Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era
Abstract
:1. Introduction
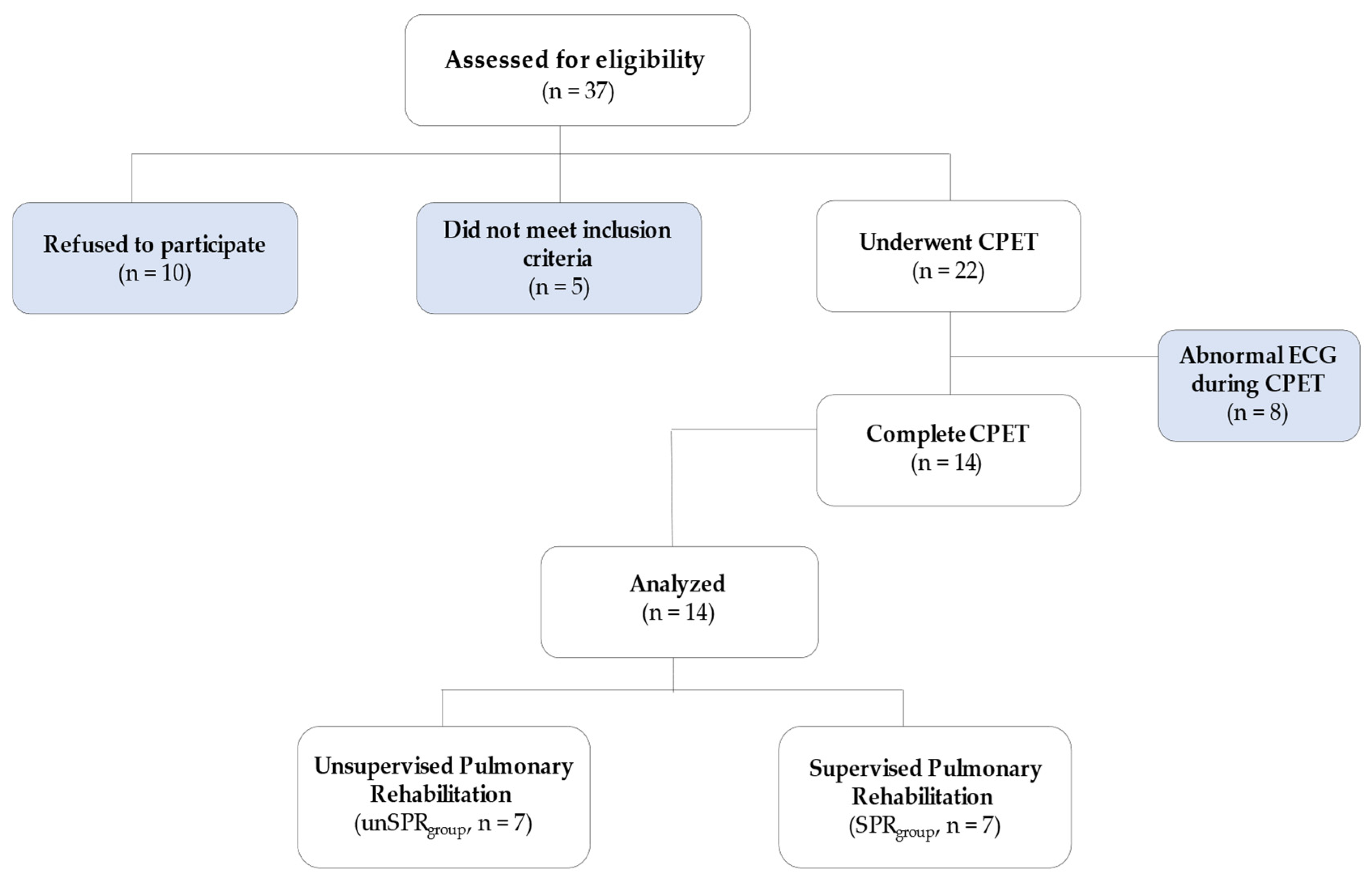
2. Materials and Methods
2.1. Study Population
2.2. Procedures
2.3. Echocardiography
2.4. Cardiopulmonary Exercise Testing
2.5. Pulmonary Rehabilitation Program
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Cardiopulmonary Exercise Testing
4.2. Quality of Life and Psychological Aspects
4.3. Sleep Quality
4.4. Implication of Rehabilitation Program
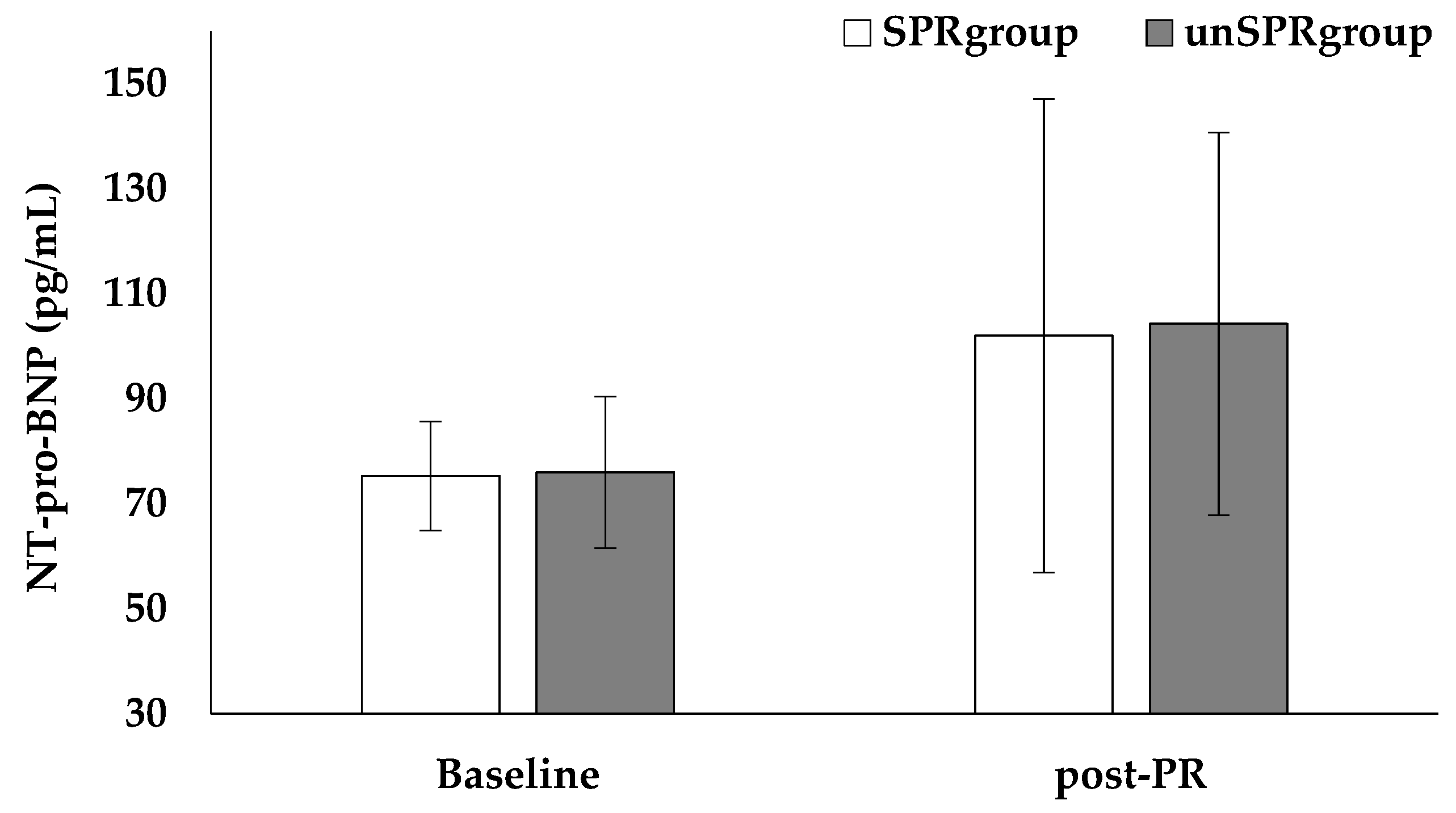
4.5. Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavorini, F.; Di Bello, V.; De Rimini, M.L.; Lucignani, G.; Marconi, L.; Palareti, G.; Pesavento, R.; Prisco, D.; Santini, M.; Sverzellati, N.; et al. Diagnosis and treatment of pulmonary embolism: A multidisciplinary approach. Multidiscip. Respir. Med. 2013, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Douma, R.A.; Kamphuisen, P.; Büller, H.R. Acute pulmonary embolism. Part 1: Epidemiology and diagnosis. Nat. Rev. Cardiol. 2010, 7, 585–596. [Google Scholar] [CrossRef]
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.; van der Hulle, T.; Exter, P.D.; Lankeit, M.; Huisman, M.; Konstantinides, S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev. 2014, 28, 221–226. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, A.; Oscullo, G.; Calvillo, P.; López-Reyes, R.; Méndez, R.; Gómez-Olivas, J.D.; Bekki, A.; Fonfría, C.; Trilles-Olaso, L.; Zaldívar, E.; et al. Incidence, risk factors, and thrombotic load of pulmonary embolism in patients hospitalized for COVID-19 infection. J. Infect. 2021, 82, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Roncon, L.; Zuin, M.; Barco, S.; Valerio, L.; Zuliani, G.; Zonzin, P.; Konstantinides, S.V. Incidence of acute pulmonary embo-lism in COVID-19 patients: Systematic review and meta-analysis. Eur. J. Intern. Med. 2020, 82, 29–37. [Google Scholar] [CrossRef]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodriguez, C.; Hunt, B.J.; Monreal, M.; et al. Incidence of VTE and Bleeding among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 543–603. [Google Scholar]
- Grünig, E.; Eichstaedt, C.; Barberà, J.-A.; Benjamin, N.; Blanco, I.; Bossone, E.; Cittadini, A.; Coghlan, G.; Corris, P.; D’Alto, M.; et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur. Respir. J. 2018, 53, 1800332. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Reference List of 100 Core Health Indicators (Plus Health-Related SDGs). WHO/HIS/IER/GPM/2018. Available online: https://apps.who.int/iris/handle/10665/259951 (accessed on 1 November 2021).
- ACSM. Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Boutou, A.K.; Pitsiou, G.G.; Argyropoulou, P. Non-invasive markers of pulmonary hypertension in interstitial lung disease: Is cardiopulmonary exercise testing the Holy Grail? Respirology 2014, 19, 621–622. [Google Scholar] [CrossRef]
- Werneke, M.W.; Deutscher, D.; Grigsby, D.; Tucker, A.C.; Mioduski, E.J.; Hayes, D. Telerehabilitation during the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys. Ther. 2021, 101, 110. [Google Scholar] [CrossRef]
- Rutkowski, S.; Szczegielniak, J.; Szczepańska-Gieracha, J. Evaluation of the Efficacy of Immersive Virtual Reality Therapy as a Method Supporting Pulmonary Rehabilitation: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 352. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomised controlled trial. Thorax 2021, 26, thoraxjnl-2021-217382. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Tourlakopoulos, K.N.; Vavougios, G.D.; Papayianni, E.; Kiribesi, K.; Maggoutas, S.; Nikolaidis, K.; Fradelos, E.C.; Dimeas, I.; Daniil, Z.; et al. Eight Weeks Unsupervised Pulmonary Rehabilitation in Previously Hospitalized of SARS-CoV-2 Infection. J. Pers. Med. 2021, 11, 806. [Google Scholar] [CrossRef]
- Mazzuca, P.; Montesi, L.; Mazzoni, G.; Grazzi, G.; Micheli, M.M.; Piergiovanni, S.; Pazzini, V.; Forlani, G.; Latessa, P.M.; Marchesini, G. Supervised vs. self-selected physical activity for individuals with diabetes and obesity: The Lifestyle Gym program. Intern. Emerg. Med. 2016, 12, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Griziotis, M.; Raptis, D.; Bardaka, F.; Karetsi, E.; Kiritsis, A.; Daniil, Z.; Tsarouhas, K.; Triposkiadis, F.; Gour-goulianis, K.I.; et al. Eight Weeks of Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Preliminary Report. Proceedings 2019, 25, 37. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ngo-Metzger, Q.; Sorkin, D.H.; Mangione, C.M.; Gandek, B.; Hays, R.D. Evaluating the SF-36 Health Survey (Version 2) in Older Vietnamese Americans. J. Aging Health 2008, 20, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Watson, P.E.; Watson, I.D.; Batt, R.D. Total body water volumes for adult males and females estimated from simple anthro-pometric measurements. Am. J. Clin. Nutr. 1980, 33, 27–39. [Google Scholar] [CrossRef]
- Hume, R. Prediction of lean body mass from height and weight. J. Clin. Pathol. 1966, 19, 389–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Whipp, B. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 4th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Albaghdadi, M.S.; Dudzinski, D.M.; Giordano, N.; Kabrhel, C.; Ghoshhajra, B.; Jaff, M.R.; Weinberg, I.; Baggish, A. Cardiopulmonary Exercise Testing in Patients Following Massive and Submassive Pulmonary Embolism. J. Am. Hear. Assoc. 2018, 7, e006841. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.R.; Akaberi, A.; Granton, J.T.; Anderson, D.R.; Wells, P.S.; Rodger, M.A.; Solymoss, S.; Kovacs, M.J.; Rudski, L.; Shimony, A.; et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: Results of the ELOPE cohort study. Am. J. Med. 2017, 130, 990.e9–990.e21. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, V.; Boutou, A.K.; Vavougios, G.D.; Pastaka, C.; Gourgoulianis, K.I.; Koutedakis, Y.; Daniil, Z.; Karetsi, E. The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir. Physiol. Neurobiol. 2019, 262, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Vanuxem, D.; Badier, M.; Guillot, C.; Delpierre, S.; Jahjah, F.; Vanuxem, P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997, 91, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Mereles, D.; Ehlken, N.; Kreuscher, S.; Ghofrani, S.; Hoeper, M.; Halank, M.; Meyer, F.J.; Karger, G.; Buss, J.; Juenger, J.; et al. Exercise and Respiratory Training Improve Exercise Capacity and Quality of Life in Patients with Severe Chronic Pulmonary Hypertension. Circulation 2006, 114, 1482–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, G.; Balady, G.J. Cardiac rehabilitation 2012: Advancing the field through emerging science. Circulation 2012, 125, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Tavoly, M.; Utne, K.K.; Jelsness-Jørgensen, L.-P.; Wik, H.S.; Klok, A.F.; Sandset, P.M.; Ghanima, W. Health-related quality of life after pulmonary embolism: A cross-sectional study. BMJ Open 2016, 6, e013086. [Google Scholar] [CrossRef] [Green Version]
- Paz-Díaz, H.; Montes de Oca, M.; López, J.M.; Celli, B.R. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am. J. Phys. Med. Rehabil. 2007, 86, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kord, Z.; Fereidouni, Z.; Mirzaee, M.S.; Alizadeh, Z.; Behnammoghadam, M.; Rezaei, M.; Abdi, N.; Delfani, F.; Zaj, P. Telenursing home care and COVID-19: A qualitative study. BMJ Support. Palliat. Care 2021, 29, bmjspcare-2021-003001. [Google Scholar] [CrossRef]
- Cooper, C.B. Desensitization to dyspnea in COPD with specificity for exercise training mode. Int. J. Chronic Obstr. Pulm. Dis. 2008, 4, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ortega, A.; Mañas, E.; López-Reyes, R.; Selma, M.J.; García-Sánchez, A.; Oscullo, G.; Jiménez, D.; Martínez-García, M. Ángel Obstructive sleep apnoea and venous thromboembolism: Pathophysiological links and clinical implications. Eur. Respir. J. 2019, 53, 1800893. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, F.; Wu, X.; Hou, W. Prevalence of pulmonary embolism in patients with obstructive sleep apnea and chronic ob-structive pulmonary disease: The overlap syndrome. Heart Lung 2019, 48, 261–265. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Seitanidis, G.; Daniil, Z.; Gourgoulianis, K.I. The effect of physical strain on breeders patients with obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2019, 260, 137–139. [Google Scholar] [CrossRef]
- Stavrou, V.; Karetsi, E.; Daniil, Z.; Gourgoulianis, I.K. Four Weeks Exercise in Obstructive Sleep Apnea Syndrome Patient with Type 2 Diabetes Mellitus and without Continuous Positive Airway Pressure Treatment: A Case Report. Sleep Med. Res. 2019, 10, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Amoury, M.; Noack, F.; Kleeberg, K.; Stoevesandt, D.; Lehnigk, B.; Bethge, S.; Heinze, V.; Schlitt, A. Prognosis of patients with pulmonary embolism after rehabilitation. Vasc. Health Risk Manag. 2018, 14, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Lakoski, S.G.; Savage, P.D.; Berkman, A.; Penalosa, L.; Crocker, A.; Ades, P.A.; Kahn, S.R.; Cushman, M. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: A randomized clinical trial. J. Thromb. Haemost. 2015, 13, 1238–1244. [Google Scholar] [CrossRef]
- Klok, E.; Mos, I.C.M.; Huisman, M.V. Brain-Type Natriuretic Peptide Levels in the Prediction of Adverse Outcome in Patients with Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2008, 178, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Donadini, M.; Gianni, M.; Bertolini, A.; Lonn, E.; Venco, A.; Cattozzo, G.; Ageno, W. Brain natriuretic peptide as a preclinical marker of chronic pulmonary hypertension in patients with pulmonary embolism. Intern. Emerg. Med. 2009, 4, 123–128. [Google Scholar] [CrossRef] [PubMed]
| SPRgroup | UnSPRgroup | p Value | |
|---|---|---|---|
| Age, years | 49.6 ± 15.4 | 51.9 ± 16.0 | 0.790 |
| Gender, M/F | 6/1 | 5/2 | 0.552 |
| Smokers, % | 28.6 | 42.9 | 0.611 |
| Body mass index, kg/m2 | 29.8 ± 3.9 | 30.1 ± 2.9 | 0.884 |
| Body surface area, m2 | 2.0 ± 0.5 | 2.2 ± 0.3 | 0.537 |
| Lean body mass, % | 60.9 ± 9.0 | 63.6 ± 6.3 | 0.531 |
| Total body water, L | 44.2 ± 8.9 | 44.7 ± 7.0 | 0.904 |
| Alcohol drinking, ml/month | 85.0 ± 13.7 | 83.3 ± 14.4 | 0.875 |
| Physical Activity, min/week | 61.7 ± 24.7 | 70.0 ± 14.1 | 0.703 |
| Systolic blood pressure, mmHg | 113.6 ± 13.8 | 117.1 ± 9.9 | 0.588 |
| Diastolic blood pressure, mmHg | 71.4 ± 6.3 | 72.9 ± 2.7 | 0.589 |
| Prevalence of CVD and diabetes, % | 42.9 | 57.1 | 0.626 |
| Prior VTE event, Y/N | 1/6 | 1/6 | 1.000 |
| Provoked event, Y/N | 3/4 | 6/1 | 0.109 |
| Under anticoagulant therapy, Y/N | 6/1 | 4/3 | 0.271 |
| MRC dyspnea scale, 0/I/II | 3/4/0 | 2/4/1 | 0.690 |
| FEV1, % of predicted | 101.0 ± 8.0 | 96.9 ± 10.2 | 0.434 |
| FVC, % of predicted | 96.8 ± 7.7 | 90.9 ± 11.6 | 0.280 |
| SPRgroup | UnSPRgroup | p Value | |
|---|---|---|---|
| Ejection fraction, % | 59.2 ± 2.0 | 59.4 ± 1.3 | 0.832 |
| End-diastolic RV diameter (4CH), cm | 3.6 ± 0.1 | 3.6 ± 0.5 | 0.763 |
| RVSP, mmHg | 23.3 ± 4.1 | 24.2 ± 5.8 | 0.777 |
| RA area, cm2 | 15.3 ± 2.5 | 15.2 ± 3.1 | 0.952 |
| TAPSE, mm | 20.5 ± 7.8 | 22.3 ± 4.3 | 0.626 |
| SPRgroup | UnSPRgroup | p Value between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-PR | p Value | Baseline | Post-PR | p Value | PRpre | PRpost | |
| Resting | ||||||||
| VO2, mL/min | 330.6 ± 91.6 | 349.0 ± 120.8 | 0.735 | 336.3 ± 84.5 | 312.0 ± 53.9 | 0.533 | 0.905 | 0.473 |
| VCO2, mL/min | 257.1 ± 67.1 | 240.6 ± 101.8 | 0.095 | 267.9 ± 102.7 | 236.1 ± 32.9 | 0.452 | 0.821 | 0.095 |
| PETCO2, mmHg | 29.9 ± 3.7 | 29.5 ± 2.4 | 0.832 | 27.2 ± 4.1 | 27.1 ± 3.6 | 0.952 | 0.227 | 0.159 |
| PETO2, mmHg | 110.0 ± 5.1 | 114.0 ± 4.2 | 0.132 | 113.8 ± 7.7 | 112.5 ± 5.4 | 0.736 | 0.305 | 0.568 |
| HR, bpm | 81.0 ± 18.2 | 82.6 ± 18.2 | 0.875 | 78.7 ± 10.1 | 73.9 ± 9.6 | 0.374 | 0.776 | 0.285 |
| MAP, mmHg | 87.6 ± 3.3 | 95.0 ± 5.5 | 0.010 | 85.5 ± 8.5 | 88.6 ± 9.2 | 0.516 | 0.549 | 0.133 |
| Maximal effort | ||||||||
| VO2, mL/min | 1559.1 ± 372.8 | 1579.3 ± 430.7 | 0.927 | 1946.0 ± 640.2 | 1896.1 ± 390.0 | 0.863 | 0.192 | 0.175 |
| VCO2, mL/min | 1536.6 ± 440.8 | 1525.5 ± 497.6 | 0.966 | 1954.1 ± 672.1 | 1791.0 ± 453.9 | 0.604 | 0.194 | 0.318 |
| PETCO2, mmHg | 37.5 ± 4.1 | 36.7 ± 3.5 | 0.710. | 35.5 ± 4.6 | 35.6 ± 4.4 | 0.970 | 0.416 | 0.615 |
| PETO2, mmHg | 108.7 ± 3.9 | 115.0 ± 7.0 | 0.059 | 112.8 ± 3.8 | 115.9 ± 4.8 | 0.199 | 0.079 | 0.788 |
| VE/MVV, % | 44.1 ± 10.8 | 48.1 ± 10.5 | 0.494 | 53.3 ± 6.3 | 53.5 ± 12.7 | 0.977 | 0.076 | 0.408 |
| VE/VCO2 | 28.6 ± 3.0 | 27.8 ± 3.0 | 0.279 | 28.5 ± 2.5 | 28.2 ± 3.3 | 0.786 | 0.357 | 0.679 |
| HR, bpm | 133.3 ± 18.6 | 133.0 ± 14.2 | 0.975 | 149.0 ± 13.3 | 138.4 ± 12.9 | 0.158 | 0.094 | 0.470 |
| MAP, mmHg | 119.5 ± 18.8 | 117.2 ± 10.6 | 0.786 | 123.3 ± 12.5 | 121.4 ± 9.4 | 0.757 | 0.660 | 0.438 |
| Leg fatigue, Borg Scale | 2.6 ± 1.4 | 3.6 ± 1.3 | 0.043 | 1.1 ± 0.7 | 1.7 ± 0.8 | 0.047 | 0.062 | 0.006 |
| Dyspnea, Borg Scale | 1.4 ± 1.0 | 2.0 ± 1.2 | 0.337 | 0.9 ± 1.1 | 1.3 ± 0.8 | 0.403 | 0.317 | 0.196 |
| SPRgroup | UnSPRgroup | p Value between Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-PR | p Value | Baseline | Post-PR | p Value | Baseline | Post-PR | ||
| Quality of life (SF-36) | Physical Health | 71.1 ± 10.7 | 92.9 ± 8.1 | 0.009 | 76.4 ± 15.7 | 93.6 ± 6.9 | 0.022 | 0.923 | 0.862 |
| Physical Functioning | 78.6 ± 26.7 | 92.9 ± 18.9 | 0.271 | 75.0 ± 25.0 | 76.4 ± 25.3 | 0.917 | 0.801 | 0.194 | |
| Body Pain | 82.5 ± 18.0 | 87.5 ± 13.4 | 0.567 | 80.0 ± 17.0 | 83.9 ± 13.8 | 0.643 | 0.794 | 0.631 | |
| General Health | 63.6 ± 9.9 | 81.4 ± 16.8 | 0.032 | 51.4 ± 15.7 | 72.1 ± 8.6 | 0.010 | 0.109 | 0.217 | |
| Vitality | 67.9 ± 16.0 | 77.1 ± 13.2 | 0.260 | 60.7 ± 13.7 | 68.6 ± 8.0 | 0.214 | 0.387 | 0.167 | |
| Social Role Functioning | 75.0 ± 20.4 | 92.9 ± 12.2 | 0.070 | 85.7 ± 18.3 | 85.4 ± 14.1 | 0.968 | 0.321 | 0.308 | |
| Emotional Role Functioning | 85.7 ± 26.2 | 98.6 ± 2.4 | 0.175 | 85.7 ± 26.2 | 90.5 ± 16.2 | 0.690 | 0.989 | 0.147 | |
| Mental Health | 73.6 ± 24.0 | 78.3 ± 19.6 | 0.635 | 77.1 ± 17.5 | 81.1 ± 12.2 | 0.629 | 0.692 | 0.749 | |
| Sleep quality (PSQI) | Cannot get to sleep within 30 min | 2.9 ± 0.4 | 1.8 ± 0.5 | 0.046 | 2.6 ± 0.5 | 2.0 ± 0.1 | 0.007 | 0.779 | 0.025 |
| Wake up in the middle of the night or early morning | 1.4 ± 0.3 | 1.4 ± 0.4 | 0.968 | 1.3 ± 0.1 | 1.3 ± 0.2 | 0.878 | 0.613 | 0.694 | |
| Have to get up to use the bathroom | 1.1 ± 0.1 | 1.0 ± 0.5 | 0.317 | 1.1 ± 0.4 | 0.9 ± 0.7 | 0.986 | 0.955 | 0.613 | |
| Cannot breathe comfortably | 1.2 ± 0.3 | 1.1 ± 0.4 | 0.867 | 1.1 ± 0.3 | 1.1 ± 0.6 | 0.831 | 0.986 | 0.978 | |
| Cough or snore loudly | 1.0 ± 0.2 | 0.9 ± 0.6 | 0.317 | 0.9 ± 0.3 | 0.8 ± 0.4 | 0.326 | 0.694 | 0.281 | |
| Feel too cold | 1.1 ± 0.3 | 1.0 ± 0.6 | 0.325 | 1.0 ± 0.4 | 0.9 ± 0.7 | 0.679 | 0.732 | 0.796 | |
| Feel too hot | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.371 | 1.1 ± 0.2 | 0.9 ± 0.3 | 0.317 | 0.698 | 0.789 | |
| Had bad dreams | 1.2 ± 0.2 | 1.1 ± 0.1 | 0.157 | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.175 | 0.121 | 0.779 | |
| Have pain | 0.8 ± 0.1 | 0.7 ± 0.4 | 0.152 | 0.8 ± 0.2 | 0.6 ± 0.8 | 0.336 | 0.956 | 0.232 | |
| …taken medicine to help you sleep | - | - | / | - | - | / | / | / | |
| …trouble staying awake (driving, eating meals, or social activity) | 0.6 ± 0.3 | 0.6 ± 0.5 | 0.317 | 0.5 ± 0.1 | 0.5 ± 0.6 | 0.307 | 0.463 | 0.397 | |
| …keep up enough enthusiasm to get things done? | 1.9 ± 0.4 | 1.0 ± 0.2 | 0.005 | 2.5 ± 0.5 | 1.3 ± 0.4 | 0.010 | 0.779 | 0.029 | |
| …sleep quality overall | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.157 | 0.2 ± 0.2 | 0.1 ± 0.4 | 0.317 | 0.294 | 0.281 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrou, V.T.; Griziotis, M.; Vavougios, G.D.; Raptis, D.G.; Bardaka, F.; Karetsi, E.; Kyritsis, A.; Daniil, Z.; Tsarouhas, K.; Triposkiadis, F.; et al. Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era. J. Funct. Morphol. Kinesiol. 2021, 6, 98. https://doi.org/10.3390/jfmk6040098
Stavrou VT, Griziotis M, Vavougios GD, Raptis DG, Bardaka F, Karetsi E, Kyritsis A, Daniil Z, Tsarouhas K, Triposkiadis F, et al. Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era. Journal of Functional Morphology and Kinesiology. 2021; 6(4):98. https://doi.org/10.3390/jfmk6040098
Chicago/Turabian StyleStavrou, Vasileios T., Michalis Griziotis, George D. Vavougios, Dimitrios G. Raptis, Fotini Bardaka, Eleni Karetsi, Athanasios Kyritsis, Zoe Daniil, Konstantinos Tsarouhas, Filippos Triposkiadis, and et al. 2021. "Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era" Journal of Functional Morphology and Kinesiology 6, no. 4: 98. https://doi.org/10.3390/jfmk6040098
APA StyleStavrou, V. T., Griziotis, M., Vavougios, G. D., Raptis, D. G., Bardaka, F., Karetsi, E., Kyritsis, A., Daniil, Z., Tsarouhas, K., Triposkiadis, F., Gourgoulianis, K. I., & Malli, F. (2021). Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era. Journal of Functional Morphology and Kinesiology, 6(4), 98. https://doi.org/10.3390/jfmk6040098










