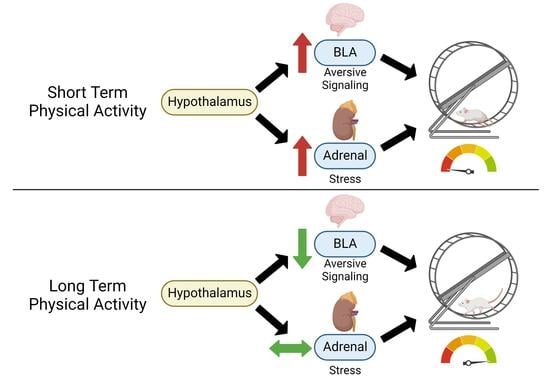
Acute Wheel-Running Increases Markers of Stress and Aversion-Related Signaling in the Basolateral Amygdala of Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
2.2. Plasma Corticosterone Levels
2.3. Novel Environment Fecal Boli Count
2.4. BLA mRNA Isolation and qPCR mRNA Analysis
2.5. Western Blotting for BLA Phospho-OPRK1 (KOR) Protein Expression
2.6. Statistical Analysis
3. Results
3.1. Male WT Rats Run Greater Daily Distances Than Lvr Rats at 4, but Not at 1 Week of Wheel-Running
3.2. Male LVR Rats Display Higher Levels of Hpa Activity across Basal and Wheel Access Conditions
3.3. LVR Rats Show Increased Bla Expression of Pdyn following 1 Week of Wheel-Running Compared to Sedentary Levels
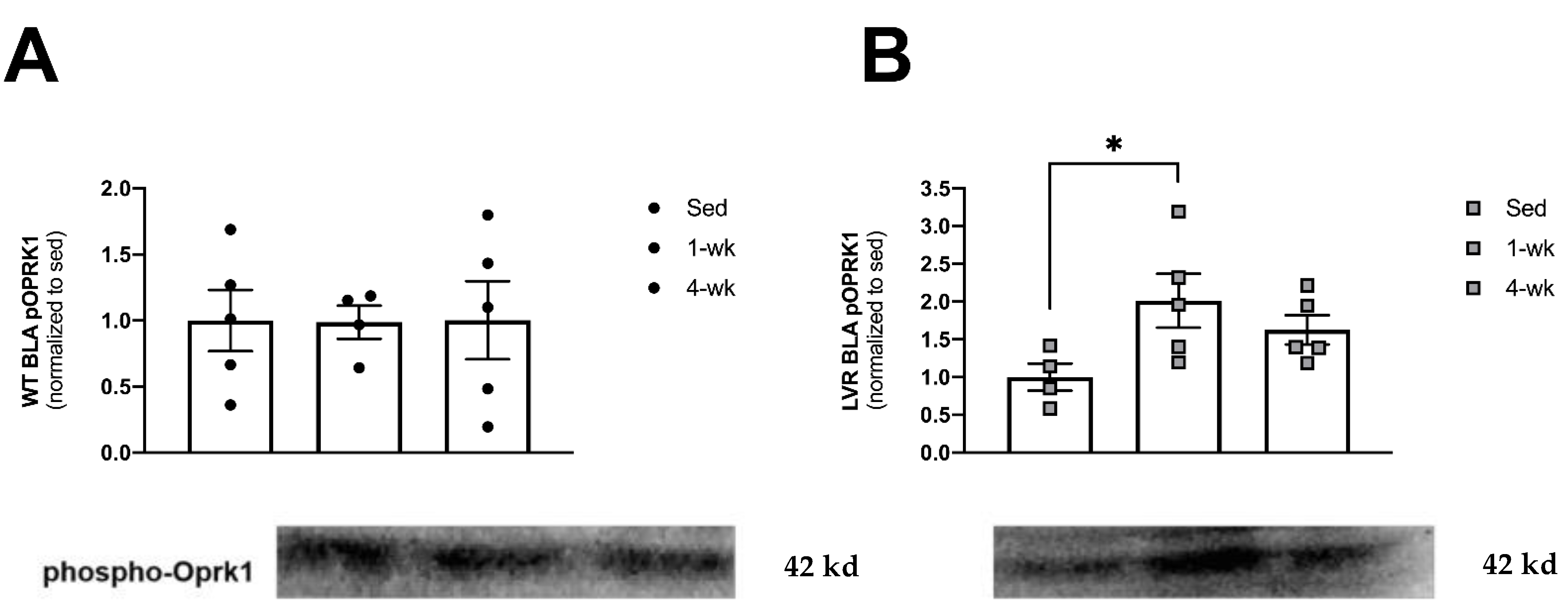
3.4. LVR Rats Show Increased Levels of Phosphorylated Oprk1 Protein Levels following 1 but Not 4 Weeks of Wheel-Running
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiank, C.; Zeden, J.-P.; Drude, S.; Domanska, G.; Fusch, G.; Otten, W.; Schuett, C. Psychological Stress-Induced, IDO1-Dependent Tryptophan Catabolism: Implications on Immunosuppression in Mice and Humans. PLoS ONE 2010, 5, e118252010-5. [Google Scholar] [CrossRef] [PubMed]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, U. Coping with Stress: Neuroendocrine Reactions and Implications for Health. Noise Health 1999, 1, 67. [Google Scholar]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef]
- Rosmond, R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology 2005, 30, 1–10. [Google Scholar] [CrossRef]
- Whitworth, J.A.; Schyvens, C.G.; Zhang, Y.; Mangos, G.J.; Kelly, J.J. Glucocorticoid-Induced Hypertension: From Mouse to Man. Clin. Exp. Pharmacol. Physiol. 2001, 28, 993–996. [Google Scholar] [CrossRef]
- Kelly, J.J.; Mangos, G.; Williamson, P.M.; Whitworth, J.A. Cortisol and Hypertension. Clin. Exp. Pharmacol. Physiol. 1998, 25, S51–S561998. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular Consequences of Cortisol Excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef]
- Hammen, C. Stress and Depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef]
- Tucker, L.A.; Clegg, A.G. Differences in health care costs and utilization among adults with selected lifestyle-related risk factors. Am. J. Health Promot. 2002, 16, 225–233. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2017, 8, a0296942017-8. [Google Scholar] [CrossRef] [PubMed]
- Stults-Kolehmainen, M.A.; Sinha, R. The Effects of Stress on Physical Activity and Exercise. Sports Med. 2014, 44, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Ginis, K.A.M.; Latimer, A.E.; McKechnie, K.; Ditor, D.S.; McCartney, N.; Hicks, A.L.; Bugaresti, J.; Craven, B.C. Using exercise to enhance subjective well-being among people with spinal cord injury: The mediating influences of stress and pain. Rehabilitation Psychol. 2003, 48, 157–164. [Google Scholar] [CrossRef]
- Knab, A.M.; Nieman, D.C.; Sha, W.; Broman-Fulks, J.J.; Canu, W.H. Exercise Frequency Is Related to Psychopathology but Not Neurocognitive Function. Med. Sci. Sports Exerc. 2012, 44, 1395–1400. [Google Scholar] [CrossRef]
- Sanchez-Villegas, A.; Ara, I.; Guillén-Grima, F.; Bes-Rastrollo, M.; Varo-Cenarruzabeitia, J.J.; Martínez-González, M.A. Physical Activity, Sedentary Index, and Mental Disorders in the SUN Cohort Study. Med. Sci. Sports Exerc. 2008, 40, 827–834. [Google Scholar] [CrossRef]
- Schnohr, P.; Kristensen, T.; Prescott, E.; Scharling, H. Stress and life dissatisfaction are inversely associated with jogging and other types of physical activity in leisure time-The Copenhagen City Heart Study. Scand. J. Med. Sci. Sports 2005, 15, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Dal-Zotto, S.; Martí, O.; Armario, A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav. Brain Res. 2000, 114, 175–181. [Google Scholar] [CrossRef]
- Kelliher, P.; Connor, T.J.; Harkin, A.; Sanchez, C.; Kelly, J.P.; Leonard, B.E. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiol. Behav. 2000, 69, 531–539. [Google Scholar] [CrossRef]
- Hauger, R.L.; Risbrough, V.; Brauns, O.; Dautzenberg, F.M. Corticotropin Releasing Factor (CRF) Receptor Signaling in the Central Nervous System: New Molecular Targets. CNS Neurol. Disord. Drug Targets 2008, 5, 453–479. [Google Scholar] [CrossRef]
- Fediuc, S.; Campbell, J.E.; Riddell, M.C. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J. Appl. Physiol. 2006, 100, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.N.; Foley, T.E.; Le, T.V.; Strong, P.V.; Loughridge, A.B.; Day, H.E.; Fleshner, M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011, 217, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Morrell, J.I. The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behav. Neurosci. 2015, 129, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, K.B.; Ruegsegger, G.N.; Childs, T.E.; Booth, F.W. Overexpression of Protein Kinase Inhibitor Alpha Reverses Rat Low Voluntary Running Behavior. Mol. Neurobiol. 2018, 56, 1782–1797. [Google Scholar] [CrossRef] [PubMed]
- Belke, T.W.; Wagner, J.P. The reinforcing property and the rewarding aftereffect of wheel running in rats: A combination of two paradigms. Behav. Process. 2005, 68, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, K.B.; Childs, T.E.; Booth, F.W. The role of nucleus accumbens CREB attenuation in rescuing low voluntary running behavior in female rats. J. Neurosci. Res. 2020, 98, 2302–2316. [Google Scholar] [CrossRef]
- Knoll, A.T.; Muschamp, J.W.; Sillivan, S.E.; Ferguson, D.; Dietz, D.M.; Meloni, E.G.; Carroll, F.I.; Nestler, E.J.; Konradi, C.; Carlezon, W.A. Kappa Opioid Receptor Signaling in the Basolateral Amygdala Regulates Conditioned Fear and Anxiety in Rats. Biol. Psychiatry 2011, 70, 425–433. [Google Scholar] [CrossRef]
- Veer, A.V.; Carlezon, W.A. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology 2013, 229, 435–452. [Google Scholar] [CrossRef]
- Taylor, G.T.; Manzella, F. Kappa Opioids, Salvinorin A and Major Depressive Disorder. Curr. Neuropharmacol. 2016, 14, 165–176. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Krystal, A.D. Kappa-Opioid Antagonists for Psychiatric Disorders: From Bench to Clinical Trials. Depression Anxiety 2016, 33, 895–906. [Google Scholar] [CrossRef]
- Schuch, F.B.; Morres, I.D.; Ekkekakis, P.; Rosenbaum, S.; Stubbs, B. A critical review of exercise as a treatment for clinically depressed adults: Time to get pragmatic. Acta Neuropsychiatr. 2017, 29, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ekkekakis, P.; Murri, M.B. Exercise as Antidepressant Treatment: Time for the Transition from Trials to Clinic? Gen. Hosp. Psychiatry 2017, 49, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, E.W. Physical activity in the prevention and treatment of anxiety and depression. Nord. J. Psychiatry 2008, 62, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Brown, J.D.; Company, J.M.; Oberle, L.P.; Heese, A.J.; Toedebusch, R.G.; Wells, K.D.; Cruthirds, C.L.; Knouse, J.A.; Ferreira, J.A.; et al. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. AJP Regul. Integr. Comp. Physiol. 2013, 304, R1024-35. [Google Scholar] [CrossRef]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Gentsch, C.; Lichtsteiner, M.; Feer, H. Locomotor activity, defecation score and corticosterone levels during an openfield exposure: A comparison among individually and group-housed rats, and genetically selected rat lines. Physiol. Behav. 1981, 27, 183–186. [Google Scholar] [CrossRef]
- Coleman, M.A.; Garland, T.; Marler, C.A.; Newton, S.S.; Swallow, J.G.; Carter, P.A. Glucocorticoid Response to Forced Exercise in Laboratory House Mice (Mus domesticus). Physiol. Behav. 1998, 63, 279–285. [Google Scholar] [CrossRef]
- Dallman, M.; Akana, S.; Bhatnagar, S.; Bell, M.; Strack, A. Bottomed out: Metabolic significance of the circadian trough in glucocorticoid concentrations. Int. J. Obes. 2000, 24, S40–S46. [Google Scholar] [CrossRef]
- Atkinson, H.C.; Waddell, B.J. Circadian Variation in Basal Plasma Corticosterone and Adrenocorticotropin in the Rat: Sexual Dimorphism and Changes across the Estrous Cycle. Endocrinology 1997, 138, 3842–3848. [Google Scholar] [CrossRef]
- Mönnikes, H.; Schmidt, B.G.; Raybould, H.E.; Tache, Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am. J. Physiol.-Gastrointest. Liver Physiol. 1992, 262, G137–G1431992. [Google Scholar] [CrossRef]
- Nakade, Y.; Fukuda, H.; Iwa, M.; Tsukamoto, K.; Yanagi, H.; Yamamura, T.; Mantyh, C.; Pappas, T.N.; Takahashi, T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G1037–G1044. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, K.B.; Kovarik, C.M.; Rottinghaus, G.E.; Booth, F.W. High and low nightly running behavior associates with nucleus accumbens N-Methyl-d-aspartate receptor (NMDAR) NR1 subunit expression and NMDAR functional differences. Neurosci. Lett. 2018, 671, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Gilpin, L.; Parker, K.E.; Childs, T.E.; Will, M.J.; Booth, F.W. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol. Behav. 2012, 105, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Toedebusch, R.G.; Wells, K.D.; Company, J.M.; Brown, J.D.; Cruthirds, C.L.; Heese, A.J.; Zhu, C.; Rottinghaus, G.E.; Childs, T.E.; et al. Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J. Physiol. 2014, 592, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Alario, P.; Gamallo, A.; Beato, M.; Trancho, G. Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiol. Behav. 1987, 40, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.T.; Cruz, F.C.; Planeta, C.S. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol. Behav. 2007, 90, 29–35. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Thompson, P.D. Exercise Is Medicine: At Any Dose? JAMA 2015, 314, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.S.; Stults-Kolehmainen, M.A.; Bartholomew, J.B. Exercise caution when stressed: Stages of change and the stress–exercise participation relationship. Psychol. Sport Exerc. 2010, 11, 560–567. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central Mechanisms of HPA Axis Regulation by Voluntary Exercise. NeuroMolecular Med. 2008, 10, 118–127. [Google Scholar] [CrossRef]
- Droste, S.K.; Chandramohan, Y.; Hill, L.E.; Linthorst, A.C.; Reul, J.M. Voluntary Exercise Impacts on the Rat Hypothalamic-Pituitary-Adrenocortical Axis Mainly at the Adrenal Level. Neuroendocrinology 2007, 86, 26–37. [Google Scholar] [CrossRef]
- Enoch, M.-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 2011, 214, 17–31. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wemm, S.E.; Sinha, R. Drug-induced stress responses and addiction risk and relapse. Neurobiol. Stress 2019, 10, 100148. [Google Scholar] [CrossRef] [PubMed]
- Land, B.B.; Bruchas, M.R.; Lemos, J.; Xu, M.; Melief, E.J.; Chavkin, C. The Dysphoric Component of Stress Is Encoded by Activation of the Dynorphin -Opioid System. J. Neurosci. 2008, 28, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Pisu, M.G.; Floris, I.; Biggio, G. Social Isolation-Induced Changes in the Hypothalamic–Pituitary–Adrenal Axis in the Rat. Stress 2005, 8, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Manrique, C.; Lacour, M. Stress axis plasticity during vestibular compensation in the adult cat. Neuroscience 2009, 160, 716–730. [Google Scholar] [CrossRef]
- Iredale, P.A.; Alvaro, J.D.; Lee, Y.; Terwilliger, R.; Chen, Y.L.; Duman, R.S. Role of Corticotropin-Releasing Factor Receptor-1 in Opiate Withdrawal. J. Neurochem. 2000, 74, 199–208. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Toedebusch, R.G.; Will, M.J.; Booth, F.W. Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology 2015, 97, 171–181. [Google Scholar] [CrossRef]
- Al-Hasani, R.; McCall, J.G.; Shin, G.; Gomez, A.M.; Schmitz, G.P.; Bernardi, J.M.; Pyo, C.-O.; Park, S.I.; Marcinkiewcz, C.M.; Crowley, N.A.; et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 2015, 87, 1063–1077. [Google Scholar] [CrossRef]
- Kelty, T.J.; Brown, J.D.; Kerr, N.R.; Roberts, M.D.; Childs, T.E.; Cabrera, O.H.; Manzella, F.M.; Miller, D.K.; Taylor, G.T.; Booth, F.W. RNA-sequencing and behavioral testing reveals inherited physical inactivity co-selects for anxiogenic behavior without altering depressive-like behavior in Wistar rats. Neurosci. Lett. 2021, 753, 135854. [Google Scholar] [CrossRef]
- Anderson, R.; Lopez, M.F.; Griffin, W.C.; Haun, H.L.; Bloodgood, D.W.; Pati, D.; Boyt, K.M.; Kash, T.L.; Becker, H.C. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology 2019, 44, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.W.; Schlosburg, J.E.; Wee, S.; Gould, A.; George, O.; Grant, Y.; Zamora-Martinez, E.R.; Edwards, S.; Crawford, E.; Vendruscolo, L.F.; et al. κ Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J. Neurosci. 2015, 35, 4296–4305. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; Orio, L.; Ghirmai, S.; Cashman, J.R.; Koob, G.F. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology 2009, 205, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.; Land, B.; Chavkin, C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010, 1314, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Hurel, I.; Mesguich, E.; Redon, B.; Stevens, C.; Georges, F.; Melis, M.; Marsicano, G.; Chaouloff, F. Exercise craving potentiates excitatory inputs to ventral tegmental area dopaminergic neurons. Addict. Biol. 2020, 26, e12967. [Google Scholar] [CrossRef]



| Gene | Forward (5′–3′) | Reverse (3′–5′) | Accession Number |
|---|---|---|---|
| 18s | GCCGCTAGAGGTGAAATTCTTG | CATTCTTGGCAAATGCTTTCG | NR_046237 |
| Pdyn | AACTGCCATAGGGGGATTTGG | GGATGGCCGATCCAAGATTCA | NM_019374 |
| Oprk1 | AAACATCAGGGACGTGGACC | CTCCCTTCCCAAATCAGCGT | XM_032906449 |
| Crhr1 | AAGGCTACCAGACTTGCTCG | GGGCTTCGCACCCTTCC | NR_126013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigsby, K.B.; Kerr, N.R.; Kelty, T.J.; Mao, X.; Childs, T.E.; Booth, F.W. Acute Wheel-Running Increases Markers of Stress and Aversion-Related Signaling in the Basolateral Amygdala of Male Rats. J. Funct. Morphol. Kinesiol. 2023, 8, 6. https://doi.org/10.3390/jfmk8010006
Grigsby KB, Kerr NR, Kelty TJ, Mao X, Childs TE, Booth FW. Acute Wheel-Running Increases Markers of Stress and Aversion-Related Signaling in the Basolateral Amygdala of Male Rats. Journal of Functional Morphology and Kinesiology. 2023; 8(1):6. https://doi.org/10.3390/jfmk8010006
Chicago/Turabian StyleGrigsby, Kolter B., Nathan R. Kerr, Taylor J. Kelty, Xuansong Mao, Thomas E. Childs, and Frank W. Booth. 2023. "Acute Wheel-Running Increases Markers of Stress and Aversion-Related Signaling in the Basolateral Amygdala of Male Rats" Journal of Functional Morphology and Kinesiology 8, no. 1: 6. https://doi.org/10.3390/jfmk8010006
APA StyleGrigsby, K. B., Kerr, N. R., Kelty, T. J., Mao, X., Childs, T. E., & Booth, F. W. (2023). Acute Wheel-Running Increases Markers of Stress and Aversion-Related Signaling in the Basolateral Amygdala of Male Rats. Journal of Functional Morphology and Kinesiology, 8(1), 6. https://doi.org/10.3390/jfmk8010006