Effects of a Supervised Nordic Walking Program on Obese Adults with and without Type 2 Diabetes: The C.U.R.I.A.Mo. Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- Gym-based exercise program for obese individuals (OB-GYM; n = 49);
- NW program for obese individuals (OB-NW; n = 37);
- Gym-based exercise program for obese individuals with DM2 (DM2-GYM; n = 10);
- NW program for obese individuals with DM2 (DM2-NW; n = 12).
2.2. Study Design
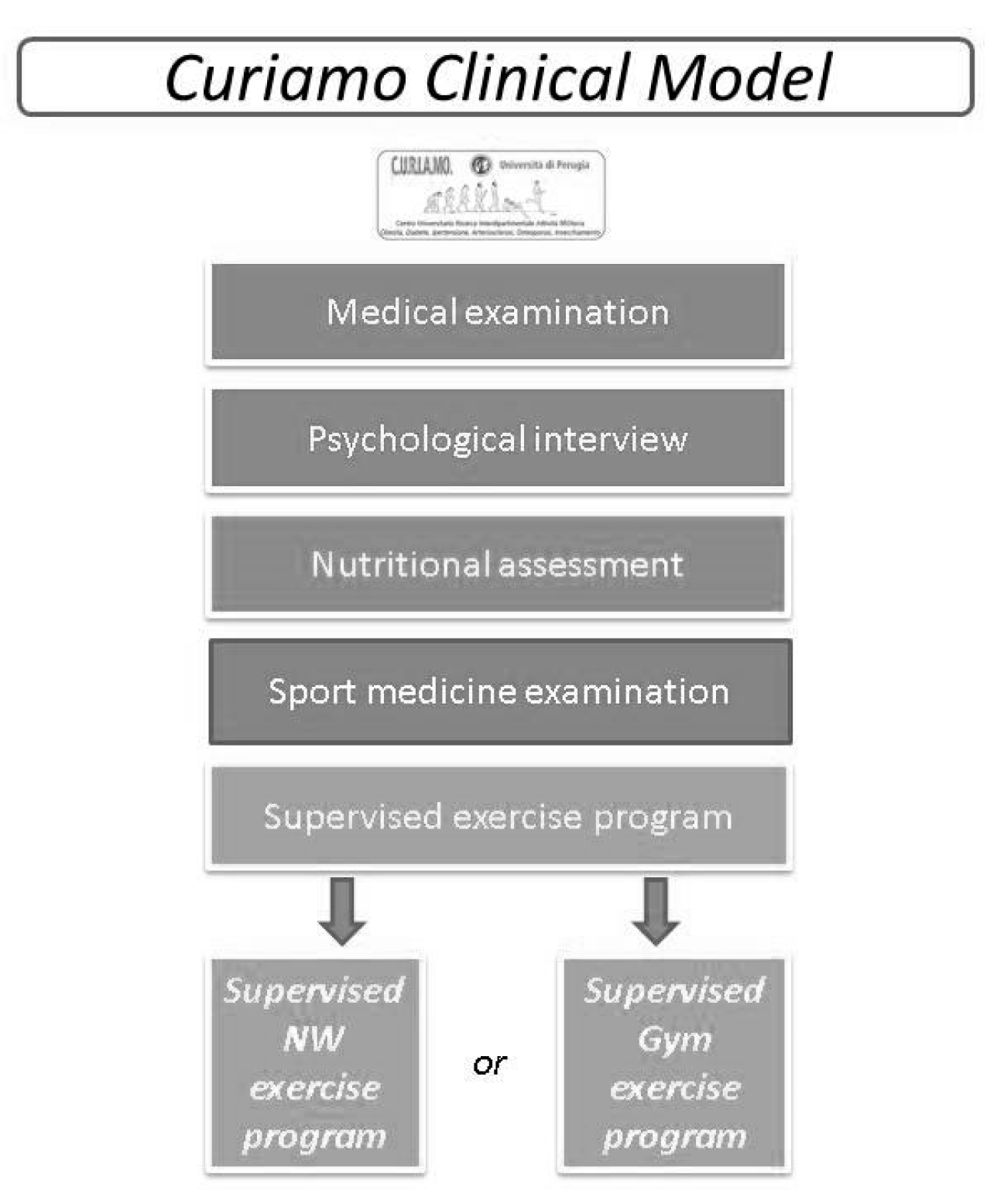
2.3. The C.U.R.I.A.Mo. Clinical Model
- A medical examination was managed by the endocrinologist to exclude the presence of clinical conditions that could contraindicate the exercise interventions. During the visit, the C.U.R.I.A.Mo. clinical model was explained to participants, and the blood tests were prescribed according to national standards of care [25].
- A psychological interview to increase the subjects’ lifestyle change and to assess their psychological status.
- A nutritional evaluation to assess the nutritional habits of the participants in order to increase their awareness of a balanced daily diet based on the Mediterranean dietary principles. The C.U.R.I.A.Mo. model provides two individual counseling sessions (before and after the interventions). Nutritional counseling aims to reduce saturated and trans-unsaturated fatty acids to under 10% of the total daily energy, promoting the consumption of fish, vegetables, legumes, fruit and whole grain cereals, to reduce calorie intake. Participants were also invited to attend educational classes focused on healthy diets and good physical activity habits.
- A complete medical examination was performed by a physician focused on assessing the individual aerobic capacity and muscle strength, and to exclude any potential contraindications to exercise. These outcomes were also used to increase the participants’ awareness of their individual physical status.
2.4. Exercise Interventions
2.4.1. Nordic Walking Program
2.4.2. GYM Program
2.5. Specific Functional and Clinical Assessments
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 2019. Available online: https://www.diabetesatlas.org/upload/resources/2019/IDF_Atlas_9th_Edition_2019.pdf (accessed on 1 March 2019).
- De Feo, P.; Di Loreto, C.; Ranchelli, A.; Fatone, C.; Gambelunghe, G.; Lucidi, P.; Santeusanio, F. Exercise and diabetes. Acta Biomed. 2006, 77, 14–17. [Google Scholar] [PubMed]
- Horton, E.S. Role and management of exercise in diabetes mellitus. Diabetes Care 1988, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Mariani, P.G.; Ministrini, S.; Pippi, R.; Aiello, C.; Reginato, E.; Siepi, D.; Innocente, S.; Lombardini, R.; Paltriccia, R.; et al. Combined aerobic and resistance training improves microcirculation in metabolic syndrome. J. Sports Med. Phys. Fit. 2019, 59, 1571–1576. [Google Scholar] [CrossRef]
- Fatone, C.; Guescini, M.; Balducci, S.; Battistoni, S.; Settequattrini, A.; Pippi, R.; Stocchi, L.; Mantuano, M.; Stocchi, V.; De Feo, P. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J. Endocrinol. Investig. 2010, 33, 489–495. [Google Scholar] [CrossRef]
- Boule, N.G.; Kenny, G.P.; Haddad, E.; Wells, G.A.; Sigal, R.J. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003, 46, 1071–1081. [Google Scholar]
- Yaribeygi, H.; Butler, A.E.; Sahebkar, A. Aerobic exercise can modulate the underlying mechanisms involved in the development of diabetic complications. J. Cell. Physiol. 2019, 234, 12508–12515. [Google Scholar] [CrossRef]
- Rachel, S.; Denise, S. The benefits of regular walking for health, well-being and the environment. C3 Collab. Health. September 2012. Available online: https://www.c3health.org/wp-content/uploads/2017/07/C3-report-on-walking-v-1-20120911.pdf (accessed on 1 May 2019).
- Katsanos, C.S. Prescribing aerobic exercise for the regulation of postprandial lipid metabolism: Current research and recommendations. Sports Med. 2006, 36, 547–560. [Google Scholar] [CrossRef]
- Slentz, C.A.; Aiken, L.B.; Houmard, J.A.; Bales, C.W.; Johnson, J.L.; Tanner, C.J.; Duscha, B.D.; Kraus, W.E. Inactivity, exercise, and visceral fat. STRRIDE: A randomized, controlled study of exercise intensity and amount. J. Appl. Physiol. 2005, 99, 1613–1618. [Google Scholar] [CrossRef]
- De Feo, P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, T.; Knicker, A.; Hoffman, U.; Harwig, B.; Hollmann, W.; Strüder, H.K. Physiological responses to nordic walking, walking and jogging. Eur. J. Appl. Physiol. 2006, 98, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.A.; Smith, G. Energy expenditure and comfort during Nordic walking with different pole lengths. J. Strength Cond. Res. 2009, 23, 1187–1194. [Google Scholar] [CrossRef]
- Cugusi, L.; Manca, A.; Dragone, D.; Deriu, F.; Solla, P.; Secci, C.; Monticone, M.; Mercuro, G. Nordic Walking for the Management of People With Parkinson Disease: A Systematic Review. PM R 2017, 9, 1157–1166. [Google Scholar] [CrossRef]
- Bullo, V.; Gobbo, S.; Vendramin, B.; Duregon, F.; Cugusi, L.; Di Blasio, A.; Bocalini, D.S.; Zaccaria, M.; Bergamin, M.; Ermolao, A. Nordic Walking Can Be Incorporated in the Exercise Prescription to Increase Aerobic Capacity, Strength, and Quality of Life for Elderly: A Systematic Review and Meta-Analysis. Rejuvenation Res. 2018, 21, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Manca, A.; Yeo, T.J.; Bassareo, P.P.; Mercuro, G.; Kaski, J.C. Nordic walking for individuals with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2017, 24, 1938–1955. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, S.; Bullo, V.; Roma, E.; Duregon, F.; Bocalini, D.S.; Rica, R.L.; Di Blasio, A.; Cugusi, L.; Vendramin, B.; Bergamo, M.; et al. Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription. J. Funct. Morphol. Kinesiol. 2019, 4, 36. [Google Scholar] [CrossRef]
- Vilchez Barrera, M.; Calvo-Arencibia, A. Scientific evidence of nordic walking in Physioterapy: Bibliographic review. Fisioterapia 2016, 38, 251–264. [Google Scholar]
- Sentinelli, F.; La Cava, V.; Serpe, R.O.B.E.R.T.O.; Boi, A.; Incani, M.I.C.H.E.L.A.; Manconi, E.T.T.O.R.E.; Solinas, A.L.D.O.; Cossu, E.; Lenzi, A.; Baroni, M.G. Positive effects of Nordic Walking on anthropometric and metabolic variables in women with type 2 diabetes mellitus. Sci. Sports 2015, 30, 25–32. [Google Scholar] [CrossRef]
- Fritz, T.; Caidahl, K.; Krook, A.; Lundström, P.; Mashili, F.; Osler, M.; Szekeres, F.L.; Östenson, C.G.; Wändell, P.; Zierath, J.R. Effects of Nordic walking on cardiovascular risk factors in overweight individuals with type 2 diabetes, impaired or normal glucose tolerance. Diabetes Metab. Res. Rev. 2013, 29, 25–32. [Google Scholar] [CrossRef]
- Gram, B.; Christensen, R.; Christiansen, C.; Gram, J. Effects of nordic walking and exercise in type 2 diabetes mellitus: A randomized controlled trial. Clin. J. Sport. Med. 2010, 20, 355–361. [Google Scholar] [PubMed]
- De Feo, P.; Fatone, C.; Burani, P.; Piana, N.; Pazzagli, C.; Battistini, D.; Capezzali, D.; Pippi, R.; Chipi, B.; Mazzeschi, C. An innovative model for changing the lifestyles of persons with obesity and/or Type 2 diabetes mellitus. J. Endocrinol. Investig. 2011, 34, e349–e354. [Google Scholar] [PubMed]
- (AMD) A.M.D.; S.I.d.D. (SID). Standard Italiani Per La Cura Del Diabete Mellito 2018; Available online: http://www.siditalia.it/pdf/Standard%20di%20Cura%20AMD%20-%20SID%202018_protetto2.pdf (accessed on 1 February 2019).
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Massarini, M.; Corigliano, G.; Nicolucci, A.; Missori, S.; Cavallo, S.; Cardelli, P.; Alessi, E.; Pugliese, G.; et al. The Italian Diabetes and Exercise Study (IDES): Design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 585–595. [Google Scholar] [CrossRef]
- American College of Sports, Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Karvonen, J.; Vuorimaa, T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 1988, 5, 303–311. [Google Scholar] [CrossRef]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Edication Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Habicht, J.P. Standardization of quantitative epidemiological methods in the field. Bol. Oficina Sani. Panam. 1974, 76, 375–384. [Google Scholar]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Linares, C.L.; Ciangura, C.; Bouillot, J.L.; Coupaye, M.; Declèves, X.; Poitou, C.; Basdevant, A.; Oppert, J.M. Validity of leg-to-leg bioelectrical impedance analysis to estimate body fat in obesity. Obes. Surg. 2011, 21, 917–923. [Google Scholar] [CrossRef]
- Weiglein, L.; Herrick, J.; Kirk, S.; Kirk, E.P. The 1-mile walk test is a valid predictor of VO2max and is a reliable alternative fitness test to the 1.5-mile run in US Air Force males. Mil. Med. 2011, 176, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Vega, D.; Merino-Marban, R.; Viciana, J. Criterion-Related Validity of Sit-and-Reach Tests for Estimating Hamstring and Lumbar Extensibility: A Meta-Analysis. J. Sports. Sci. Med. 2014, 13, 1–14. [Google Scholar] [PubMed]
- Kucio, C.; Narloch, D.; Kucio, E.; Kurek, J. The application of Nordic walking in the treatment hypertension and obesity. Fam. Med. Prim. Care Rev. 2017, 19, 144–148. [Google Scholar] [CrossRef]
- Venojarvi, M.; Wasenius, N.; Manderoos, S.; Heinonen, O.J.; Hernelahti, M.; Lindholm, H.; Surakka, J.; Lindström, J.; Aunola, S.; Atalay, M.; et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann. Med. 2013, 45, 162–170. [Google Scholar] [CrossRef]
- Jain, R.; Olejas, S.; Feh, A.R.; Edwards, A.; Abigo, I.; Zietek, W.; Khan, Z.; Ragoonanan, S.; Benoy, N.; Bramble, D. Review the evidence for lifestyle management in the prevention of type 2 diabetes and compare them to pharmacological interventions. EC Diabetes Metab. Res. 2020, 4, 161–166. [Google Scholar] [CrossRef]
- Ried-Larsen, M.; MacDonald, C.S.; Johansen, M.Y.; Hansen, K.B.; Christensen, R.; Almdal, T.P.; Pedersen, B.K.; Karstoft, K. Why prescribe exercise as therapy in type 2 diabetes? We have a pill for that! Diabetes Metab. Res. Rev. 2018, 34, e2999. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Johansen, M.Y.; MacDonald, C.S.; Hansen, K.B.; Karstoft, K.; Christensen, R.; Pedersen, M.; Hansen, L.S.; Zacho, M.; Wedell-Neergaard, A.S.; Nielsen, S.T.; et al. Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 637–646. [Google Scholar] [CrossRef]
- Russo, A.; Pirisinu, I.; Vacca, C.; Reginato, E.; Tomaro, E.S.; Pippi, R.; Aiello, C.; Talesa, V.N.; De Feo, P.; Romani, R. An intensive lifestyle intervention reduces circulating oxidised low-density lipoprotein and increases human paraoxonase activity in obese subjects. Obes. Res. Clin. Pract. 2018, 12, 108–114. [Google Scholar] [CrossRef]
- Tomaro, E.S.; Pippi, R.; Reginato, E.; Aiello, C.; Buratta, L.; Mazzeschi, C.; Perrone, C.; Ranucci, C.; Tirimagni, A.; Russo, A.; et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 688–694. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W.; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.S.; Gibbons, L.W. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Cheng, Y.J.; Earnest, C.P.; Barlow, C.E.; Gibbons, L.W.; Priest, E.L.; Blair, S.N. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Myers, J.; Nylen, E.; Panagiotakos, D.B.; Manolis, A.; Pittaras, A.; Blackman, M.R.; Jacob-Issac, R.; Faselis, C.; Abella, J.; et al. Exercise capacity and all-cause mortality in African American and Caucasian men with type 2 diabetes. Diabetes Care 2009, 32, 623–628. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Lahart, I.; Darcy, P.; Gidlow, C.; Calogiuri, G. The Effects of Green Exercise on Physical and Mental Wellbeing: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1352. [Google Scholar] [CrossRef]
- McArthur, D.; Dumas, A.; Woodend, K.; Beach, S.; Stacey, D. Factors influencing adherence to regular exercise in middle-aged women: A qualitative study to inform clinical practice. BMC Womens Health 2014, 14, 49. [Google Scholar] [CrossRef]
- Gladwell, V.F.; Brown, D.K.; Wood, C.; Sandercock, G.R.; Barton, J.L. The great outdoors: How a green exercise environment can benefit all. Extrem. Physiol. Med. 2013, 2, 3. [Google Scholar] [CrossRef]
- Fluery-Bahi, G.; Pol, E.; Navarro, O. International Handbooks of Quality-of-life. In Handbook of Environmental Psychology and Quality of Life Research, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume XIII, p. 574. [Google Scholar]
- Hoare, E.; Stavreski, B.; Jennings, G.L.; Kingwell, B.A. Exploring Motivation and Barriers to Physical Activity among Active and Inactive Australian Adults. Sports 2017, 5, 47. [Google Scholar] [CrossRef]
- Moreno, J.; Johnson, C. Barriers to physical activity in women. Am. J. Lifestyle Med. 2014, 8, 164–166. [Google Scholar] [CrossRef]
- Eyler, A.E.; Wilcox, S.; Matson-Koffman, D.; Evenson, K.R.; Sanderson, B.; Thompson, J.; Wilbur, J.; Rohm-Young, D. Correlates of physical activity among women from diverse racial/ethnic groups. J. Womens Health Gend. Based Med. 2002, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; D’Errico, V.; Haxhi, J.; Sacchetti, M.; Orlando, G.; Cardelli, P.; Vitale, M.; Bollanti, L.; Conti, F.; Zanuso, S.; et al. Effect of a Behavioral Intervention Strategy on Sustained Change in Physical Activity and Sedentary Behavior in Patients With Type 2 Diabetes: The IDES_2 Randomized Clinical Trial. JAMA 2019, 321, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; De Feo, P.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Fallucca, F.; Pugliese, G. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: A randomized controlled trial: The Italian Diabetes and Exercise Study (IDES). Arch. Intern. Med. 2010, 170, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Fritz, T.; Caidahl, K.; Osler, M.; Östenson, C.G.; Zierath, J.R.; Wändell, P. Effects of Nordic walking on health-related quality of life in overweight individuals with type 2 diabetes mellitus, impaired or normal glucose tolerance. Diabet. Med. 2011, 28, 1362–1372. [Google Scholar] [CrossRef]
- Cugusi, L.; Manca, A.; Bassareo, P.P.; Crisafulli, A.; Deriu, F.; Mercuro, G. Supervised aquatic-based exercise for men with coronary artery disease: A meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Cadeddu, C.; Nocco, S.; Orrù, F.; Bandino, S.; Deidda, M.; Caria, A.; Bassareo, P.P.; Piras, A.; Cabras, S.; et al. Effects of an aquatic-based exercise program to improve cardiometabolic profile, quality of life, and physical activity levels in men with type 2 diabetes mellitus. PM R 2015, 7, 141–148. [Google Scholar] [CrossRef]
| Outcomes | Total Sample n = 108 (F = 73; M = 35) | OB-GYM n = 49 (F = 33; M = 16) | OB-NW n = 37 (F = 30; M = 7) | DM2-GYM n = 10 (F = 4; M = 6) | DM2-NW n = 12 (F = 6; M = 6) | F | p Values |
|---|---|---|---|---|---|---|---|
| Age | 56.44 ± 5.94 | 55.29 ± 5.67 | 56.62 ± 5.8 | 59.6 ± 6.5 | 58 ± 6.35 | 1.89 | 0.14 |
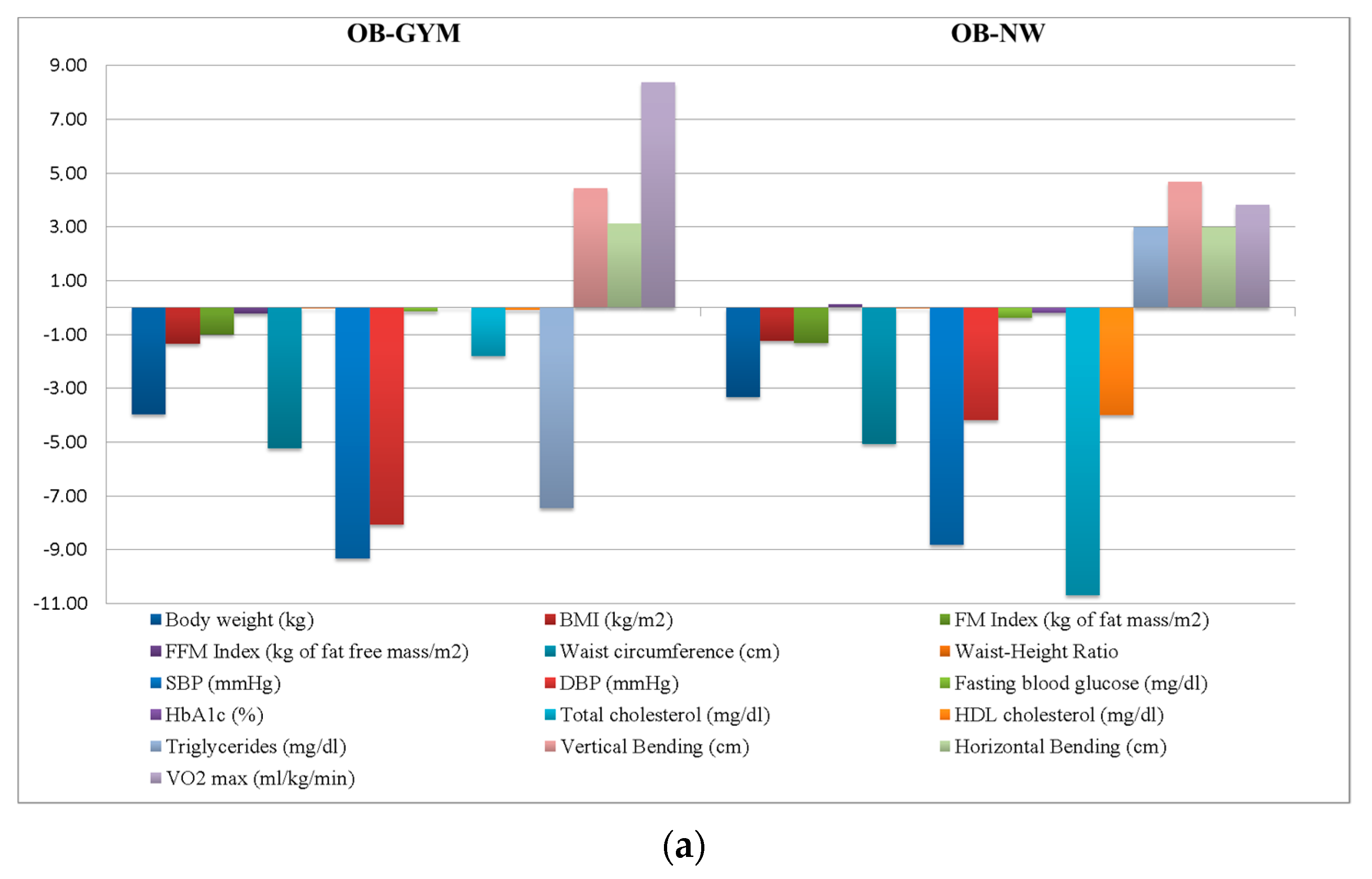
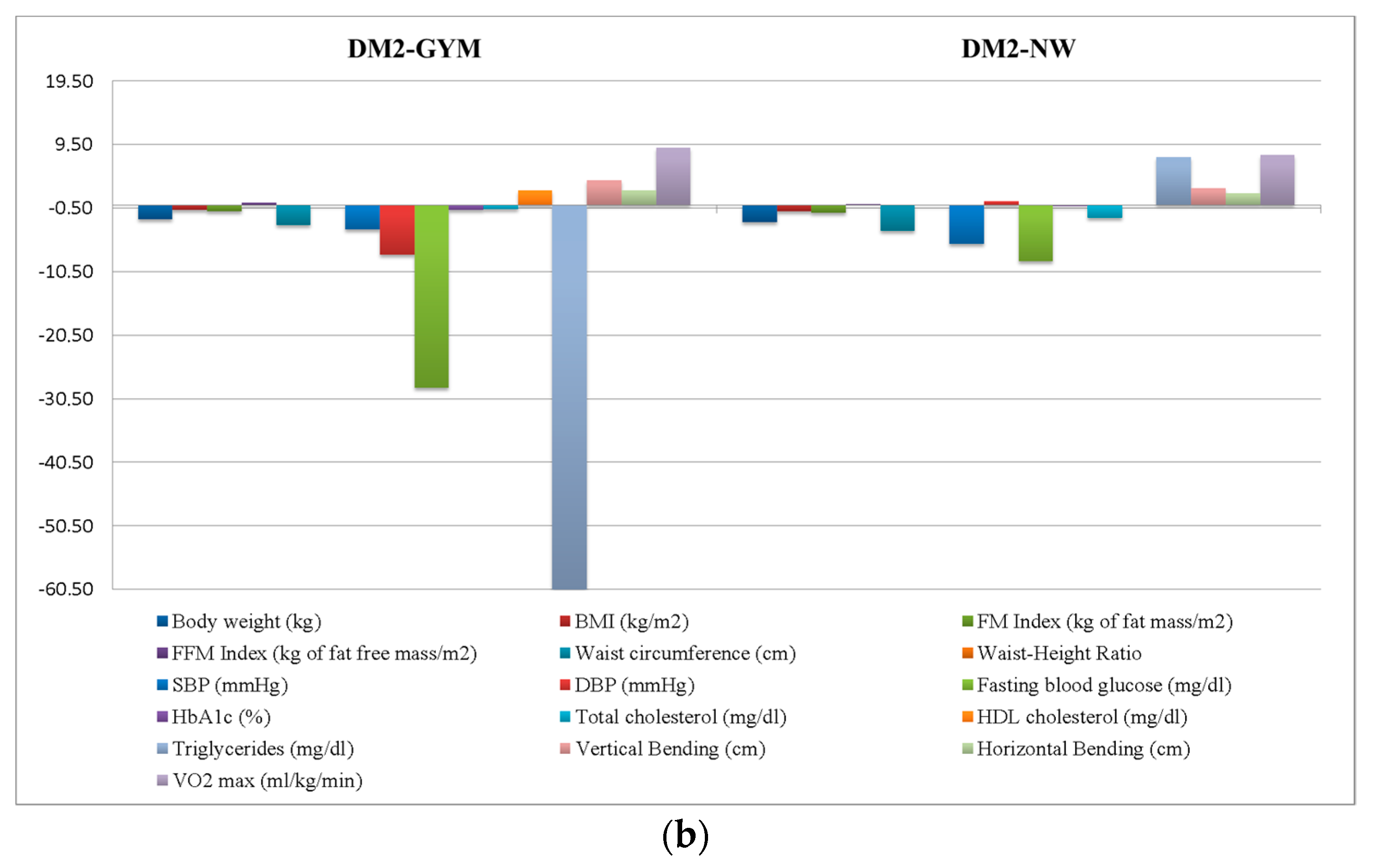
| Body weight (kg) | 100.75 ± 15.4 | 101.07 ± 16.71 | 98.50 ± 14.87 | 104.51 ± 14.38 | 103.23 ± 12.58 | 0.57 | 0.64 |
| BMI (kg/m2) | 36.47 ± 5.11 | 36.6 ± 4.99 | 36.37 ± 5.85 | 35.48 ± 4.32 | 37.07 ± 4.07 | 0.19 | 0.90 |
| FM index (kg of fat mass/m2) | 15.27 ± 4.24 | 15.22 ± 3.91 | 15.74 ± 4.64 | 13.62 ± 4.16 | 15.46 ± 4.6 | 0.65 | 0.58 |
| FFM index (kg of fat free mass/m2) | 19.87 ± 2.27 | 20.05 ± 2.23 | 19.15 ± 1.99 | 20.77 ± 2.91 | 20.49 ± 2.36 | 2.17 | 0.08 |
| Waist circumference (cm) | 115.93 ± 10.52 | 114.88 ± 11.26 | 115.5 ± 9.83 | 118.5 ± 9.88 | 119.25 ± 10.22 | 0.77 | 0.51 |
| Waist–Height Ratio | 0.7 ± 0.06 | 0.69 ± 0.07 | 0.7 ± 0.07 | 0.69 ± 0.06 | 0.72 ± 0.07 | 0.49 | 0.69 |
| SBP (mmHg) | 135 ± 13.08 | 134.69 ± 10.43 | 135 ± 12.95 | 137 ± 16.02 | 134.55 ± 21.27 | 0.09 | 0.97 |
| DBP (mmHg) | 82.52 ± 8.94 | 83.64 ± 7.83 | 80.86 ± 9.66 | 85 ± 7.07 | 80.46 ± 12.14 | 1.13 | 0.34 |
| Fasting blood glucose (mg/dL) | 105.13 ± 30.83 | 95 ± 12.64 | 94.62 ± 11.66 | 149 ± 58.25 | 138.83 ± 35.45 | 24.58 | <0.01 |
| HbA1c (%) | 6.04 ± 0.99 | 5.71 ± 0.52 | 5.76 ± 0.41 | 7.17 ± 1.91 | 7 ± 1.07 | 15.17 | <0.01 |
| Total cholesterol (mg/dL) | 210.76 ± 36.78 | 218.06 ± 35.26 | 207.91 ± 35.67 | 201.9 ± 41.52 | 196.67 ± 39.9 | 1.52 | 0.22 |
| HDL cholesterol (mg/dL) | 49.65 ± 11.48 | 49.83 ± 9.99 | 53.06 ± 13.86 | 43.1 ± 8.89 | 44.75 ± 8.07 | 2.98 | 0.04 |
| Triglycerides (mg/dL) | 151.81 ± 82.19 | 142.49 ± 66.51 | 130.67 ± 46.22 | 253.9 ± 169.79 | 162.92 ± 53.07 | 7.28 | <0.01 |
| Vertical bending (cm) | −9.67 ± 9.34 | −9.89 ± 9.31 | −6 ± 2 | −12 ± 10.61 | −8.6 ± 13.33 | 0.51 | 0.68 |
| Horizontal bending (cm) | 24.89 ± 9.72 | 24.26 ± 8.83 | 33.33 ± 7.06 | 21 ± 9.53 | 27 ± 15.68 | 2.26 | 0.09 |
| VO2 max (mL/kg/min) | 13.98 ± 9.25 | 14.40 ± 8.77 | 11.88 ± 12.85 | 14.77 ± 12 | 11.6 ± 2.85 | 0.16 | 0.92 |
| Nordic Walking | |||||
|---|---|---|---|---|---|
| Phase | Exercise | Duration | Sets | Repetitions | Intensity |
| Warm up | 10 min | ||||
| Main part | Nordic walking | 60–65 min | 40 to 60% HRR | ||
| Cool down | Stretching | 15 min | |||
| GYM Program | |||||
| Warm up | Treadmill, or walking, or cycling | 10 min | |||
| Main part | Treadmill | 12 min | 40 to 60% HRR | ||
| Leg press | 2 | 20 | 55 to 70% 1-RM | ||
| Cycle ergometer | 4 min | 40 to 60% HRR | |||
| Lat machine | 2 | 20 | 55 to 70% 1-RM | ||
| Arm ergometer | 4 min | 40 to 60% HRR | |||
| Chest press | 2 | 20 | 55 to 70% 1-RM | ||
| Cardio | 4 min | 40 to 60% HRR | |||
| Abdominal | 3 | 10–15 | 55 to 70% 1-RM | ||
| Cardio | 4 min | 40 to 60% HRR | |||
| Leg extension | 2 | 20 | 55 to 70% 1-RM | ||
| Cool down | Treadmill, or walking, or cycling Stretching | 15 min | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pippi, R.; Di Blasio, A.; Aiello, C.; Fanelli, C.; Bullo, V.; Gobbo, S.; Cugusi, L.; Bergamin, M. Effects of a Supervised Nordic Walking Program on Obese Adults with and without Type 2 Diabetes: The C.U.R.I.A.Mo. Centre Experience. J. Funct. Morphol. Kinesiol. 2020, 5, 62. https://doi.org/10.3390/jfmk5030062
Pippi R, Di Blasio A, Aiello C, Fanelli C, Bullo V, Gobbo S, Cugusi L, Bergamin M. Effects of a Supervised Nordic Walking Program on Obese Adults with and without Type 2 Diabetes: The C.U.R.I.A.Mo. Centre Experience. Journal of Functional Morphology and Kinesiology. 2020; 5(3):62. https://doi.org/10.3390/jfmk5030062
Chicago/Turabian StylePippi, Roberto, Andrea Di Blasio, Cristina Aiello, Carmine Fanelli, Valentina Bullo, Stefano Gobbo, Lucia Cugusi, and Marco Bergamin. 2020. "Effects of a Supervised Nordic Walking Program on Obese Adults with and without Type 2 Diabetes: The C.U.R.I.A.Mo. Centre Experience" Journal of Functional Morphology and Kinesiology 5, no. 3: 62. https://doi.org/10.3390/jfmk5030062
APA StylePippi, R., Di Blasio, A., Aiello, C., Fanelli, C., Bullo, V., Gobbo, S., Cugusi, L., & Bergamin, M. (2020). Effects of a Supervised Nordic Walking Program on Obese Adults with and without Type 2 Diabetes: The C.U.R.I.A.Mo. Centre Experience. Journal of Functional Morphology and Kinesiology, 5(3), 62. https://doi.org/10.3390/jfmk5030062





