 1992; pp. 317–332. [Google Scholar]
- Raney, G.E.; Fischler, I.; Hardonk, M. ERP evidence of lexical and message level priming in sentences. Presented at the meeting of the Psychonomic Society, St. Louis, MO, USA, November 1992. [Google Scholar]
- Kliegl, R.; Nuthmann, A.; Engbert, R. Tracking the mind during reading: The influence of past, present, and future words on fixation durations. J. Exp. Psychol. 2006, 135, 12–35. [Google Scholar] [CrossRef]
- Dambacher, M.; Kliegl, R.; Hofmann, M.; Jacobs, A.M. Frequency and predictability effects on event-related potentials during reading. Brain Res. 2006, 1084, 89–103. [Google Scholar] [CrossRef]
- Balota, D.A.; Chumbley, J.I. Where are the effects of frequency in visual word recognition tasks? Right where we said they were! Comment on Monsell, Doyle, and Haggard (1989). J. Exp. Psychol. 1990, 119, 231–237. [Google Scholar] [CrossRef][Green Version]
- Baccino, T. Eye movements and concurrent event-related potentials’: Eye fixation-related potential investigations in reading. In The Oxford Handbook of Eye Movements; Liversedge, S.P., Gilchrist, I., Everling, S., Eds.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Himmelstoss, N.A.; Schuster, S.; Hutzler, F.; Moran, R.; Hawelka, S. Co-registration of eye movements and neuroimaging for studying contextual predictions in natural reading. Lang. Cogn. Neurosci. 2019, 1–18. [Google Scholar] [CrossRef]
- Kliegl, R.; Dambacher, M.; Dimigen, O.; Sommer, W. Oculomotor control, brain potentials, and timelines of word recognition during natural reading. In Current Trends in Eye Tracking Research; Horsley, M., Eliot, M., Knight, B.A., Reilly, R., Eds.; Springer: Cham, Switzlerland, 2014; pp. 141–155. [Google Scholar]
- Nikolaev, A.R.; Meghanathan, R.N.; van Leeuwen, C. Combining EEG and eye movement recording in free viewing: Pitfalls and possibilities. Brain Cogn. 2016, 107, 55–83. [Google Scholar] [CrossRef]
- Ehinger, B.V.; Gross, K.; Ibs, I.; Koenig, P. A new comprehensive Eye-Tracking Test Battery concurrently evaluating the Pupil Labs Glasses and the EyeLink 1000. bioRxiv 2019, 536243. [Google Scholar] [CrossRef]
- Holmqvist, K. Common Predictors of Accuracy, Precision and Data Loss in 12 Eye-Trackers. 2017. Available online: https://www.researchgate.net/publication/321678981 (accessed on 11 February 2019).
- Holmqvist, K.; Nystrom, M.; Andersson, R.; Dewhurst, R.; Jarodzka, H.; van de Weijer, J. Eye Tracking. A Comprehensive Guide to Methods and Measures; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Holmqvist, K.; Nyström, M.; Mulvey, F. Eye tracker data quality: What it is and how to measure it. In Proceedings of the Symposium on Eye Tracking Research and Applications, Santa Barbara, CA, USA, March 2012; Association for Computing Machinery: New York, NY, USA, 2012; pp. 45–52. [Google Scholar] [CrossRef]
- Croft, R.J.; Barry, R.J. Removal of ocular artifact from the EEG: A review. Clin. Neurophysiol. 2000, 30, 5–19. [Google Scholar] [CrossRef]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 2007, 34, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G. Dealing with artifacts: The EOG contamination of the event-related brain potential. Behav. Res. Methods Instrum. Comput. 1998, 30, 44–53. [Google Scholar] [CrossRef]
- Plöchl, M.; Ossandón, J.P.; König, P. Combining EEG and eye tracking: Identification, characterization, and correction of eye movement artifacts in electroencephalographic data. Front. Hum. Neurosci. 2012, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Ogata, M. Measurement of work load using brain potentials during VDT tasks. In Advances in Human Factors/Ergonomics; Anzai, Y., Ogawa, K., Mori, H., Eds.; Elsevier: Tokyo, Japan, 1995; Volume 20, pp. 823–826. [Google Scholar] [CrossRef]
- Frey, A.; Lemaire, B.; Vercueil, L.; Guérin-Dugué, A. An eye fixation-related potential study in two reading tasks: Reading to memorize and reading to make a decision. Brain Topogr. 2018, 31, 640–660. [Google Scholar] [CrossRef]
- Kornrumpf, B.; Dimigen, O.; Sommer, W. Lateralization of posterior alpha EEG reflects the distribution of spatial attention during saccadic reading. Psychophysiology 2017, 54, 809–823. [Google Scholar] [CrossRef]
- Kretzschmar, F.; Pleimling, D.; Hosemann, J.; Füssel, S.; Bornkessel-Schlesewsky, I.; Schlesewsky, M. Subjective Impressions Do Not Mirror Online Reading Effort: Concurrent EEG-Eyetracking Evidence from the Reading of Books and Digital Media. PLoS ONE 2013, 8, e56178. [Google Scholar] [CrossRef]
- Metzner, P.; von der Malsburg, T.; Vasishth, S.; Rösler, F. Brain Responses to World Knowledge Violations: A Comparison of Stimulus- and Fixation-triggered Event-related Potentials and Neural Oscillations. J. Cogn. Neurosci. 2015, 27, 1017–1028. [Google Scholar] [CrossRef]
- Vignali, L.; Himmelstoss, N.A.; Hawelka, S.; Richlan, F.; Hutzler, F. Oscillatory brain dynamics during sentence reading: A fixation-related spectral perturbation analysis. Front. Hum. Neurosci. 2016, 10, 191. [Google Scholar] [CrossRef]
- Baccino, T.; Manunta, Y. Eye-fixation-related potentials: Insight into parafoveal processing. J. Psychophysiol. 2005, 19, 204–215. [Google Scholar] [CrossRef]
- Hutzler, F.; Braun, M.; Võ, M.L.H.; Engl, V.; Hofmann, M.; Dambacher, M.; Jacobs, A.M. Welcome to the real world: Validating fixation-related brain potentials for ecologically valid settings. Brain Res. 2007, 1172, 124–129. [Google Scholar] [CrossRef]
- Kretzschmar, F.; Bornkessel-Schlesewsky, I.; Schlesewsky, M. Parafoveal versus foveal N400s dissociate spreading activation from contextual fit. NeuroReport 2009, 20, 1613–1618. [Google Scholar] [CrossRef]
- Simola, J.; Holmqvist, K.; Lindgren, M. Right visual field advantage in parafoveal processing: Evidence from eye-fixation-related potentials. Brain Lang. 2009, 111, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Dimigen, O.; Kliegl, R.; Sommer, W. Trans-saccadic parafoveal preview benefits in fluent reading: A study with fixation-related brain potentials. NeuroImage 2012, 62, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Luke, S.G.; Schmidt, J.; Richards, J.E. Co-registration of eye movements and event-related potentials in connected-text paragraph reading. Front. Syst. Neurosci. 2013, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, F.; Fuchs, I.; Gagl, B.; Schuster, S.; Richlan, F.; Braun, M.; Hawelka, S. Parafoveal X-masks interfere with foveal word recognition: Evidence from fixation-related brain potentials. Front. Syst. Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, F.; Schlesewsky, M.; Staub, A. Dissociating word frequency and predictability effects in reading: Evidence from coregistration of eye movements and EEG. J. Exp. Psychol. 2015, 41, 1648–1662. [Google Scholar] [CrossRef]
- Kornrumpf, B.; Niefind, F.; Sommer, W.; Dimigen, O. Neural correlates of word recognition: A systematic comparison of natural reading and rapid serial visual presentation. J. Cogn. Neurosci. 2016, 28, 1374–1391. [Google Scholar] [CrossRef]
- López-Peréz, P.J.; Dampuré, J.; Hernández-Cabrera, J.A.; Barber, H.A. Semantic parafoveal-on-foveal effects and preview benefits in reading: Evidence from fixation related potentials. Brain Lang. 2016, 162, 29–34. [Google Scholar] [CrossRef]
- Metzner, P.; Von Der Malsburg, T.; Vasishth, S.; Rösler, F. The importance of reading naturally: Evidence from combined recordings of eye movements and electric brain potentials. Cogn. Sci. 2016, 41, 1232–1263. [Google Scholar] [CrossRef]
- Niefind, F.; Dimigen, O. Dissociating parafoveal preview benefit and parafovea-on-fovea effects during reading: A combined eye tracking and EEG study. Psychophysiology 2016, 53, 1784–1798. [Google Scholar] [CrossRef]
- Weiss, B.; Knakker, B.; Vidnyánszky, Z. Visual processing during natural reading. Sci. Rep. 2016, 6, 26902. [Google Scholar] [CrossRef] [PubMed]
- Loberg, O.; Hautala, J.; Hämäläinen, J.A.; Leppänen, P.H. Semantic anomaly detection in school-aged children during natural sentence reading–A study of fixation-related brain potentials. PLoS ONE 2018, 13, e0209741. [Google Scholar] [CrossRef] [PubMed]
- Degno, F.; Loberg, O.; Zang, C.; Zhang, M.; Donnelly, N.; Liversedge, S.P. Parafoveal previews and lexical frequency in natural reading: Evidence from eye movements and fixation-related potentials. J. Exp. Psychol. 2019, 148, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Degno, F.; Loberg, O.; Zang, C.; Zhang, M.; Donnelly, N.; Liversedge, S.P. A co-registration investigation of inter-word spacing and parafoveal preview: Eye movements and fixation-related potentials. PLoS ONE 2019, 14, e0225819. [Google Scholar] [CrossRef]
- Loberg, O.; Hautala, J.; Hämäläinen, J.A.; Leppänen, P.H. Influence of reading skill and word length on fixation-related brain activity in school-aged children during natural reading. Vis. Res. 2019, 165, 109–122. [Google Scholar] [CrossRef]
- Frey, A.; Ionescu, G.; Lemaire, B.; López-Orozco, F.; Baccino, T.; Guérin-Dugué, A. Decision-making in information seeking on texts: An eye-fixation-related potentials investigation. Front. Syst. Neurosci. 2013, 7, 39. [Google Scholar] [CrossRef]
- Marx, C.; Hawelka, S.; Schuster, S.; Hutzler, F. An incremental boundary study on parafoveal preprocessing in children reading aloud: Parafoveal masks overestimate the preview benefit. J. Cogn. Psychol. 2015, 27, 549–561. [Google Scholar] [CrossRef]
- Hutzler, F.; Schuster, S.; Marx, C.; Hawelka, S. An investigation of parafoveal masks with the incremental boundary paradigm. PLoS ONE 2019, 14, e0203013. [Google Scholar] [CrossRef]
- Kliegl, R.; Hohenstein, S.; Yan, M.; McDonald, S.A. How preview space/time translates into preview cost/benefit for fixation durations during reading. Q. J. Exp. Psychol. 2013, 66, 581–600. [Google Scholar] [CrossRef]
- Kazai, K.; Yagi, A. Comparison between the lambda response of eye-fixation–related potentials and the P100 component of pattern- reversal visual evoked potentials. Cogn. Affect. Behav. Neurosci. 2003, 3, 46–56. [Google Scholar] [CrossRef]
- Hauk, O.; Davis, M.H.; Ford, M.; Pulvermüller, F.; Marslen-Wilson, W.D. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage 2006, 30, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Sereno, S.C.; Brewer, C.C.; O’Donnell, P.J. Context effects in word recognition: Evidence for early interactive processing. Psychol. Sci. 2003, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Ehinger, B.V.; Dimigen, O. Unfold: An integrated toolbox for overlap correction, non-linear modeling, and regression-based EEG analysis. PeerJ 2019, 7, e7838. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, S.; Matuschek, H.; Kliegl, R. Linked linear mixed models: A joint analysis of fixation locations and fixation durations in natural reading. Psychon. Bull. Rev. 2017, 24, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Clifton, C., Jr.; Staub, A.; Rayner, K. Eye movements in reading words and sentences. In Eye Movements: A Window on Mind and Brain; van Gompel, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 341–372. [Google Scholar]
- Amsel, B.D. Tracking real-time neural activation of conceptual knowledge using single-trial event-related potentials. Neuropsychologia 2011, 49, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, T.; Sassenhagen, J.; Võ, M.L.H. Improving free-viewing fixation-related EEG potentials with continuous-time regression. J. Neurosci. Methods 2019, 313, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Guerin-Dugué, A.; Rivet, B. Regularization and a general linear model for event-related potential estimation. Behav. Res. Methods 2017, 49, 2255–2274. [Google Scholar] [CrossRef]
- Kristensen, E.; Rivet, B.; Guérin-Dugué, A. Estimation of overlapped Eye Fixation Related Potentials: The General Linear Model, a more flexible framework than the ADJAR algorithm. J. Eye Mov. Res. 2017, 10, 1–27. [Google Scholar] [CrossRef]
- Smith, N.J.; Kutas, M. Regression-based estimation of ERP waveforms: I. The rERP framework. Psychophysiology 2015, 52, 157–168. [Google Scholar] [CrossRef]
- Smith, N.J.; Kutas, M. Regression-based estimation of ERP waveforms: II. Nonlinear effects, overlap correction, and practical considerations. Psychophysiology 2015, 52, 169–181. [Google Scholar] [CrossRef]
- Woldorff, M.G. Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology 1993, 30, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Hauk, O.; Giraud, A.L.; Clarke, A. Brain oscillations in language comprehension. Lang. Cogn. Neurosci. 2017, 32, 533–535. [Google Scholar] [CrossRef]
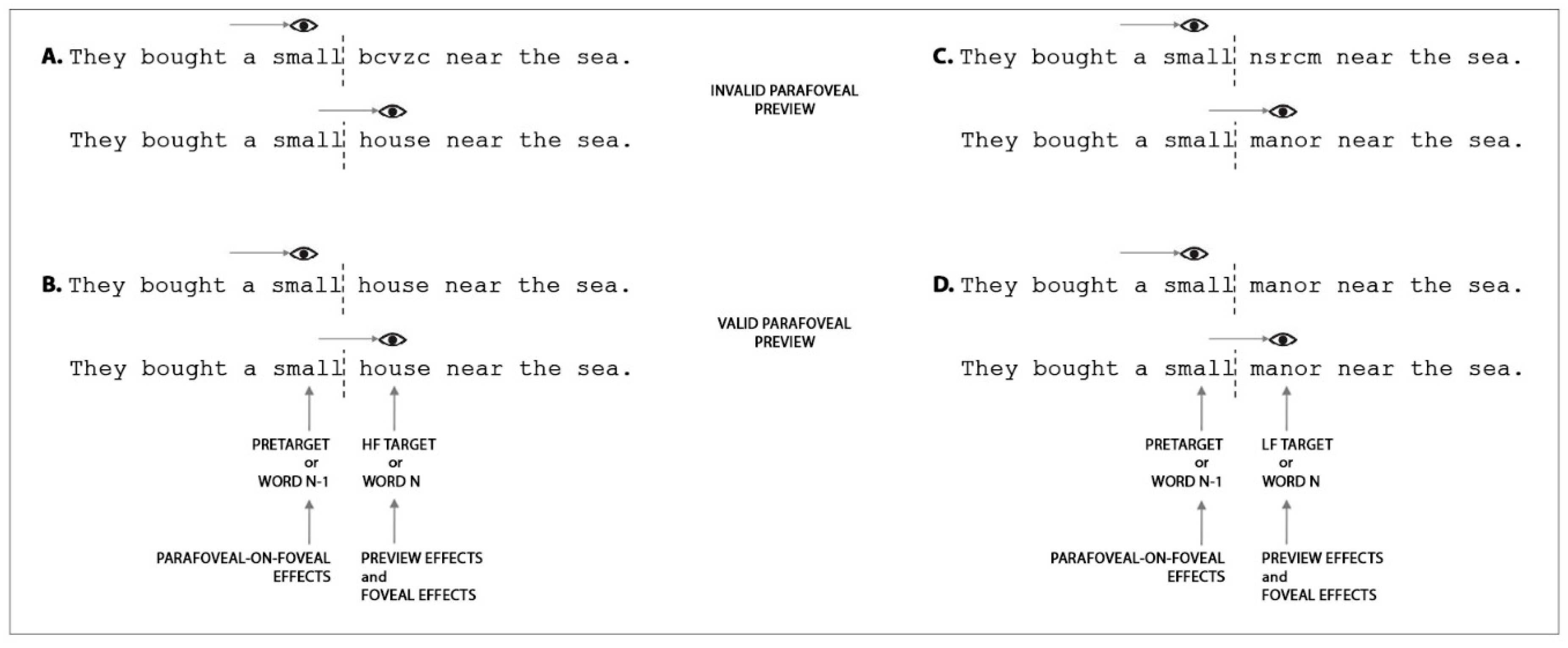
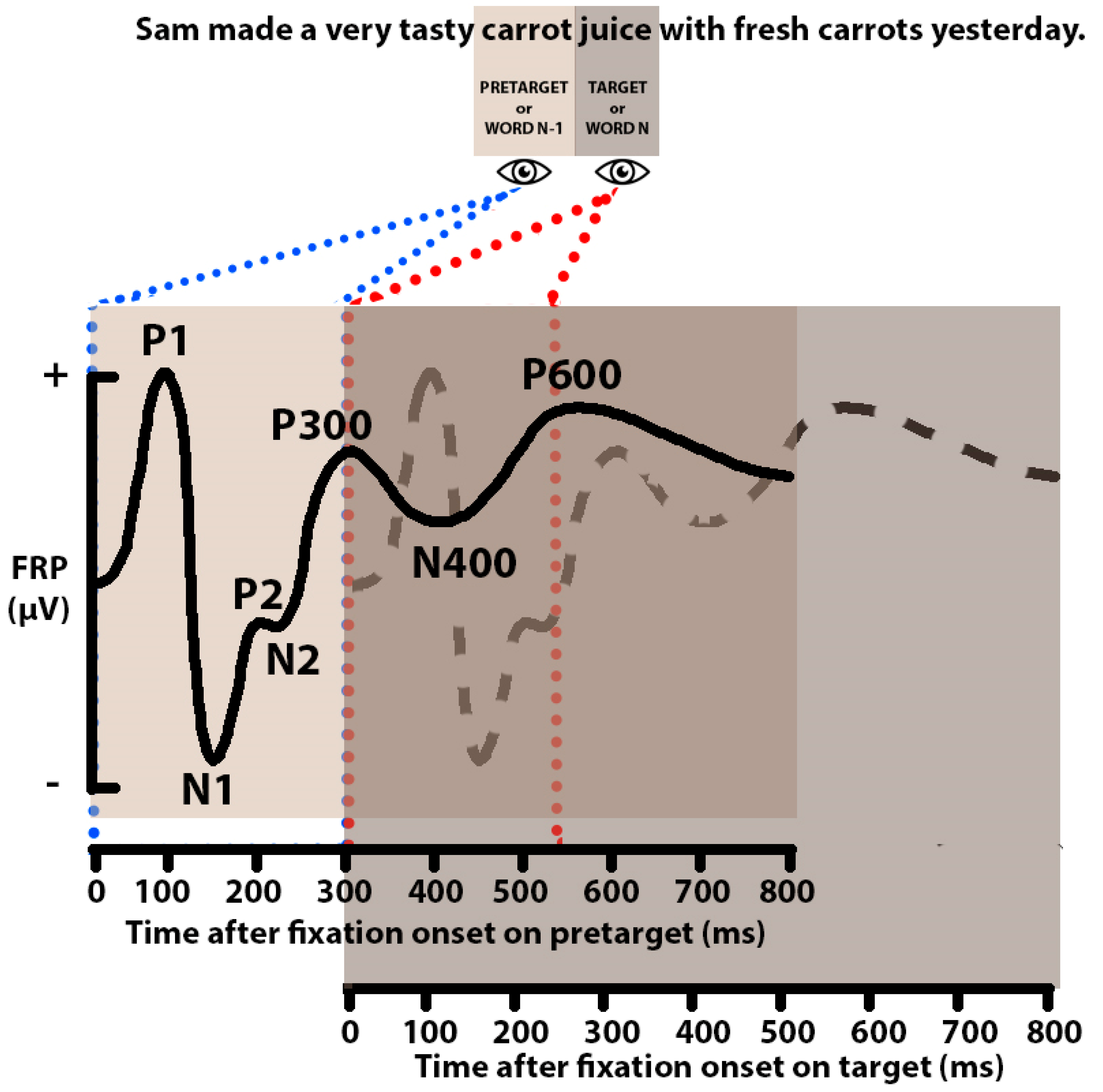
| Study | Language | Participants | Paradigm | Task | Variables | Investigated Effects |
|---|---|---|---|---|---|---|
| BM2005 + [106] | French | Age range: 22–38 Average age: 27.0 Typical readers | Priming | Semantic association judgment | Parafoveal preview, Semantic relatedness | Parafoveal processing (PoF) |
| Hetal2007 ++ [107] | German | Age range not reported Average age: 24.6 Typical readers | Free reading RSVP | Recognition | Reading modality, Repetition | Foveal processing |
| KBSS2009 * [108] | German | Age range: 19–31 Average age: 23.7 Typical readers | Free reading | Reading for comprehension | Target word predictability, Semantic relatedness | Foveal processing, Parafoveal processing (PoF) |
| SHL2009 + [109] | Swedish | Age range not reported Average age: 27.5 Typical readers | Priming | Semantic association judgment | Parafoveal preview, Visual field of presentation | Parafoveal processing (PoF) |
| DSHJK2011 * [26] | German | Age range: 17–37 Average age: 23.0 Typical readers | Free reading | Reading for comprehension | Target word predictability | Foveal processing |
| DKS2012 ++ [110] | German | Age range: 19–36 Average age: 24.4 Typical readers | Boundary, Free reading | Semantic decision | Parafoveal preview, Semantic relatedness, Repetition | Foveal processing, Parafoveal processing (PoF, Preview benefit) |
| HLSR2013 ** [111] | English | Age not reported Typical readers | Free reading | Reading | Text type | Foveal processing |
| Hetal2013 ++ [112] | German | Age range not reported Average age: 24.0 Typical readers | Boundary, RSVP fixed-pace, RSVP self-pace | Recognition | Parafoveal preview, Reading modality, Repetition | Foveal processing, Parafoveal processing (Preview baseline) |
| KSS2015 * [113] | English | Age range: 18–29 Average age: 20.3 Typical readers | Free reading | Reading for comprehension | Target word frequency, Target word predictability | Foveal processing, Parafoveal processing (PoF) |
| MvdMVR2015 * [104] | German | Age range: 19–34 Average age: 25 Typical readers | Free reading, RSVP | Reading for comprehension | Target word predictability | Foveal processing, Parafoveal processing (PoF) |
| KNSD2016 ++ [114] | German | Age range: 18–34 Average age: 24.8 Typical readers | Boundary, RSVP-with-flankers | Semantic decision | Parafoveal preview, Reading modality, Pretarget word frequency | Foveal processing, Parafoveal processing (Preview benefit) |
| LPDHCB2016 + [115] | Spanish | Age range: 18–29 Average age: 20.0 Typical readers | Priming, Boundary | Semantic association judgment | Preview semantic relatedness, Target semantic relatedness | Parafoveal processing (PoF, Preview benefit) |
| MvdMVR2016 * [116] | German | Age range not reported Average age: 25.0 Typical readers | Free reading, RSVP | Sentence well-formedness judgment | Syntactic/semantic violations, Violation position, Reading modality | Foveal processing |
| ND2016 ++ [117] | German | Age range: 18–34 Average age: 26.1 Typical readers | Boundary, RSVP-with-flankers | Semantic decision | Parafoveal preview, Foveal load, Target word frequency | Foveal processing, Parafoveal processing (PoF, Preview benefit) |
| WKV2016 * [118] | Hungarian | Age range: 20–26 Average age: 22.3 Typical readers Fast and slow readers | Free reading | Reading for comprehension | Inter-letter spacing, Reading ability | Foveal processing |
| LHHL2018 * [119] | Finnish | Age range: 12–13.5 Average age not reported Typical readers | Free reading | Sentence plausibility judgm0nt | Semantic violations | Foveal processing, Parafoveal processing (PoF for EM data only) |
| DLZZDL2019 * [120] | English | Age range: 18–26 Average age: 19.3 Typical readers | Boundary | Reading for comprehension | Parafoveal preview, Target word frequency | Foveal processing, Parafoveal processing (PoF, Preview benefit) |
| DLZZDL2019 * [121] | English | Age range: 18–26 Average age: 19.3 Typical readers | Boundary | Reading for comprehension | Inter-word spacing, Parafoveal preview | Foveal processing, Parafoveal processing (Preview benefit) |
| LHHL2019 * [122] | Finnish | Age range: 12–13.5 Average age not reported Slow and typical readers | Free reading | Sentence plausibility judgment | Word length, Fixation order, Reading ability | Foveal processing |
| Investigated Effect | Study | FRP Data | EM Data | |||||
|---|---|---|---|---|---|---|---|---|
| Significant | Time-Window | Electrode Sites | Direction of the Effect | Significant | EM Measure | Direction of the Effect | ||
| Word form | BM2005 [106] | yes | peak 119 ms (N1) | LO | related words > letter-string | yes | TFD | words > letter-string |
| from 100 ms, peak 140 ms (P) | RC, RF | unrelated words > letter-string | ||||||
| SHL2009 [109] | yes | 200–280 ms (P2) | O | RVF words > RVF letter-string | yes | FFD | RVF unrelated words > letter-string | |
| TRT | words > letter-string | |||||||
| KNSD2016 [114] | no | 200–280 ms (N1) | OT | not analysed | ||||
| DLZZDL2019 [120] | yes | 70–120 ms (P1) | RO, RP, MO, MP | X-string > identity | yes | (FFD) | X-string > identity | |
| RO, RP, MO, MP | X-string > letter-string | SFD | X-string > identity | |||||
| LO, LP, T, F | letter-string > X-string | GD | X-string > identity and letter-string | |||||
| C | identity > letter-string | |||||||
| 120–300 ms (N1) | RO, RP, MO, MP | identity > X-string | ||||||
| RO, RP, MO, MP | letter-string > X-string | |||||||
| LO, LP, T, F | X-string > letter-string | |||||||
| C | letter-string > identity | |||||||
| F, C, T | X-string > identity | |||||||
| 300–500 ms (N400) | RO, RP, MO, MP | identity > X-string | ||||||
| RO, RP, MO, MP | letter-string > X-string | |||||||
| LO, LP, T, F | X-string > letter-string | |||||||
| C | letter-string > identity | |||||||
| F, C, T | X-string > identity | |||||||
| Word repetition | DKS012 [110] | no | from 0–40 ms to 560–600 ms | all | X | yes | FFD | repeated < different |
| SFD | repeated < different | |||||||
| GD | repeated < different | |||||||
| Preview frequency | KSS2015 [113] | no† | from 150–200 to 350–400 ms (N400) | CP, M | X | no | LFD | X |
| ND2016 [117] | yes | 130–140 ms (P) | RF | LF > HF | yes | GD | LF > HF | |
| 630–640 ms (P) | LP | HF > LF | ||||||
| DLZZDL2019 [120] | no | 70–120 ms (P1), 120–300 ms (N1), 300–500 ms (N400) | all | X | no | FFD, SFD, GD | X | |
| Semantic relatedness | BM2005 [106] | yes | from 160 ms, peak 215 ms (P2) | C, F | related words > unrelated words | yes | TFD | unrelated words > related words |
| SHL2009 [109] | no | 90–140 ms (P1), 140–200 ms (N1), 200–280 ms (P2) | O | X | no | FFD, GD, TRT | X | |
| 70–120 ms (N1), 140–280 ms (P2) | FP | X | ||||||
| KBS2009 [108] | yes | 250–400 ms (N400) | P, C | unpredicted unrelated > predicted antonym | no | LFD | X | |
| P, C | unpredicted unrelated > unpredicted related | |||||||
| DKS2012 [110] | no | from 0–40 ms to 560–600 ms | all | X | no | FFD, SFD, GD | X | |
| LDHB2016 [115] * | yes | 400–550 ms (N400) | all | unrelated > related | no | FFD, GD | X | |
| Preview predictability | KBS2009 [108] | no | 250–400 ms (N400) | P, C | X | no | LFD | X |
| DSHJK2011 [26] | no | 300–500 ms (N400) | X | X | not analysed | X | X | |
| KSS2015 [113] | † | from 150–200 to 350–400 ms (N400) | CP, M | X | no | LFD | X | |
| MvdMVR2015 [104] | + | 334–826 ms, peak 608 ms (N) peak 658 ms (P) | CP F | incongruent > congruent incongruent > congruent | no | FFD, GD, RP | X | |
| Investigated Effect | Study | FRP Data | EM Data | |||||
|---|---|---|---|---|---|---|---|---|
| Signicant | Time-Window | Electrode Sites | Direction of the Effect | Signicant | EM Measure | Direction of the Effect | ||
| Identity parafoveal preview | DKS012 [110] | yes | 200–240 ms (N1) | OT | invalid > identity | yes | FFD | invalid > valid previews |
| 240–280 ms (N1) | OT | invalid > identity | SFD | invalid > valid previews | ||||
| 360–400 ms (N400) | CP | invalid > identity | GD | invalid > valid previews | ||||
| KNSD2016 [114] | yes | 200–280 ms (N1) | OT | X-string > 1 letter | yes | FFD | X-string > 3 letters | |
| X-string > 2 letters | X-string > full preview | |||||||
| X-string > 3 letters | ||||||||
| X-string > full preview | ||||||||
| 400–500 ms (N400) | CP | invalid > identity | ||||||
| ND2016 [117] | yes | 140–200 ms (N1) | OT | invalid > identity | yes | FFD | invalid > valid previews | |
| 200–300 ms (N1) | OT | invalid > identity | SFD | invalid > valid previews | ||||
| GD | invalid > valid previews | |||||||
| LDHB2016 [115] | yes | 300–500 ms (N400) | not reported | invalid > identity | not analysed | X | X | |
| 500–800 ms (P600) | C | invalid > identity | ||||||
| DLZZDL2019 [120] | yes | 0–70 ms (N) | RO, MO, RP, MP | identity > X-string | yes | FFD | invalid > valid previews | |
| RO, MO, RP, MP | letter-string > X-string | SFD | invalid > valid previews | |||||
| RO, T, P | identity > letter-string | GD | invalid > valid previews | |||||
| C | letter-string > X-string | |||||||
| C | letter-string > identity | |||||||
| T, F | X-string > letter-string | |||||||
| 70–120 ms (P1) | RO, RP, MO, MP | X-string > identity | ||||||
| RO, RP, MO, MP | X-string > letter-string | |||||||
| RO, T, P | letter-string > identity | |||||||
| C | X-string > letter-string | |||||||
| C | identity > letter-string | |||||||
| RT, RF | letter-string > X-string | |||||||
| 120–300 ms (N1) | O, P (120–200 ms) | identity > X-string | ||||||
| O, T, P (200–300 ms) | X-string > identity | |||||||
| O, T, P | X-string > letter-string | |||||||
| O, T, P | identity > letter-string | |||||||
| T, C, F (120–200 ms) | X-string > identity | |||||||
| M, C | letter-string > X-string | |||||||
| M, C (200–300 ms) | identity > X-string | |||||||
| C | letter-string > identity | |||||||
| 300–500 ms (N400) | O, T, P | X-string > identity | ||||||
| O, T, P | X-string > letter-string | |||||||
| O, P | identity > letter-string | |||||||
| M, C | letter-string > X-string | |||||||
| M, C | identity > X-string | |||||||
| DLZZDL2019 [121] | yes | 0–70 ms | X | yes | FFD | invalid > valid previews | ||
| 70–120 ms (P1) | O, P | string > identity | SFD | invalid > valid previews | ||||
| C, F | identity > string | |||||||
| 120–300 ms (N1) | O, RT, LT, RP, LP (120–180 ms) | identity > string | GD | invalid > valid previews | ||||
| C, F (120–180 ms) | identity < string | |||||||
| O, RT, LT, RP, LP (185–300 ms) | identity < string | |||||||
| MP, C, LF, MF (185–300 ms) | identity > string | |||||||
| 300–500 ms (N400) | O, LP, LC, MC | identity > string | ||||||
| RT, RC, MC, F | identity < string | |||||||
| Semantic relatedness | DKS2012 [110] | no | from 0–40 ms to 560–600 ms | all | X | no | FFD, SFD, GD | |
| LDHB2016 [115] | yes | 0–200 ms (N) | O, P, C | unrelated > related | no | FFD, | ||
| 300–500 ms (N400) | all | unrelated > related | GD | |||||
| 500–750 ms (P600) | all | unrelated > related | ||||||
| Investigated Effect | Study | FRP Data | EM Data | |||||
|---|---|---|---|---|---|---|---|---|
| Significant | Time-Window | Electrode Sites | Direction of the Effect | Significant | EM Measure | Direction of the Effect | ||
| Text type | HLSR2013 [111] | yes | 75–125 ms (P1) | O, T | text > pseudotext | yes | FFD | pseudotext > text |
| 125–210 ms (N1) | O, T | text > pseudotext | ||||||
| Inter-word spacing X | DLZZDL2019 [121] | yes | 0–70 ms^ (N) | LP, LO | unspaced > spaced | yes | FFD | unspaced > spaced |
| 70–120 ms (P1) | O, P | spaced > unspaced | SFD | unspaced > spaced | ||||
| F, LC, MC | unspaced > spaced | GD | unspaced > spaced | |||||
| 120–300 ms (N1) | O, P (135215– ms) | spaced > unspaced | ||||||
| F, C, T (145205– ms) | spaced < unspaced | |||||||
| O, P (215300– ms) | spaced < unspaced | |||||||
| RC (145300– ms) | spaced < unspaced | |||||||
| F, C, MP (220300– ms) | spaced > unspaced | |||||||
| 300–500 ms (N400) | X | X | ||||||
| Inter-letter spacing | WKV2016 [118] | yes | 120–175 ms (N) | OT, P | normal spacing >reduced and double spacing | yes | FD * | reduced > normal spacing |
| 155–220 ms (N) | ROT, RP | reduced > normal > double spacing | normal > double spacing | |||||
| 230–265 ms (P) | ROT, P | normal spacing >reduced and double spacing | SA * | double > normal spacing | ||||
| 345–380 ms (N) | LOT | normal spacing >reduced and double spacing | normal > reduced spacing | |||||
| Word length | LHHL2019 [122] | yes | 130–300 ms (P) | F | TP: long > short words for additional fixation | yes | FFD | long > short words |
| 130–300 ms (N) | O | TP: long > short words for additional fixation | GD | long > short words | ||||
| 170–280 ms (N) | RO | SR: long > short words for additional fixation | REFIX | long > short words | ||||
| Word frequency | KSS2015 [113] | no | from 150–200 to 650–700 ms (N400) | CP, M | X | yes | FFD | LF > HF |
| GD | LF > HF | |||||||
| skipping | LF < HF | |||||||
| ND2016 [117] | yes | 140–200 ms (N1) | OT | LF > HF | yes | FFD | LF > HF | |
| 200–300 ms (N1) | OT | LF > HF | SFD | LF > HF | ||||
| GD | LF > HF | |||||||
| DLZZDL2019 [120] | no | 0–70 ms ^, 70–120 ms (P1), | all | X | yes | FFD | LF > HF | |
| 120–300 ms (N1), 300–500 ms (N400) | SFD | LF > HF | ||||||
| GD | LF > HF | |||||||
| Repetition (old/new) | Hetal2007 [107] | yes | 250–600 ms (P) | P, C, F + | old > new | not analysed | ||
| DKS2012 [110] | yes | 80–480 ms (N400) | CP | new > old | yes | FFD | new > old | |
| SFD | new > old | |||||||
| GD | new > old | |||||||
| Hetal2013 [112] | yes | 176–390 ms (P) | P, C, F + | old > new | not analysed | |||
| Semantic Relatedness | KBS2009 [108] | yes | 450–740 ms (P600) | P | unpredicted unrelated > unpredicted related | no | FFD | X |
| P, C | unpredicted unrelated > predicted antonyms | |||||||
| LC | unpredicted related > predicted antonyms | |||||||
| DKS2012 [110] | yes | 160–480 ms (N400) | CP | unrelated > related | yes | FFD | unrelated > related | |
| SFD | unrelated > related | |||||||
| GD | unrelated > related | |||||||
| LDHB2016 [115] | yes | 300–500 ms (N400) | all | unrelated > related | no | FFD, GD | X | |
| 500–750 ms (P600) | all | unrelated > related | ||||||
| Word predictability | KBS2009 [108] | yes | 250–400 ms (N400) | RP | unpredicted related > predicted antonym | yes | FFD | unpredicted > predicted |
| P | unpredicted unrelated > predicted antonym | |||||||
| DSHJK2011 [26] | yes | 248–500 ms (N400) | CP | low predictable > high predictable | yes | FFD | LP > HP | |
| GD | LP > HP | |||||||
| TSR | LP > HP | |||||||
| KSS2015 [113] | yes | 150–250 ms (P200) | CP, M | predictable > unpredictable | yes | FFD | LP > HP | |
| 250–650 ms (N400) | CP, M | unpredictable > predictable | GD | LP > HP | ||||
| regressions | LP > HP | |||||||
| skipping | LP < HP | |||||||
| MvddMVR2015 [104] | yes | 222–514 ms, peak 378 ms (N400) | ROT | incongruent > congruent | yes | FFD | incongruent > congruent | |
| 318–626 ms, peak 476 ms (P) | F, FC | incongruent > congruent | GD | incongruent > congruent | ||||
| 692–1400 ms, peak 1382 ms(P) | CP | incongruent > congruent | RP | incongruent > congruent | ||||
| Syntactic & semantic violations | MvdMVR2016 [116] | yes | 290–1000 ms (P600) | X | Mid-sentence syntactic violations regression trials > control | yes | FFD | violations > control |
| 540–1000 ms (P600) | X | Mid-sentence semantic violations regression trials > control | GD | violations > control | ||||
| 24–378 ms (N400) | CP | Sentence-final syntactic violations regression trials > control | RP | violations > control | ||||
| 244–1000 ms (P600) | CP | Sentence-final syntactic violations regression trials > control | ||||||
| 98–392 ms (N400) | OT | Sentence-final semantic violations regression trials > control | ||||||
| 412–1000 ms (P600) | CP | Sentence-final semantic violations regression trials > control | ||||||
| 310–1000 ms (N) | CP | Sentence-final syntactic violations no-regression trials > control | ||||||
| 336–646 ms (N) | CP | Sentence-final semantic violations no-regression trials > control | ||||||
| 652–774 ms (N) | CP | Sentence-final semantic violations no-regression trials > control | ||||||
| LHHL2018 [119] | yes | 167–547 ms (N) | RFEF | anomalous word neighbour > plausible | yes | FFD | anomalous word neighbour > plausible | |
| 238–738 ms (N) | RFEF | unrelated anomalous > plausible | unrelated anomalous > plausible | |||||
| 254–445 ms (N400) | LP | unrelated anomalous > plausible | GD | anomalous word neighbour > plausible | ||||
| 263–447 ms (N400) | CP | unrelated anomalous > plausible | unrelated anomalous > plausible | |||||
| 309–535 ms (N400) | LP | anomalous word neighbour > plausible | REFIX | anomalous word neighbour > plausible | ||||
| 484–683 ms (P600) | LP | unrelated anomalous > anomalous word neighbour | unrelated anomalous > plausible | |||||
| 558–899 ms (P600) | LP | unrelated anomalous > plausible | ||||||
| 564–709 ms (P600) | RP | unrelated anomalous > plausible | ||||||
| 648–739 ms (P600) | CP | anomalous word neighbour > plausible | ||||||
| 710–899 ms (P600) | LP | anomalous word neighbour > plausible | ||||||
| 739–813 ms (P600) | RP | unrelated anomalous > plausible | ||||||
| 792–869 ms (P600) | CP | unrelated anomalous > plausible | ||||||
| Foveal load | KNSD2016 [114] | yes | 200–280 ms (N1) | OT | HF > LF | yes | FFD | LF > HF |
| Reading ability | LHHL2019 [122] | yes | 140–250 ms (P) | C | Slow > Typical readers | yes | FFD | Slow > Typical readers |
| 250–300 ms (P) | O | Slow > Typical readers | GD | Slow > Typical readers | ||||
| REFIX | Slow > Typical readers |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degno, F.; Liversedge, S.P. Eye Movements and Fixation-Related Potentials in Reading: A Review. Vision 2020, 4, 11. https://doi.org/10.3390/vision4010011
Degno F, Liversedge SP. Eye Movements and Fixation-Related Potentials in Reading: A Review. Vision. 2020; 4(1):11. https://doi.org/10.3390/vision4010011
Chicago/Turabian StyleDegno, Federica, and Simon P. Liversedge. 2020. "Eye Movements and Fixation-Related Potentials in Reading: A Review" Vision 4, no. 1: 11. https://doi.org/10.3390/vision4010011
APA StyleDegno, F., & Liversedge, S. P. (2020). Eye Movements and Fixation-Related Potentials in Reading: A Review. Vision, 4(1), 11. https://doi.org/10.3390/vision4010011






