Impact of Exercise Training at Maximal Fat Oxidation Intensity on Metabolic and Epigenetic Parameters in Patients with Overweight and Obesity: Study Protocol of a Randomized Controlled Trial
Abstract
1. Introduction
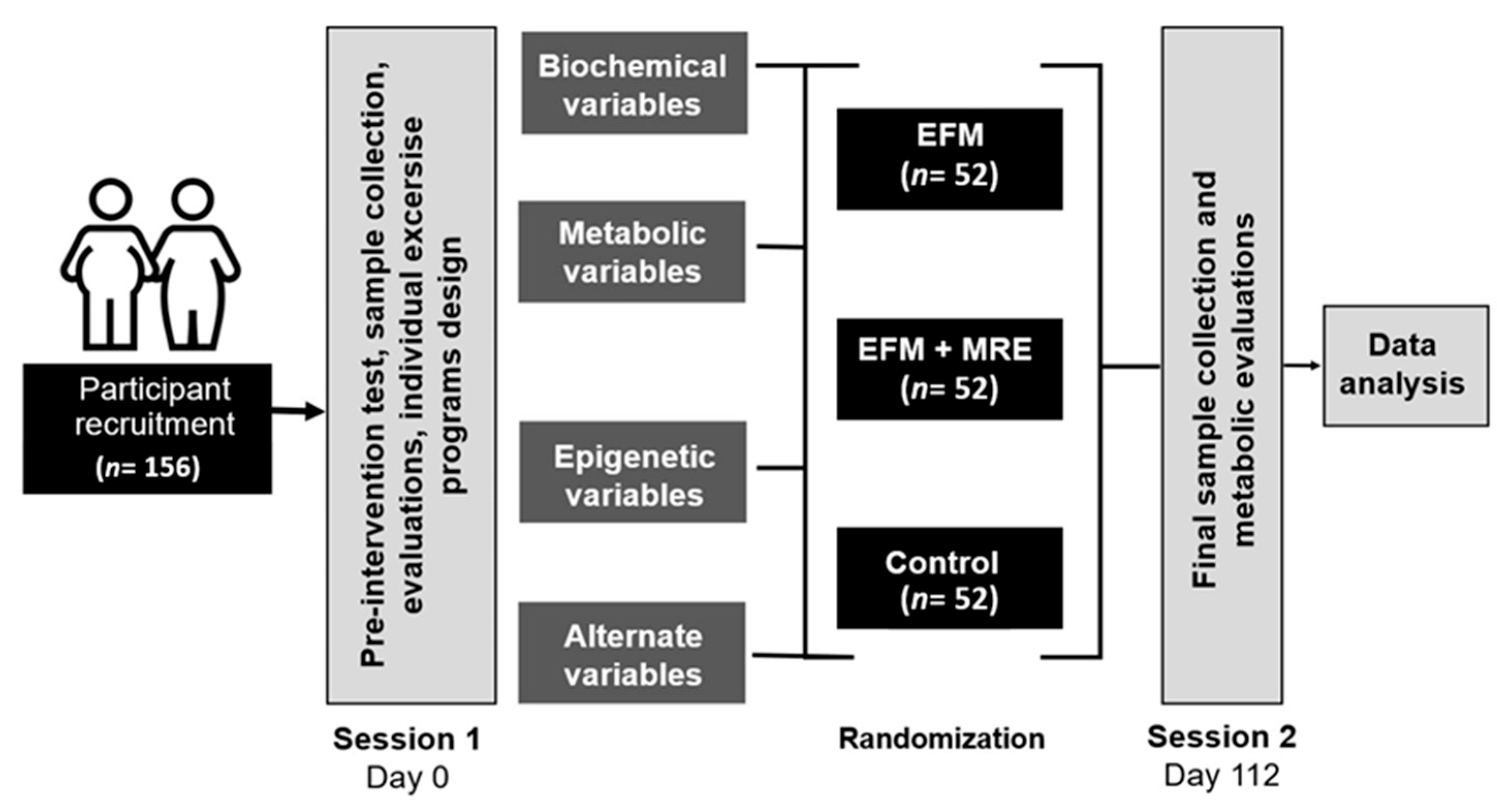
2. Experimental Design
2.1. Type of Study
2.2. Participants Eligibility Criteria
2.3. Interventions or Treatments
2.4. Dependent Variables
2.5. Groups and Randomization
2.6. Duration and Timeline
2.7. Control Procedures
3. Materials and Equipment
3.1. Biochemical Variables
3.2. Body Composition
3.3. Epigenetic Variables
3.4. Metabolic Flexibility
4. Detailed Procedure
4.1. Sample Size Calculation
4.2. Randomization and Blinding
4.3. Pre Intervention Exercise Test
4.4. FatMax and Resistance Training: Frequency, Intensity, Time and Type
4.5. Baseline and Follow-Up Measurements
4.5.1. Biochemical Variables
4.5.2. Metabolic Variables
4.5.3. Epigenetic Variables
4.5.4. Dietary and Psychological Counseling
4.5.5. Data Analysis
4.5.6. Ethics Concerns
5. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2021).
- Dixon, B.N.; Ugwoaba, U.A.; Brockmann, A.N.; Ross, K.M. Associations between the built environment and dietary intake, physical activity, and obesity: A scoping review of reviews. Obes. Rev. 2021, 22, e13171. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016. [Google Scholar]
- Chávez-Guevara, I.A.; Urquidez-Romero, R.; Pérez-León, J.A.; González-Rodríguez, E.; Moreno-Brito, V.; Ramos-Jiménez, A. Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2020, 17, 7888. [Google Scholar] [CrossRef]
- Brun, J.-F.; Myzia, J.; Varlet-Marie, E.; de Mauverger, E.R.; Mercier, J. Beyond the Calorie Paradigm: Taking into Account in Practice the Balance of Fat and Carbohydrate Oxidation during Exercise? Nutrients 2022, 14, 1605. [Google Scholar] [CrossRef]
- Keating, S.E.; Johnson, N.A.; Mielke, G.I.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017, 18, 943–964. [Google Scholar] [CrossRef]
- Sizoo, D.; de Heide, L.J.; Emous, M.; van Zutphen, T.; Navis, G.; van Beek, A.P. Measuring muscle mass and strength in obesity: A review of various methods. Obes. Surg. 2021, 31, 384–393. [Google Scholar] [CrossRef]
- Forbes, G.B.; Welle, S.L. Lean body mass in obesity. Int. J. Obes. 1983, 7, 99–107. [Google Scholar]
- Chávez-Guevara, I.A.; Hernández-Torres, R.P.; González-Rodríguez, E.; Ramos-Jiménez, A.; Amaro-Gahete, F.J. Biomarkers and genetic polymorphisms associated with maximal fat oxidation during physical exercise: Implications for metabolic health and sports performance. Eur. J. Appl. Physiol. 2022, 122, 1773–1795. [Google Scholar] [CrossRef]
- Chavez-Guevara, I.A.; Amaro-Gahete, F.J.; Ramos-Jimenez, A.; Brun, J.F. Toward exercise guidelines for optimizing fat oxidation during exercise in obesity: A systematic review and meta-regression. Spor. Med. 2023, 53, 2399–2416. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Vangelakoudi, A.; Maridaki, M. Peak fat oxidation rate during walking in sedentary overweight men and women. J. Sports Sci. Med. 2008, 7, 525–531. [Google Scholar] [PubMed]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2014, 36, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Chávez-Guevara, I.A.; Peric, R.; Amaro-Gahete, F.J.; Ramos-Jiménez, A. Reliability of the Metabolic Response During Steady-State Exercise at FATmax in Young Men with Obesity. Res. Q. Exerc. Sport 2024, 95, 766–774. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation During Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2004, 26, S28–S37. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; Sanchez-Delgado, G.; Jurado-Fasoli, L.; De-La-O, A.; Castillo, M.J.; Helge, J.W.; Ruiz, J.R. Assessment of maximal fat oxidation during exercise: A systematic review. Scand. J. Med. Sci. Sports 2019, 29, 910–921. [Google Scholar] [CrossRef]
- Chávez-Guevara, I.A. Assessment of metabolic flexibility by measuring maximal fat oxidation during submaximal intensity exercise: Can we improve the analytical procedures? Sports Med. Health Sci. 2023, 5, 156–158. [Google Scholar] [CrossRef]
- Keir, D.A.; Iannetta, D.; Maturana, F.M.; Kowalchuk, J.M.; Murias, J.M. Identification of Non-Invasive Exercise Thresholds: Methods, Strategies, and an Online App. Sports Med. 2021, 52, 237–255. [Google Scholar] [CrossRef]
- Wasserman, K.; McIlroy, M.B. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am. J. Cardiol. 1964, 14, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, F.; Verdi, M.; Schlemper, B.R., Jr.; Caponi, S. 50th Anniversary of the Declaration of Helsinki: The Double Standard Was Introduced. Arch. Med. Res. 2014, 45, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Guess, N. A qualitative investigation of attitudes towards aerobic and resistance exercise amongst overweight and obese individuals. BMC Res. Notes 2012, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22, e13273. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion/Elimination Criteria |
|---|---|
| Both genders | ≥100 mL of alcohol consumption per week |
| 18–35 years old | Consume a dietary supplement or pharmacological treatment |
| Energy expenditure < 600 METs a week | Have limitations to perform regular physical activity |
| Body mass index ≥ 25 kg/m2 | Have a chronic non-communicable disease |
| Acceptance of informed consent | Answer “yes” to any of the questions on the Physical Activity Readiness-Questionnaire for Everyone (PAR-Q+), which represents a health risk. |
| <80% attendance at exercise sessions |
| Treadmill | Cycle Ergometer | |
|---|---|---|
| Warm up | Duration: 5 min Intensity: 3 km/h; 0% inclination | Duration: 5 min; 40 Watts; 60 RPM |
| Phase 1 | Duration of stages: 3 min Initial load: 3 km/h; 1% inclination Progression of the load: 1 km/h Conclusion: RER = 1.0 for 30 s | Duration of stages: 3 min Initial load: 40 Watts; 60 RPM Progression of load: 20 Watts Conclusion: RER = 1.0 for 30 s |
| Phase 2 | Duration of stages: 1 min Load progression: 1 km/h Conclusion: (i) Rate of perceived exertion = 10; (ii) Heart rate ≥ 90% MHR | Duration of stages: 1 min Load progression: 20 Watts Conclusion: (i) Rate of perceived exertion = 10 Effort = 10; (ii) Heart rate ≥ 90% MHR |
| Training | Frequency | Intensity | Time | Type |
|---|---|---|---|---|
| FatMax | Fout times a week | Individualized according to the Heart Rate at thetMax | Sessions between 1–1.5 h | Aerobic exercise |
| Resistance Training | Two times a week | Individualized, with a total of 3 sets of 8–12 reps per muscle group a week | Sessions between 40–60 min | Resistance training (weight lifting) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Lepe, M.A.; Hernández-Ontiveros, D.A.; Chávez-Guevara, I.A.; Ramos-Jiménez, A.; Hernández-Torres, R.P.; López-Fregoso, R.J.; Ramos-Lopez, O.; Amaro-Gahete, F.J.; Muñiz-Salazar, R.; Olivas-Aguirre, F.J. Impact of Exercise Training at Maximal Fat Oxidation Intensity on Metabolic and Epigenetic Parameters in Patients with Overweight and Obesity: Study Protocol of a Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2024, 9, 214. https://doi.org/10.3390/jfmk9040214
Hernández-Lepe MA, Hernández-Ontiveros DA, Chávez-Guevara IA, Ramos-Jiménez A, Hernández-Torres RP, López-Fregoso RJ, Ramos-Lopez O, Amaro-Gahete FJ, Muñiz-Salazar R, Olivas-Aguirre FJ. Impact of Exercise Training at Maximal Fat Oxidation Intensity on Metabolic and Epigenetic Parameters in Patients with Overweight and Obesity: Study Protocol of a Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2024; 9(4):214. https://doi.org/10.3390/jfmk9040214
Chicago/Turabian StyleHernández-Lepe, Marco Antonio, David Alfredo Hernández-Ontiveros, Isaac Armando Chávez-Guevara, Arnulfo Ramos-Jiménez, Rosa Patricia Hernández-Torres, Reymond Josué López-Fregoso, Omar Ramos-Lopez, Francisco José Amaro-Gahete, Raquel Muñiz-Salazar, and Francisco Javier Olivas-Aguirre. 2024. "Impact of Exercise Training at Maximal Fat Oxidation Intensity on Metabolic and Epigenetic Parameters in Patients with Overweight and Obesity: Study Protocol of a Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 9, no. 4: 214. https://doi.org/10.3390/jfmk9040214
APA StyleHernández-Lepe, M. A., Hernández-Ontiveros, D. A., Chávez-Guevara, I. A., Ramos-Jiménez, A., Hernández-Torres, R. P., López-Fregoso, R. J., Ramos-Lopez, O., Amaro-Gahete, F. J., Muñiz-Salazar, R., & Olivas-Aguirre, F. J. (2024). Impact of Exercise Training at Maximal Fat Oxidation Intensity on Metabolic and Epigenetic Parameters in Patients with Overweight and Obesity: Study Protocol of a Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 9(4), 214. https://doi.org/10.3390/jfmk9040214











