Iliotibial Band Behavior Assessed Through Tensor Fasciae Latae Electromyographic Activity with Different Foot Orthoses in Recreational Runners According to Foot Type: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
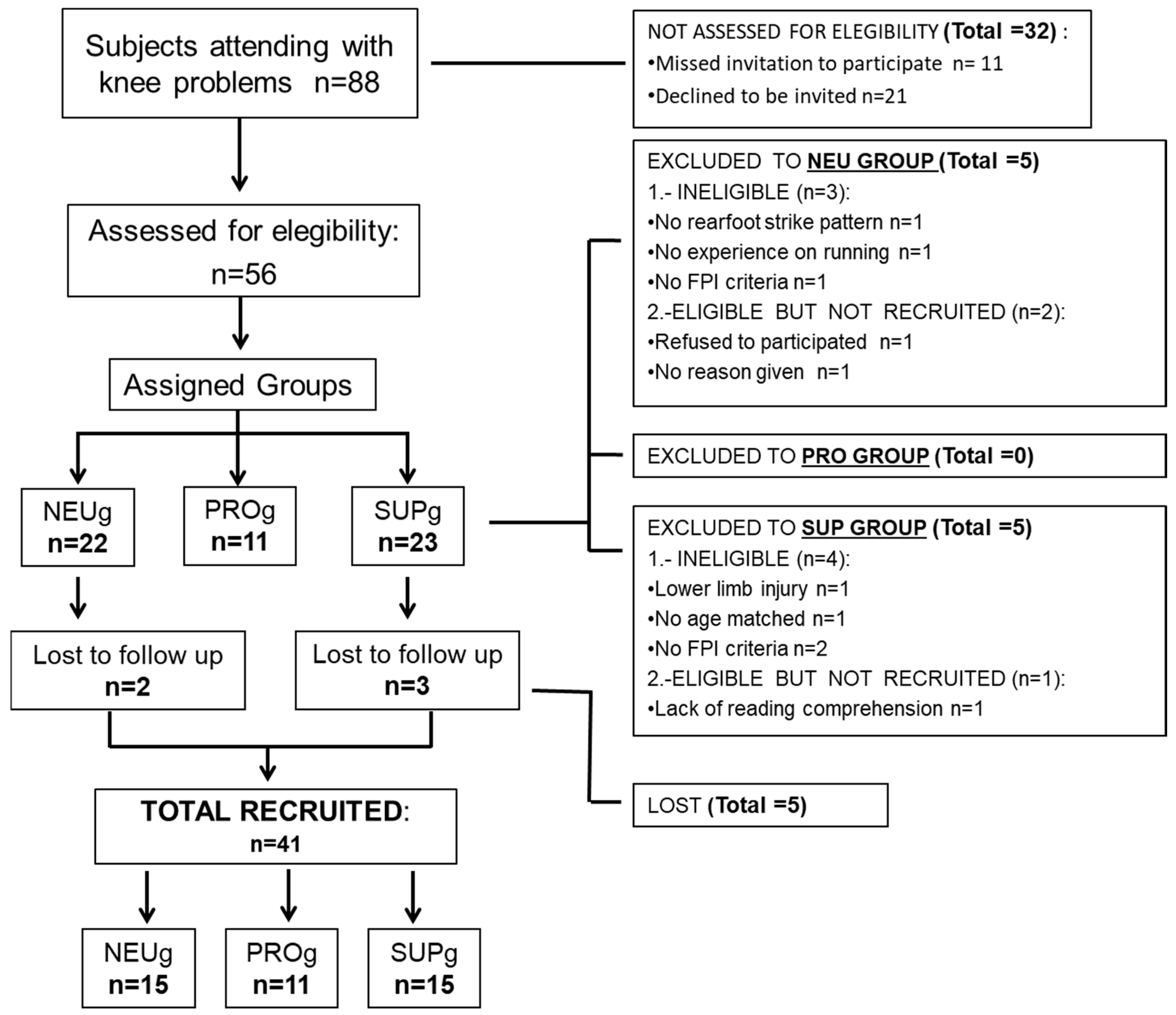
2.1. Sample Size and Study Design
2.2. Participants
2.3. Instruments and Assessments
2.4. Materials
2.5. Procedure
Running Test
2.6. Statistical Analysis
3. Results
3.1. Results for Neutral Group Foot Type
3.2. Results for Supinated Group Foot Type
- -
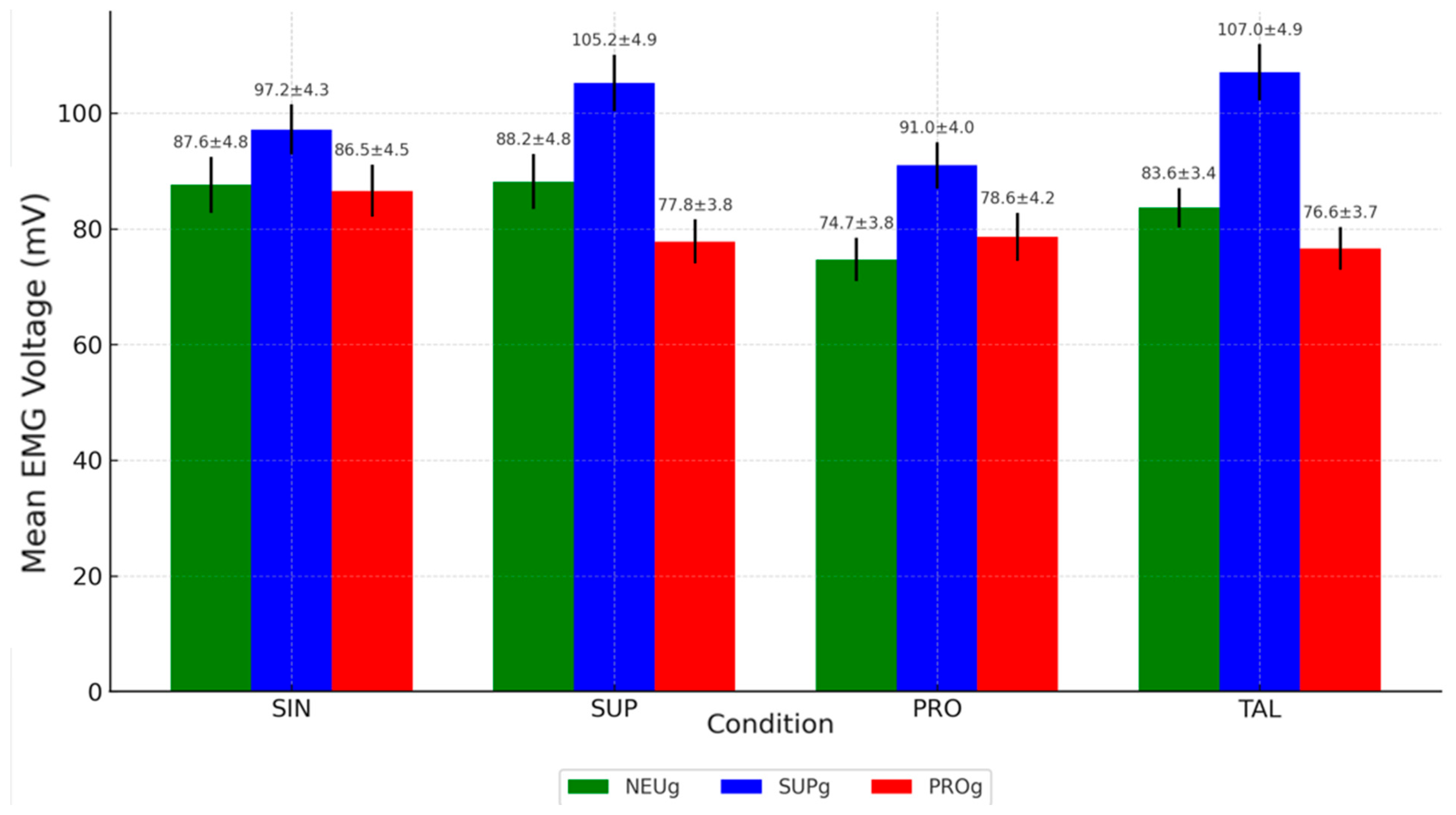
- EMG activity was significantly higher in the SUP (105.22 ± 4.9 mV) and TAL (107.02 ± 4.88 mV) conditions compared to the SIN condition (97.17 ± 4.3 mV), both with p-values < 0.05.
- -
- Both SUP and TAL conditions also showed significantly higher values than PRO (90.96 ± 4 mV), with p-values < 0.05 and <0.001, respectively.
3.3. Results for Pronated Group Foot Type
4. Discussion
Limitations and Future Lines of Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clement, D.B.; Taunton, J.E.; Smart, G.W.; McNicol, K.L. A Survey of Overuse Running Injuries. Physician Sportsmed. 1981, 9, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Friede, M.C.; Innerhofer, G.; Fink, C.; Alegre, L.M.; Csapo, R. Conservative Treatment of Iliotibial Band Syndrome in Runners: Are We Targeting the Right Goals? Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2022, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.B. The Iliotibial Tract; Clinical and Morphological Significance. J. Bone Jt. Surg. Am. 1958, 40-A, 817–832. [Google Scholar] [CrossRef]
- Fairclough, J.; Hayashi, K.; Toumi, H.; Lyons, K.; Bydder, G.; Phillips, N.; Best, T.M.; Benjamin, M. The Functional Anatomy of the Iliotibial Band during Flexion and Extension of the Knee: Implications for Understanding Iliotibial Band Syndrome. J. Anat. 2006, 208, 309–316. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Lichtwark, G.A.; Willy, R.W.; Kelly, L.A. The Iliotibial Band: A Complex Structure with Versatile Functions. Sports Med. 2022, 52, 995–1008. [Google Scholar] [CrossRef]
- Baker, R.L.; Fredericson, M. Iliotibial Band Syndrome in Runners: Biomechanical Implications and Exercise Interventions. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 53–77. [Google Scholar] [CrossRef]
- Jelsing, E.J.; Finnoff, J.T.; Cheville, A.L.; Levy, B.A.; Smith, J. Sonographic Evaluation of the Iliotibial Band at the Lateral Femoral Epicondyle: Does the Iliotibial Band Move? J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2013, 32, 1199–1206. [Google Scholar] [CrossRef]
- Lavine, R. Iliotibial Band Friction Syndrome. Curr. Rev. Musculoskelet. Med. 2010, 3, 18–22. [Google Scholar] [CrossRef]
- Fredericson, M.; Weir, A. Practical Management of Iliotibial Band Friction Syndrome in Runners. Clin. J. Sport. Med. 2006, 16, 261–268. [Google Scholar] [CrossRef]
- Fairclough, J.; Hayashi, K.; Toumi, H.; Lyons, K.; Bydder, G.; Phillips, N.; Best, T.M.; Benjamin, M. Is Iliotibial Band Syndrome Really a Friction Syndrome? J. Sci. Med. Sport. 2007, 10, 74–76; discussion 77–78. [Google Scholar] [CrossRef]
- van der Worp, M.P.; van der Horst, N.; de Wijer, A.; Backx, F.J.G.; Nijhuis-van der Sanden, M.W.G. Iliotibial Band Syndrome in Runners: A Systematic Review. Sports Med. 2012, 42, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Geisler, P.R. Iliotibial Band Pathology: Synthesizing the Available Evidence for Clinical Progress. J. Athl. Train. 2020, 56, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the Hip: A Focus on Muscular Actions. J. Orthop. Sports Phys. Ther. 2010, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Selkowitz, D.M.; Beneck, G.J.; Powers, C.M. Comparison of Electromyographic Activity of the Superior and Inferior Portions of the Gluteus Maximus Muscle During Common Therapeutic Exercises. J. Orthop. Sports Phys. Ther. 2016, 46, 794–799. [Google Scholar] [CrossRef]
- Huerta, J.P.; Moreno, J.M.R.; Kirby, K.A.; Carmona, F.J.G.; García, A.M.O. Effect of 7-Degree Rearfoot Varus and Valgus Wedging on Rearfoot Kinematics and Kinetics during the Stance Phase of Walking. J. Am. Podiatr. Med. Assoc. 2009, 99, 415–421. [Google Scholar] [CrossRef]
- Stickley, C.D.; Presuto, M.M.; Radzak, K.N.; Bourbeau, C.M.; Hetzler, R.K. Dynamic Varus and the Development of Iliotibial Band Syndrome. J. Athl. Train. 2018, 53, 128–134. [Google Scholar] [CrossRef]
- Nguyen, A.P.; Detrembleur, C.; Van Cant, J. Conservative Treatment for Iliotibial Band Syndrome: Are We Facing a Research Gap? A Scoping Review of 98 Studies with Clinical Perspectives. Phys. Ther. Sport. 2023, 62, 25–31. [Google Scholar] [CrossRef]
- Noble, C.A. The Treatment of Iliotibial Band Friction Syndrome. Br. J. Sports Med. 1979, 13, 51–54. [Google Scholar] [CrossRef]
- Geraci, M.C.; Brown, W. Evidence-Based Treatment of Hip and Pelvic Injuries in Runners. Phys. Med. Rehabil. Clin. North. Am. 2005, 16, 711–747. [Google Scholar] [CrossRef]
- Gordon, J.E.; Davis, L.E. Leg Length Discrepancy: The Natural History (And What Do We Really Know). J. Pediatr. Orthop. 2019, 39, S10–S13. [Google Scholar] [CrossRef]
- Valmassy, R.L. Clinical Biomechanics of the Lower Extremities; Mosby: St. Louis, MO, USA, 1995. [Google Scholar]
- Dananberg, H.J. Gait Style as an Etiology to Chronic Postural Pain. Part I. Functional Hallux Limitus. J. Am. Podiatr. Med. Assoc. 1993, 83, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.A.; Howe, E.E.; Perry, S.D. The Effects of Foot Orthoses and Sensory Facilitation on Lower Limb Electromyography: A Scoping Review. Foot 2022, 52, 101904. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J.; Harnett, M.C.; Pinfold, S.C. Does Short-Term Gluteal Activation Enhance Muscle Performance? Res. Sports Med. 2017, 25, 156–165. [Google Scholar] [CrossRef]
- Xie, P.; István, B.; Liang, M. The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis. Healthc. 2022, 11, 99. [Google Scholar] [CrossRef]
- Smallcomb, M.; Khandare, S.; Vidt, M.E.; Simon, J.C. Therapeutic Ultrasound and Shockwave Therapy for Tendinopathy: A Narrative Review. Am. J. Phys. Med. Rehabil. 2022, 101, 801–807. [Google Scholar] [CrossRef]
- Muyor, J.M.; Martín-Fuentes, I.; Rodríguez-Ridao, D.; Antequera-Vique, J.A. Electromyographic Activity in the Gluteus Medius, Gluteus Maximus, Biceps Femoris, Vastus Lateralis, Vastus Medialis and Rectus Femoris during the Monopodal Squat, Forward Lunge and Lateral Step-Up Exercises. PLoS ONE 2020, 15, e0230841. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Orien, W.P.; Root, J.W.M. Normal and Abnormal Function of the Foot; Corp, C.B., Ed.; Clinical Biomechanics Corp: Los Angeles, CA, USA, 1977; Volume II. [Google Scholar]
- Sánchez-Gómez, R.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Calvo-Lobo, C.; Navarro-Flores, E.; Palomo-López, P.; Romero-Morales, C.; López-López, D. Reliability Study of Diagnostic Tests for Functional Hallux Limitus. Foot Ankle Int. 2020, 41, 457–462. [Google Scholar] [CrossRef]
- Redmond, A.C.; Crosbie, J.; Ouvrier, R.A. Development and Validation of a Novel Rating System for Scoring Standing Foot Posture: The Foot Posture Index. Clin. Biomech. 2006, 21, 89–98. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Becerro-de-Bengoa-Vallejo, R.; Morales, C.R.; Losa-Iglesias, M.E.; de la Fuente, A.C.; López-López, D.; Vega, I.D.; Calvo-Lobo, C. Muscle Activity of the Triceps Surae with Novel Propulsion Heel-Lift Orthotics in Recreational Runners. Orthop. J. Sports Med. 2020, 8, 2325967120956914. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Romero-Morales, C.; Gómez-Carrión, Á.; De-La-Cruz-Torres, B.; Zaragoza-García, I.; Anttila, P.; Kantola, M.; Ortuño-Soriano, I. Effects of Novel Inverted Rocker Orthoses for First Metatarsophalangeal Joint on Gastrocnemius Muscle Electromyographic Activity during Running: A Cross-Sectional Pilot Study. Sensors 2020, 20, 3205. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, M.B.; de Pedro, A.I.B.; Rubio, Á.A.; Muñoz, J.L.M.; Lougedo, J.H. Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria. Sensors 2024, 24, 1223. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.; Lin, K.-L.; Shiang, T.-Y. Is the Foot Striking Pattern More Important than Barefoot or Shod Conditions in Running? Gait Posture 2013, 38, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.L.; Souza, R.B.; Rauh, M.J.; Fredericson, M.; Rosenthal, M.D. Differences in Knee and Hip Adduction and Hip Muscle Activation in Runners with and Without Iliotibial Band Syndrome. Phys. Med. Rehabil. 2018, 10, 1032–1039. [Google Scholar] [CrossRef]
- Fleming, N.; Walters, J.; Grounds, J.; Fife, L.; Finch, A. Acute Response to Barefoot Running in Habitually Shod Males. Human. Mov. Sci. 2015, 42, 27–37. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Saunders, P.U.; Pyne, D.B.; Telford, R.D.; Hawley, J.A. Reliability and Variability of Running Economy in Elite Distance Runners. Med. Sci. Sports Exerc. 2004, 36, 1972–1976. [Google Scholar] [CrossRef]
- Levinger, P.; Menz, H.B.; Fotoohabadi, M.R.; Feller, J.A.; Bartlett, J.R.; Bergman, N.R. Foot Posture in People with Medial Compartment Knee Osteoarthritis. J. Foot Ankle Res. 2010, 3, 29. [Google Scholar] [CrossRef]
- Abourazzak, F.E.; Kadi, N.; Azzouzi, H.; Lazrak, F.; Najdi, A.; Nejjari, C.; Harzy, T. A Positive Association between Foot Posture Index and Medial Compartment Knee Osteoarthritis in Moroccan People. Open Rheumatol. J. 2014, 8, 96–99. [Google Scholar] [CrossRef]
- Hadley, A.; Griffiths, S.; Griffiths, L.; Vicenzino, B. Antipronation Taping and Temporary Orthoses. Effects on Tibial Rotation Position after Exercise. J. Am. Podiatr. Med. Assoc. 1999, 89, 118–123. [Google Scholar] [CrossRef]
- Duval, K.; Lam, T.; Sanderson, D. The Mechanical Relationship between the Rearfoot, Pelvis and Low-Back. Gait Posture 2010, 32, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Kakouris, N.; Yener, N.; Fong, D.T.P. A Systematic Review of Running-Related Musculoskeletal Injuries in Runners. J. Sport Health Sci. 2021, 10, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Vijittrakarnrung, C.; Mongkolpichayaruk, A.; Limroongreungrat, W.; Chuckpaiwong, B. Comparison of Foot Kinematics Between Normal Arch and Flexible Flatfoot Using the Oxford Foot Model: A Matched Case-Control Study. Foot Ankle Orthop. 2024, 9, 24730114241231245. [Google Scholar] [CrossRef]
- Orchard, J.W.; Fricker, P.A.; Abud, A.T.; Mason, B.R. Biomechanics of Iliotibial Band Friction Syndrome in Runners. Am. J. Sports Med. 1996, 24, 375–379. [Google Scholar] [CrossRef]
- Noehren, B.; Schmitz, A.; Hempel, R.; Westlake, C.; Black, W. Assessment of Strength, Flexibility, and Running Mechanics in Men with Iliotibial Band Syndrome. J. Orthop. Sports Phys. Ther. 2014, 44, 217–222. [Google Scholar] [CrossRef]
- Foch, E.; Reinbolt, J.A.; Zhang, S.; Fitzhugh, E.C.; Milner, C.E. Associations between Iliotibial Band Injury Status and Running Biomechanics in Women. Gait Posture 2015, 41, 706–710. [Google Scholar] [CrossRef]
- Mucha, M.D.; Caldwell, W.; Schlueter, E.L.; Walters, C.; Hassen, A. Hip Abductor Strength and Lower Extremity Running Related Injury in Distance Runners: A Systematic Review. J. Sci. Med. Sport. 2017, 20, 349–355. [Google Scholar] [CrossRef]
- Pettitt, R.; Dolski, A. Corrective Neuromuscular Approach to the Treatment of Iliotibial Band Friction Syndrome: A Case Report. J. Athl. Train. 2000, 35, 96–99. [Google Scholar]
- Foch, E.; Aubol, K.; Milner, C.E. Relationship between Iliotibial Band Syndrome and Hip Neuromechanics in Women Runners. Gait Posture 2020, 77, 64–68. [Google Scholar] [CrossRef]
- Distefano, L.J.; Blackburn, J.T.; Marshall, S.W.; Padua, D.A. Gluteal Muscle Activation during Common Therapeutic Exercises. J. Orthop. Sports Phys. Ther. 2009, 39, 532–540. [Google Scholar] [CrossRef]
- Lehecka, B.J.; Stoffregen, S.; May, A.; Thomas, J.; Mettling, A.; Hoover, J.; Hafenstine, R.; Hakansson, N.A. Gluteal Muscle Activation During Common Yoga Poses. Int. J. Sports Phys. Ther. 2021, 16, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Gasibat, Q.; Alexe, C.I.; Raveica, G.; Tohănean, D.I.; Vasilios, K.; Alexe, D.I. Decoding Hip Muscle Activation: A Comparative Electromyographic Analysis of Turn-Out Bent Knee Pulse and Single-Leg Banded Glute Bridge Exercises in Healthy Female Subjects. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.P.L.; Rodrigues, H.L.d.N.; Coelho, B.A.L.; Rodrigues, C.A.S.; de Paula Lima, P.O. Anteromedial versus Posterolateral Hip Musculature Strengthening with Dose-Controlled in Women with Patellofemoral Pain: A Randomized Controlled Trial. Phys. Ther. Sport. 2021, 49, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Giorgi, F.; Donati, D. Footwear and Foot Health: Unveiling the Role of Proper Shoe Fit in Preventing Podiatric Issues and Enhancing Well-Being. Appl. Sci. 2024, 14, 9938. [Google Scholar] [CrossRef]
- Stevens, P.M.; Rheinstein, J.; Wurdeman, S.R. Prosthetic Foot Selection for Individuals with Lower-Limb Amputation: A Clinical Practice Guideline. J. Prosthet. Orthot. 2018, 30, 175–180. [Google Scholar] [CrossRef]
- Vogt, B.; Gosheger, G.; Wirth, T.; Horn, J.; Rödl, R. Leg Length Discrepancy- Treatment Indications and Strategies. Dtsch. Arztebl. Int. 2020, 117, 405–411. [Google Scholar] [CrossRef]
- Harvey, W.F.; Yang, M.; Cooke, T.D.V.; Segal, N.A.; Lane, N.; Lewis, C.E.; Felson, D.T. Association of Leg-Length Inequality with Knee Osteoarthritis: A Cohort Study. Ann. Intern. Med. 2010, 152, 287–295. [Google Scholar] [CrossRef]
- Grill, F.; Chochole, M.; Schultz, A. Pelvic tilt and leg length discrepancy. Orthopade 1990, 19, 244–262. [Google Scholar]
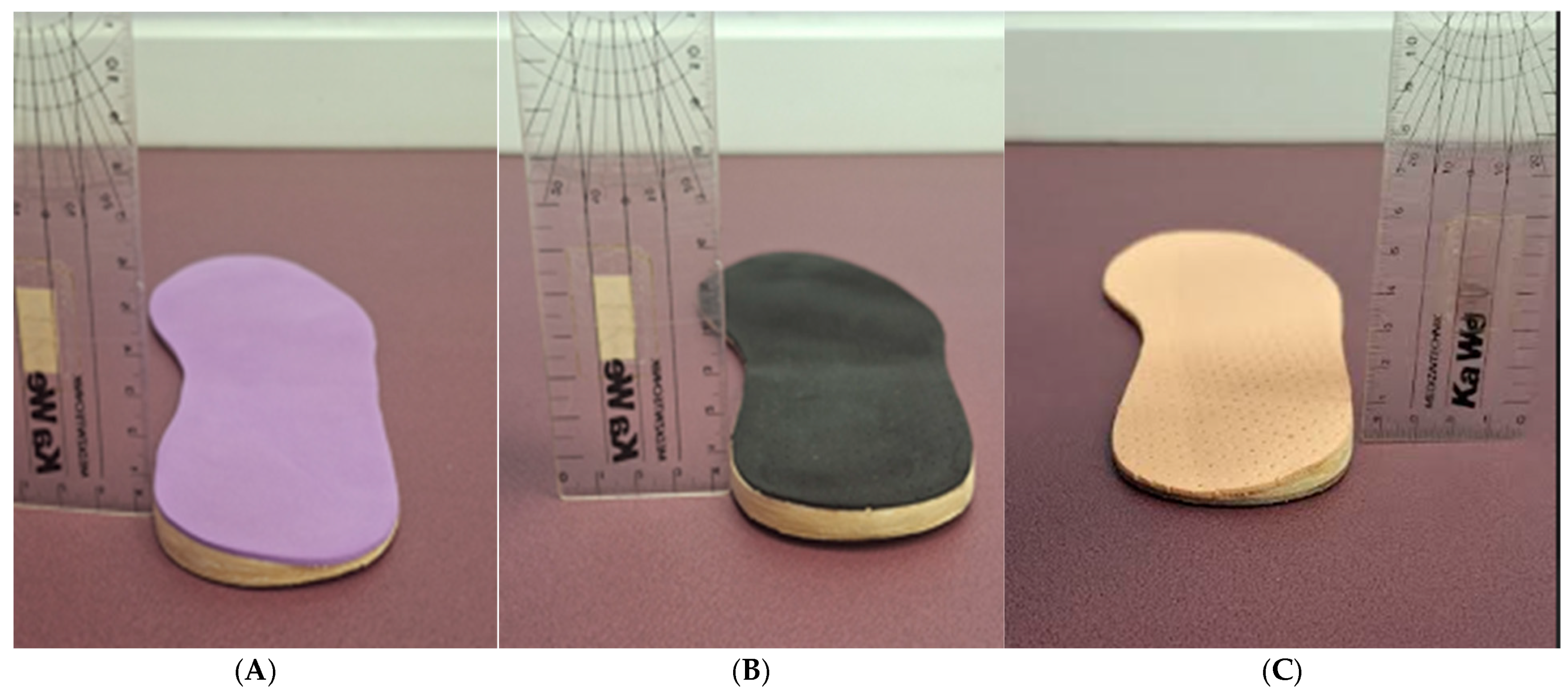
| Total Population N = 41 | Neutral Group (NEUg) | Supinated Group (SUPg) | Pronated Group (PROg) | ||
|---|---|---|---|---|---|
| n = 15 | n = 15 | n = 11 | |||
| Variable | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | F p-Value |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| % | % | % | % | ||
| age (years) | 32.66 ± 3.51 | 33 ± 3 | 36 ± 7 | 29 ± 4 | 7.62 p < 0.001 ** |
| (31.76–33.56) | (31.73–34.27) | (33.03–38.97) | (27.02–30.98) | ||
| height (cm) | 170 ± 1.43 | 168.87 ± 4 | 170 ± 2 | 171.72 ± 3 | 5.95 p < 0.001 ** |
| (169.64–170.36) | (167.18–170.56) | (169.16–170.84) | (170.2–173.2) | ||
| weight (kg) | 67 ± 3.17 | 61.4 ± 6 | 65.3 ± 2 | 67 ± 7 | 3.66 p < 0.05 * |
| (66.19–67.81) | (58.86–63.94) | (64.46–66.14) | (63.53–70.47) | ||
| foot size (Es) | 41.2 ± 0.9 | 41.2 ± 2.2 | 42.1 ± 1.8 | 40.3 ± 1.2 | 4.56 p < 0.001 ** |
| (40.97–41.43) | (40.27–42.13) | (41.34–42.86) | (39.71–40.89) | ||
| BMI (kg/m2) | 20.24 ± 0.19 | 20.3 ± 1.76 | 20.02 ± 2.04 | 20.4 ± 0.4 | 4.2 p < 0.05 * |
| (20.2–20.28) | (19.56–21.04) | (19.16–20.88) | (20.21–20.59) |
| Variable | Group 1 | Group 2 | Cohen’s d |
|---|---|---|---|
| Age | NEUg | SUPg | −0.557 |
| Age | NEUg | PROg | 1.159 |
| Age | SUPg | PROg | 1.179 |
| Height | NEUg | SUPg | −0.357 |
| Height | NEUg | PROg | −0.788 |
| Height | SUPg | PROg | −0.697 |
| Weight | NEUg | SUPg | −0.872 |
| Weight | NEUg | PROg | −0.87 |
| Weight | SUPg | PROg | −0.356 |
| Foot Size | NEUg | SUPg | −0.448 |
| Foot Size | NEUg | PROg | 0.486 |
| Foot Size | SUPg | PROg | 1.141 |
| BMI | NEUg | SUPg | 0.147 |
| BMI | NEUg | PROg | −0.073 |
| BMI | SUPg | PROg | −0.241 |
| Neutral Group (NEUg) | Supinated Group (SUPg) | Pronated Group (PROg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 15 | n = 15 | n = 11 | ||||||||
| Intervention | Mean (mV) ± SD (95% CI) | ICC 95% IC | SEM | Mean (mV) ± SD (95% CI) | ICC 95% IC | SEM | Mean (mV) ± SD (95% CI) | ICC 95% IC | SEM | F p-Value |
| (Li-Ls) | (Li-Ls) | (Li-Ls) | ||||||||
| SIN | 87.58 ± 4.81 (85.16–90) | 1 | 0.574 | 97.17 ± 4.3 (95.35–98.99) | 0.999 | 1.02 | 86.55 ± 4.5 (84.32–88.78) | 0.988 | 5.12 | 22.07 p < 0.001 ** |
| (1–1) | (0.999–1) | (0.966–0.996) | ||||||||
| SUP | 88.16 ± 4.76 (86.14–90.18) | 1 | 0.536 | 105.22 ± 4.9 (103.14–107.3) | 1 | 0.882 | 77.81 ± 3,8 (75.93–79.69) | 0.998 | 1.74 | 154.01 p < 0.001 ** |
| (1–1) | (0.999–1) | (0.994–0.999) | ||||||||
| PRO | 74.69 ± 3.77 (73.09–76.29) | 0.999 | 1.028 | 90.96 ± 4 (89.27–92.65) | 1 | 0.63 | 78.58 ± 4.2 (76.5–80.66) | 0.998 | 2 | 65.29 p < 0.001 ** |
| (0.998–1) | (0.999–1) | (0.994–0.999) | ||||||||
| TAL | 83.64 ± 3.4 (76.61–80.49) | 1 | 0.684 | 107.02 ± 4.88 (107.2–111.16) | 0.999 | 1.116 | 76.62 ± 3.7 (74.79–78.45) | 1 | 0.332 | 203.86 p < 0.001 ** |
| (0.999–1) | (0.999–1) | (1–1) | ||||||||
| Intervention | Group 1 | Group 2 | Cohen’s d |
|---|---|---|---|
| SIN | NEUg | SUPg | −2.102 |
| SIN | NEUg | PROg | 0.22 |
| SIN | SUPg | PROg | 2.422 |
| SUP | NEUg | SUPg | −3.532 |
| SUP | NEUg | PROg | 2.36 |
| SUP | SUPg | PROg | 6.126 |
| PRO | NEUg | SUPg | −4.186 |
| PRO | NEUg | PROg | −0.984 |
| PRO | SUPg | PROg | 3.031 |
| TAL | NEUg | SUPg | −5.559 |
| TAL | NEUg | PROg | 1.99 |
| TAL | SUPg | PROg | 6.867 |
|
NEUg SIN Mean (mV) ± SD (95% CI) | SUPg SIN Mean (mV) ± SD (95% CI) |
PROg SIN Mean (mV) ± SD (95% CI) | p-Value SIN NEUg vs. SUPg | p-Value SIN NEUg vs. PROg | p-Value SIN SUPg vs. PROg |
|---|---|---|---|---|---|
| 87.58 ± 4.81 (85.16–90) | 97.17 ± 4.3 (95.35–98.99) | 86.55 ± 4.5 (84.32–88.78) | <0.05 * | 1 | <0.05 * |
|
SIN Mean (mV) ± SD (95% CI) |
SUP Mean (mV) ± SD (95% CI) |
PRO Mean (mV) ± SD (95% CI) |
TAL Mean (mV) ± SD (95% CI) | p-Value SIN vs. SUP | p-Value SIN vs. PRO | p
-Value SIN vs. TAL | p-Value SUP vs. PRO | p
-Value SUP vs. TAL | p-Value PRO vs. TAL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral group (NEUg) | 87.58 ± 4.81 (85.16–90) | 88.16 ± 4.76 (86.14–90.18) | 74.69 ± 3.77 (73.09–76.29) | 83.64 ± 3.4 (76.61–80.49) | 1 | <0.001 ** | 0.86 | 0.08 | 1 | 1 |
| Supinated group (SUPg) | 97.17 ± 4.3 (95.35–98.99) | 105.22 ± 4.9 (103.14–107.3) | 90.96 ± 4 (89.27–92.65) | 107.02 ± 4.88 (107.2–111.16) | <0.05 * | <0.001 ** | <0.05 * | <0.05 * | 1 | <0.001 ** |
| Pronated group (PROg) | 86.55 ± 4.5 (84.32–88.78) | 77.81 ± 3.8 (75.93–79.69) | 78.58 ± 4.2 (76.5–80.66) | 76.62 ± 3.7 (74.79–78.45) | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Gomez, R.; Carrión, Á.G.; Ortuño Soriano, I.; Sanz Wozniak, P.; Zaragoza García, I.; Waer, F.B.; Alexe, C.I.; Alexe, D.I. Iliotibial Band Behavior Assessed Through Tensor Fasciae Latae Electromyographic Activity with Different Foot Orthoses in Recreational Runners According to Foot Type: A Cross-Sectional Study. J. Funct. Morphol. Kinesiol. 2025, 10, 237. https://doi.org/10.3390/jfmk10030237
Sanchez-Gomez R, Carrión ÁG, Ortuño Soriano I, Sanz Wozniak P, Zaragoza García I, Waer FB, Alexe CI, Alexe DI. Iliotibial Band Behavior Assessed Through Tensor Fasciae Latae Electromyographic Activity with Different Foot Orthoses in Recreational Runners According to Foot Type: A Cross-Sectional Study. Journal of Functional Morphology and Kinesiology. 2025; 10(3):237. https://doi.org/10.3390/jfmk10030237
Chicago/Turabian StyleSanchez-Gomez, Ruben, Álvaro Gómez Carrión, Ismael Ortuño Soriano, Paola Sanz Wozniak, Ignacio Zaragoza García, Fatma Ben Waer, Cristina Iona Alexe, and Dan Iulian Alexe. 2025. "Iliotibial Band Behavior Assessed Through Tensor Fasciae Latae Electromyographic Activity with Different Foot Orthoses in Recreational Runners According to Foot Type: A Cross-Sectional Study" Journal of Functional Morphology and Kinesiology 10, no. 3: 237. https://doi.org/10.3390/jfmk10030237
APA StyleSanchez-Gomez, R., Carrión, Á. G., Ortuño Soriano, I., Sanz Wozniak, P., Zaragoza García, I., Waer, F. B., Alexe, C. I., & Alexe, D. I. (2025). Iliotibial Band Behavior Assessed Through Tensor Fasciae Latae Electromyographic Activity with Different Foot Orthoses in Recreational Runners According to Foot Type: A Cross-Sectional Study. Journal of Functional Morphology and Kinesiology, 10(3), 237. https://doi.org/10.3390/jfmk10030237









