Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise †
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Procedures
2.3.1. Biometric Measures
2.3.2. Maximal Strength Testing
2.3.3. Assessment of Peak Power during Back Squat
2.3.4. Assessment of Maximal Voluntary Contraction and Voluntary Activation
2.3.5. High Volume Squat Exercise
2.3.6. Assessment of Heart Rate
2.3.7. Assessment of Perceived Exertion
2.3.8. Assessment of Blood Lactate Concentration
2.3.9. External to Internal Load Ratios
2.4. Statistical Analysis
3. Results
3.1. Biometric Measures and Training History
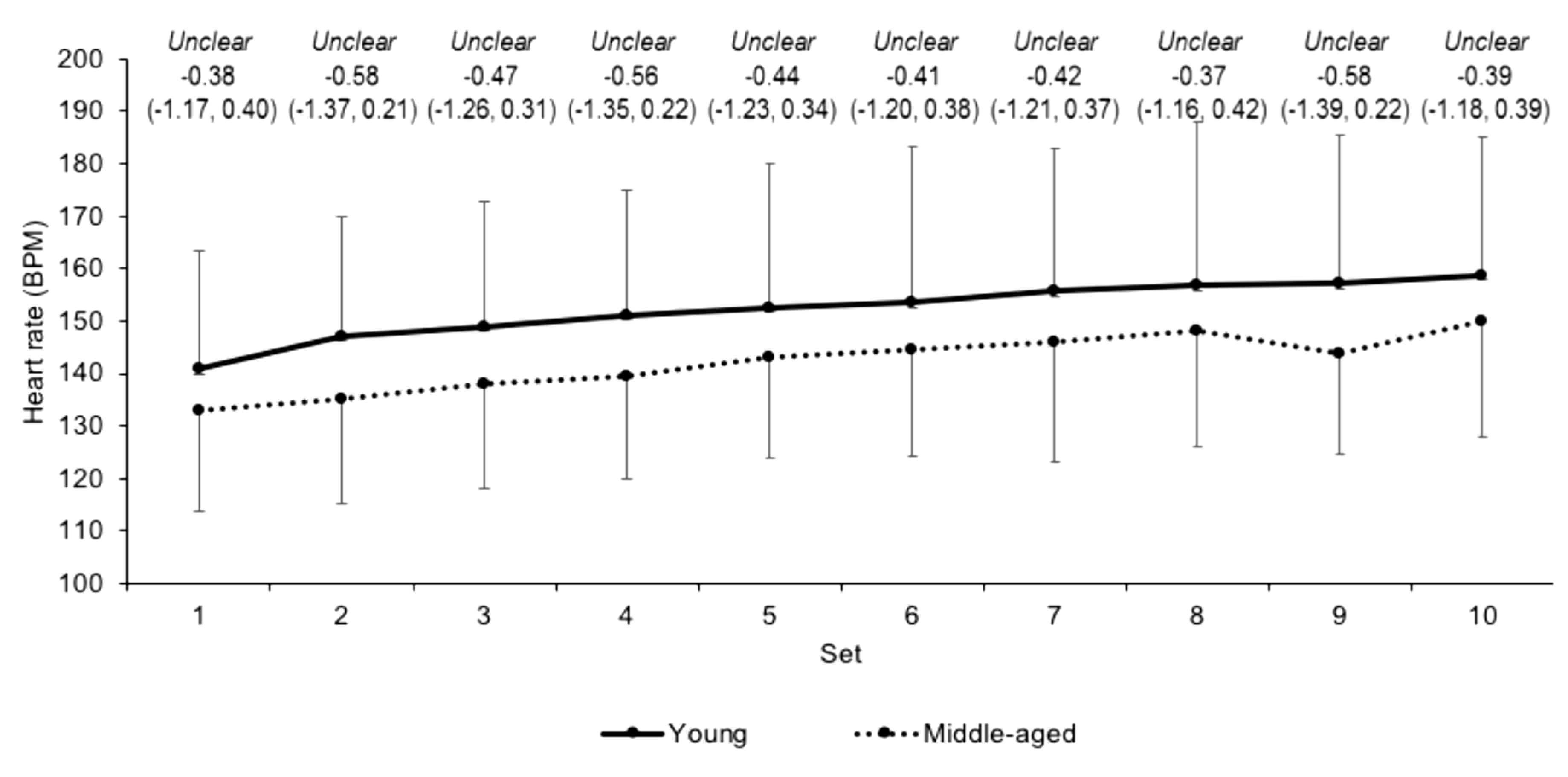
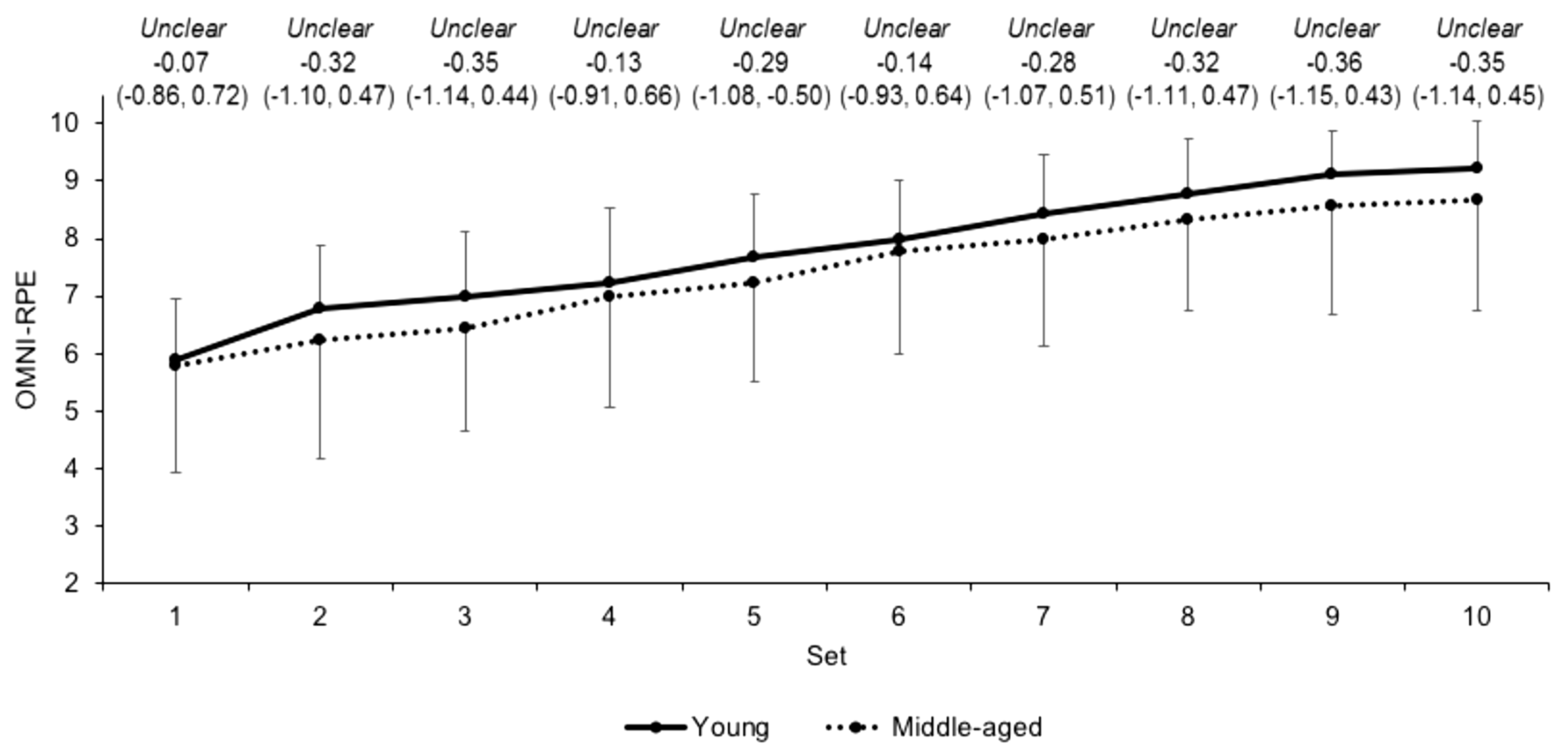
3.2. Internal Load Measures
3.3. External Load Measures
3.4. External to Internal Load Ratios
3.5. Markers of Fatigue after Squatting Exercise
3.6. Relationship between Internal and External Load Markers with Fatigue
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Newton, R.U.; Hakkinen, K.; Hakkinen, A.; McCormick, M.; Volek, J.; Kraemer, W.J. Mixed-methods resistance training increases power and strength of young and older men. Med. Sci. Sports Exerc. 2002, 34, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Kosek, D.J.; Kim, J.S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006, 101, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Reeves, N.D.; Morse, C.I.; Maganaris, C.N. Muscular adaptations to resistance exercise in the elderly. J. Musculoskelet. Neuronal. Interact. 2004, 4, 161–164. [Google Scholar] [PubMed]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. A comparison of load-velocity and load-power relationships between well-trained young and middle-aged males during three popular resistance exercises. J. Strength Cond. Res. 2018, 32, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, P.D.; De Villarreal, E.S.; Brisswalter, J.; Peyré-Tartaruga, L.A.; Morin, J.B. Sprint acceleration mechanics in master athletes. Med. Sci. Sports Exerc. 2016, 48, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Duthie, G.M.; Thornton, H.R.; Dascombe, B.J. Training monitoring for resistance exercise: Theory and applications. Sports Med. 2016, 46, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Smolander, J.; Aminoff, T.; Korhonen, I.; Tervo, M.; Shen, N.; Korhonen, O.; Louhevaara, V. Heart rate and blood pressure responses to isometric exercise in young and older men. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Parlavantzas, A.; Tokmakidis, S.P. Hormonal responses after a strength endurance resistance exercise protocol in young and elderly males. Int. J. Sports Med. 2007, 28, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Mani, D.; Pierpoint, L.A.; Enoka, R.M. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp. Gerontol. 2014, 55, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M. Older adults underestimate RPE and knee extensor torque as compared with young adults. Med. Sci. Sports Exerc. 2011, 43, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Timmons, M.K.; Elsing, D. RPE angle effects in young and middle-aged adults. Int. J. Sports Med. 2010, 31, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Tanimoto, M.; Yamamoto, K.; Sanada, K.; Gando, Y.; Tabata, I.; Higuchi, M.; Miyachi, M. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp. Physiol. 2008, 93, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Akubat, I.; Barrett, S.; Abt, G. Integrating the internal and external training loads in soccer. Int. J. Sports Physiol. Perform. 2014, 9, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.; Doran, D.; Akubat, I.; Collins, K. The integration of internal and external training load metrics in hurling. J. Hum. Kinet. 2016, 53, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.H.T. Human muscle function and fatigue. Ciba Found. Symp. 1981, 82, 1–18. [Google Scholar] [PubMed]
- Avin, K.G.; Frey Law, L.A. Age-related differences in muscle fatigue vary by contraction type: A meta-analysis. Phys. Ther. 2011, 91, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Snook, E.M.; Kent-Braun, J.A. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med. Sci. Sports Exerc. 2011, 43, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.H.; Power, G.A.; Vandervoort, A.A.; Rice, C.L. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp. Gerontol. 2012, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.H.; Power, G.A.; Paturel, J.R.; Rice, C.L. Older men are more fatigable than young when matched for maximal power and knee extension angular velocity is unconstrained. Age 2015, 37, 49. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.K.; Kim, J.S.; Tuggle, S.C.; Hall, S.R.; Bamman, M.M. Age differences in knee extension power, contractile velocity, and fatigability. J. Appl. Physiol. 2005, 98, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Brandon, R.; Howatson, G.; Strachan, F.; Hunter, A.M. Neuromuscular response differences to power vs strength back squat exercise in elite athletes. Scand. J. Med. Sci. Sports 2015, 25, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Brandon, R.; Hunter, A.M. The response to and recovery from maximum-strength and-power training in elite track and field athletes. Int. J. Sports Physiol. Perform. 2016, 11, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.T.; Lamb, L.K.; Twist, C. The intra- and inter-day reproducibility of the FitroDyne as a measure of multi-jointed muscle function. Isokinet. Exerc. Sci. 2016, 24, 39–49. [Google Scholar] [CrossRef]
- MacDonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam rolling as a recovery tool after an intense bout of physical activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Pollock, M. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Heyward, V.H.; Wagner, D.H. Applied Body Composition Assessment; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Wathen, D. Load assignment. In Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 1994; pp. 435–446. [Google Scholar]
- LeSuer, D.A.; McCormick, J.H.; Mayhew, J.L.; Wasserstein, R.L.; Arnold, M.D. The accuracy of prediction equations for estimating 1-RM Perf in the bench press, squat, and deadlift. J. Strength Cond. Res. 1997, 11, 211–213. [Google Scholar]
- Morton, J.P.; Atkinson, G.; MacLaren, D.P.; Cable, N.T.; Gilbert, G.; Broome, C.; McArdle, A.; Drust, B. Reliability of maximal muscle force and voluntary activation as markers of exercise-induced muscle damage. Eur. J. Appl. Physiol. 2005, 94, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.W.; Foster, C.; McGuigan, M.R.; Brice, G. Quantitation of resistance training using the session rating of perceived exertion method. J. Strength Cond. Res. 2004, 18, 796–802. [Google Scholar] [PubMed]
- Day, M.L.; McGuigan, M.R.; Brice, G.; Foster, C. Monitoring exercise intensity during resistance training using the session RPE scale. J. Strength Cond. Res. 2004, 18, 353–358. [Google Scholar] [PubMed]
- Baldari, C.; Bonavolontà, V.; Emerenziani, G.P.; Gallotta, M.C.; Silva, A.J.; Guidetti, L. Accuracy, reliability, linearity of Accutrend and Lactate Pro versus EBIO plus analyzer. Eur. J. Appl. Physiol. 2009, 107, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.; Batterham, A.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Twist, C.; Eston, R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur. J. Appl. Physiol. 2005, 94, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience 2007, 11, 16–20. [Google Scholar]
- DeMorree, H.M.; Klein, C.; Marcora, S.M. Perception of effort reflects central motor command during movement execution. Psychophysiology 2012, 49, 1242–1253. [Google Scholar]
- Genner, K.M.; Weston, M. A comparison of workload quantification methods in relation to physiological responses to resistance exercise. J. Strength Cond. Res. 2014, 28, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, F.M.; Rampinini, E.; Coutts, A.J.; Sassi, A.; Marcora, S.M. Use of RPE-based training load in soccer. Med. Sci. Sports Exerc. 2004, 36, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Avela, J.; Kyröläinen, H.; Komi, P.V.; Rama, D. Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J. Appl. Physiol. 1999, 86, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Horita, T.; Komi, P.V.; Nicol, C.; Kyröläinen, H. Effect of exhausting stretch-shortening cycle exercise on the time course of mechanical behaviour in the drop jump: Possible role of muscle damage. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Doguet, V.; Jubeau, M.; Dorel, S.; Couturier, A.; Lacourpaille, L.; Guével, A.; Guilhem, G. Time-course of neuromuscular changes during and after maximal eccentric contractions. Front. Physiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Trinity, J.D.; Hart, C.R.; Kim, S.E.; Groot, H.J.; Le Fur, Y.; Sorensen, J.R.; Jeong, E.K.; Richardson, R.S. In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin. Sci. 2014, 126, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Rezk, C.C.; Marrache, R.C.B.; Tinucci, T.; Mion, D.; Forjaz, C.L.M. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: Influence of exercise intensity. Eur. J. Appl. Physiol. 2006, 98, 105–112. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Young (n = 9) | Middle-Aged (n = 9) | Effect Size |
|---|---|---|---|
| Age (y) | 22.3 ± 1.7 | 39.9 ± 6.2 | Most likely ↑ |
| 3.70 (2.87, 4.53) | |||
| Mass (kg) | 82.0 ± 9.0 | 79.1 ± 10.3 | Unclear |
| 0.29 (−1.10, 0.52) | |||
| Fat-free mass (kg) | 71.4 ± 7.9 | 63.9 ± 6.5 | Very likely ↓ |
| −1.02 (−1.83, −0.22) | |||
| Fat-mass (kg) | 10.5 ± 4.5 | 15.2 ± 5.7 | Likely ↑ |
| 0.89 (0.09, 1.70) | |||
| Body fat (%) | 12.8 ± 4.7 | 18.8 ± 5.8 | Very likely ↑ |
| 1.13 (0.32, 1.94) | |||
| Sum of skinfolds (mm) | 82.3 ± 24.6 | 102.4 ± 31.9 | Likely ↑ |
| 0.69 (−0.12, 1.50) | |||
| Squat 1RM (kg) | 130.8 ± 26.8 | 109.3 ± 22.5 | Unclear |
| −0.85 (−1.65, −0.04) |
| Load Ratio | Young | Middle-Aged | Effect Size |
|---|---|---|---|
| HR: peak velocity | 0.7 ± 0.2 | 0.7 ± 0.2 | Unclear |
| 0.10 (−0.71, 0.90) | |||
| HR:peak power | 5.2 ± 2.0 | 4.3 ± 1.3 | Unclear |
| −0.51 (−1.32, 0.30) | |||
| HR:volume load | 52.2 ± 11.8 | 47.0 ± 13.0 | Unclear |
| −0.41 (−1.22, 0.39) | |||
| OMNI-RPE: peak velocity | 12.6 ± 3.3 | 13.3 ± 2.7 | Unclear |
| 0.21 (−0.60, 1.01) | |||
| OMNI-RPE: peak power | 99.5 ± 36.6 | 84.8 ± 23.1 | Unclear |
| −0.47 (−1.28, 0.34) | |||
| OMNI-RPE: volume load | 1030.2 ± 244.6 | 968.5 ± 451.2 | Unclear |
| −0.14 (−0.95, 0.68) |
| Fatigue Indicators | Group | Pre | Post | Comparison |
|---|---|---|---|---|
| MVC (N/m) | Young | 265.7 ± 95.8 | 179.2 ± 60.7 | Unclear |
| Middle-aged | 199.1 ± 63.3 | 144.9 ± 55.4 | −0.56 (−1.37, 0.25) | |
| VA (%) | Young | 93.4 ± 5.8 | 85.3 ± 9.4 | Unclear |
| Middle-aged | 93.6 ± 5.6 | 82.9 ± 12.9 | −0.20 (−1.00, 0.61) | |
| Resting doublet (N/m) | Young | 85.1 ± 10.4 | 64.2 ± 10.4 | Very likely ↓ |
| Middle-aged | 69.2 ± 21.1 | 48.3 ± 9.3 | −1.53 (−2.34, −0.71) | |
| 20% 1RM peak power (W) | Young | 507.9 ± 134.6 | 486.6 ± 112.7 | Very likely ↓ |
| Middle-aged | 387.4 ± 87.9 | 357.6 ± 86.2 | −1.21 (−2.03, −0.39) | |
| 80% 1RM peak power (W) | Young | 1295.3 ± 369.1 | 1098.5 ± 307.1 | Very likely ↓ |
| Middle-aged | 977.1 ± 211.1 | 831.9 ± 215.2 | −0.94 (−1.76, −0.12) | |
| Blood lactate (mmol·L−1) | Young | 1.9 ± 0.7 | 9.8 ± 2.9 | Unclear |
| Middle-aged | 1.6 ± 0.4 | 8.1 ± 5.2 | −0.39 (−1.18, 0.40) |
| Load | Load Markers | MVC | Peak Power | |
|---|---|---|---|---|
| 20% 1RM | 80% 1RM | |||
| Internal | ∆Heart rate | Likely | Unclear | Very likely |
| 0.45 (0.06, 0.72) | 0.28 (−0.14, 0.61) | 0.50 (0.13, 0.75) | ||
| Mean OMNI-RPE | Unclear | Likely | Likely | |
| −0.06 (−0.45, 0.35) | 0.36 (−0.05, 0.66) | 0.32 (−0.09, 0.64) | ||
| sRPE | Unclear | Unclear | Unclear | |
| 0.07 (−0.34, 0.46) | 0.18 (−0.24, 0.54) | 0.29 (−0.13, 0.62) | ||
| BLA increase | Unclear | Unclear | Unclear | |
| 0.22 (−0.57, 0.2) | −0.20 (−0.55, 0.22) | −0.19 (−0.55, 0.23) | ||
| External | Mean peak velocity | Unclear | Unclear | Unclear |
| −0.05 (−0.44, 0.36) | 0.04 (−0.37, 0.43) | 0.02 (−0.38, 0.42) | ||
| Mean peak power | Likely | Likely | Likely | |
| 0.38 (−0.03, 0.68) | 0.43 (0.03, 0.71) | 0.35 (−0.06, 0.66) | ||
| Volume load | Very likely | Very likely | Very likely | |
| 0.59 (0.24, 0.80) | 0.55 (0.19, 0.78) | 0.50 (0.13, 0.75) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise †. J. Funct. Morphol. Kinesiol. 2018, 3, 45. https://doi.org/10.3390/jfmk3030045
Fernandes JFT, Lamb KL, Twist C. Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise †. Journal of Functional Morphology and Kinesiology. 2018; 3(3):45. https://doi.org/10.3390/jfmk3030045
Chicago/Turabian StyleFernandes, John F. T., Kevin L. Lamb, and Craig Twist. 2018. "Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise †" Journal of Functional Morphology and Kinesiology 3, no. 3: 45. https://doi.org/10.3390/jfmk3030045
APA StyleFernandes, J. F. T., Lamb, K. L., & Twist, C. (2018). Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise †. Journal of Functional Morphology and Kinesiology, 3(3), 45. https://doi.org/10.3390/jfmk3030045







